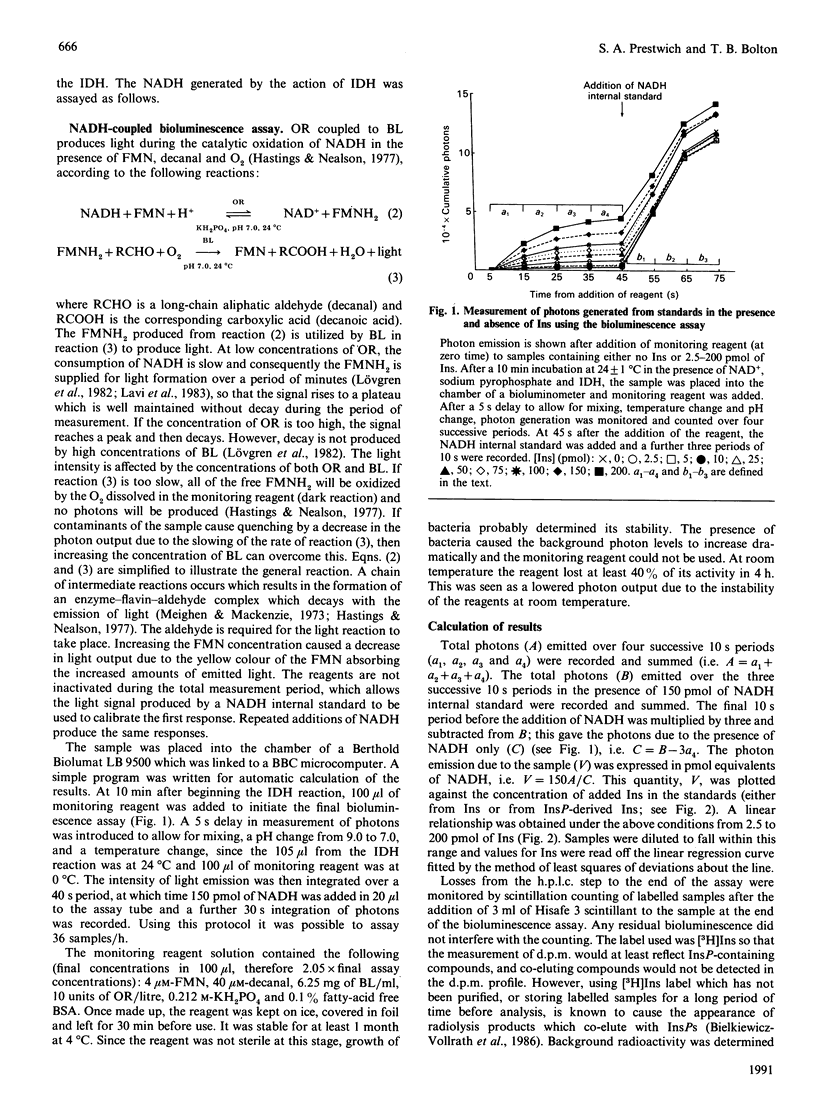
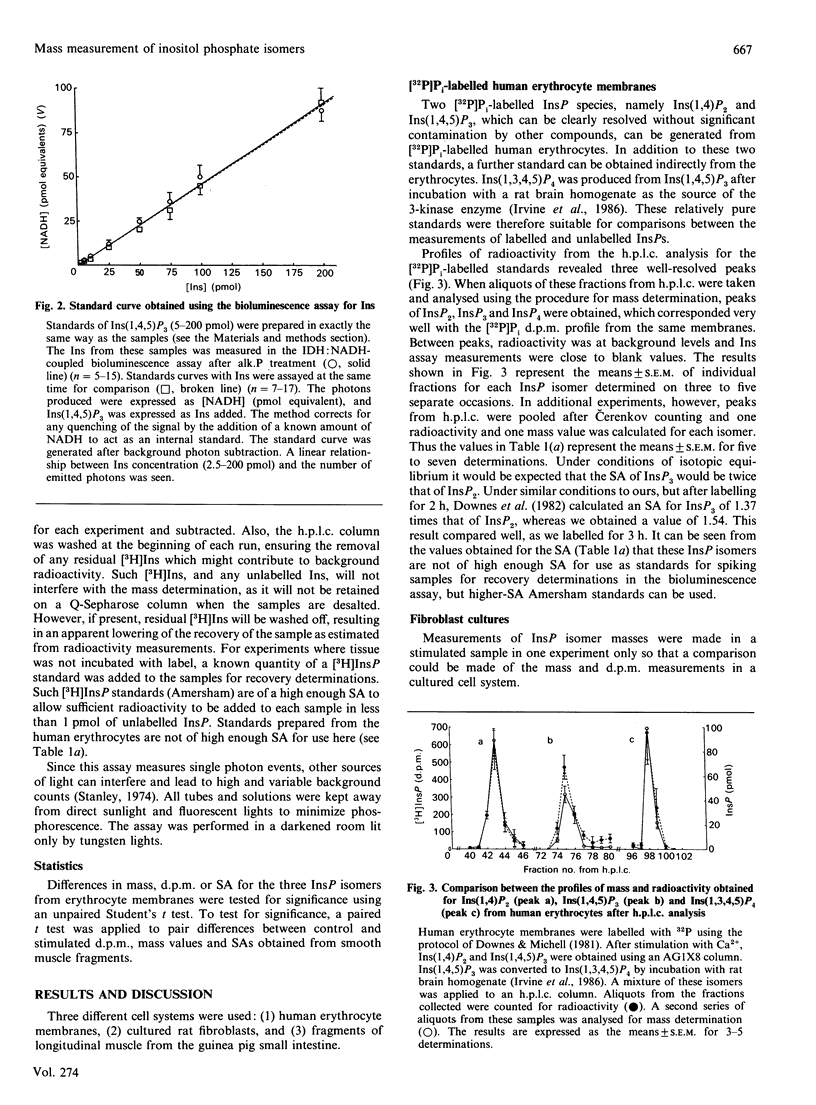
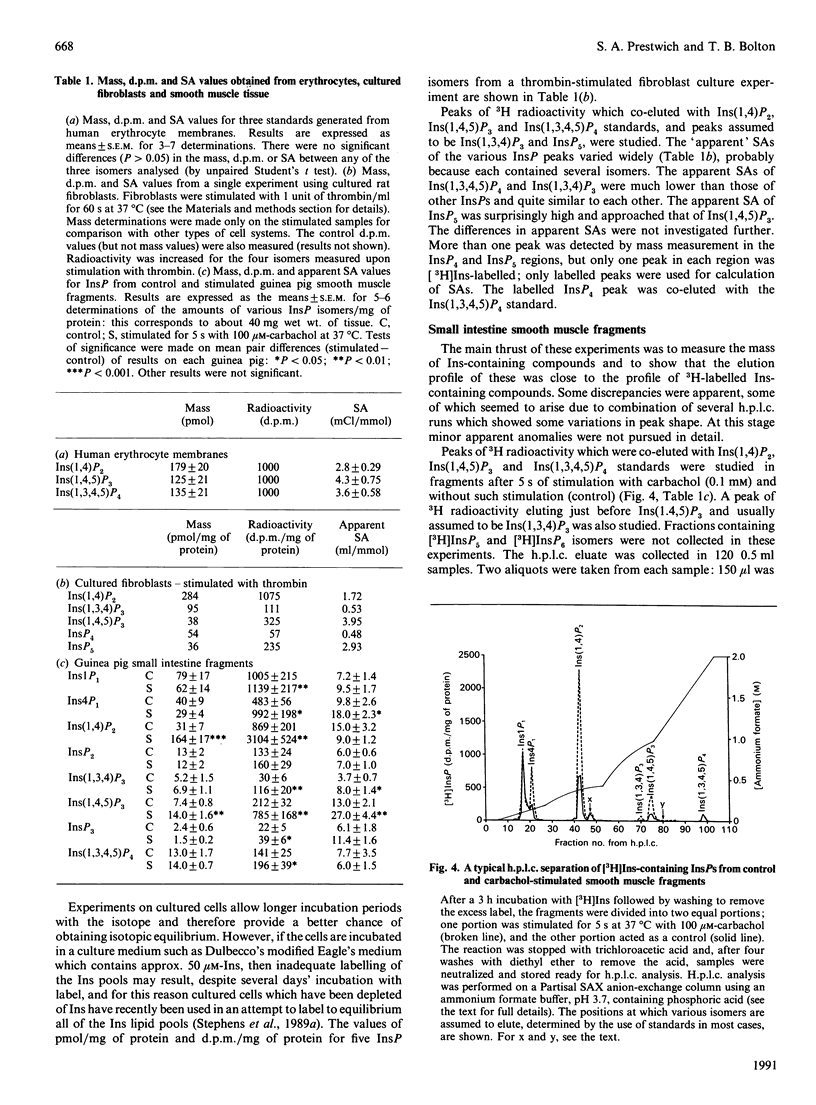
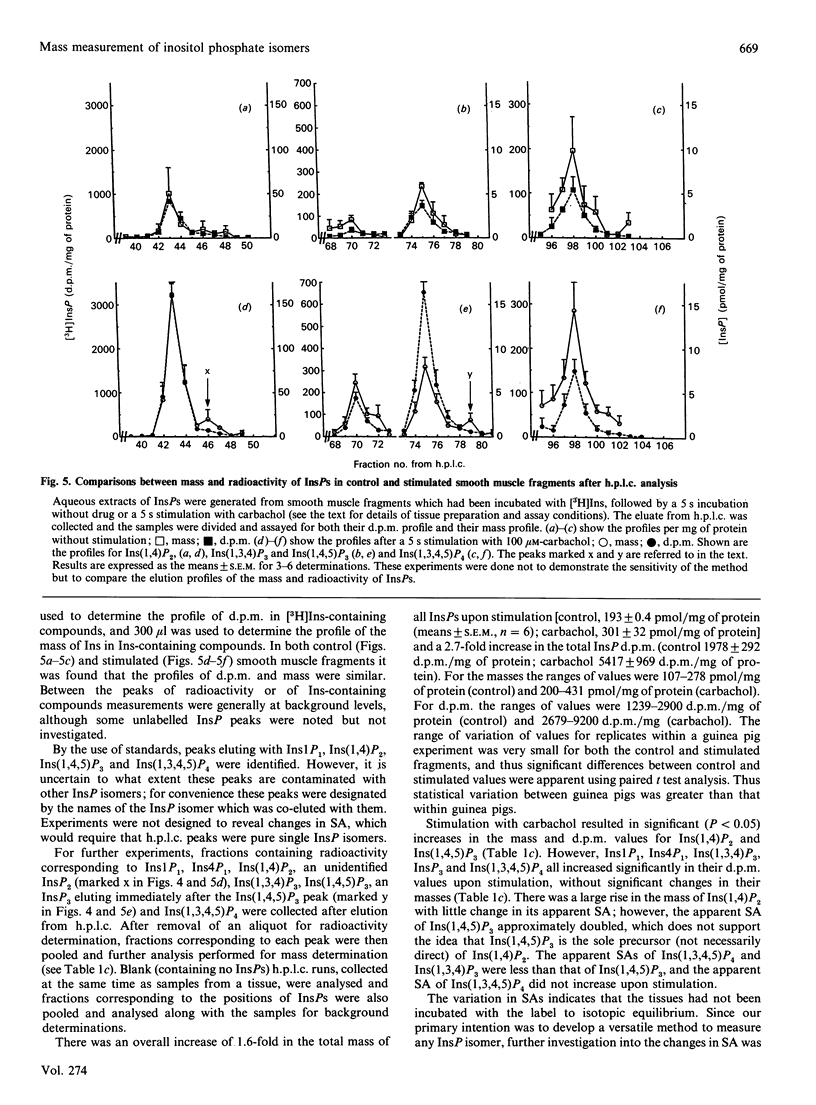
Abstract
An assay is described which allows the determination of the mass of any individual inositol phosphate (InsP) isomer by combining a popular h.p.l.c. separation method with simple desalting, dephosphorylation and final measurement of Ins liberated using an inositol dehydrogenase-NADH-linked bioluminescence reaction. The limit of sensitivity of this assay is about 1 pmol of Ins. routinely 5 pmol. About 40 mg wet wt. of guinea pig small intestine longitudinal smooth muscle contains 5 pmol of Ins(1,4,5)P3. For Inst(1,3,4)P3 or Ins(1,3,4,5)P4 slightly more smooth muscle is needed, and for major isomers of InsP1 or InsP2 10 mg wet wt. or less of tissue can be used. A 35 mm tissue culture plate with a confluent layer of rat fibroblasts contains about 30 pmol of Ins(1,4,5)P3. The method was applied to the measurement of the masses of Ins1P1. Ins4P1, Ins(1,4)P2, Ins(1,3,4)P3, Ins(1,4,5)P3, Ins(1,3,4,5)P4 and InsP5. The h.p.l.c. elution profiles of radiolabelled InsPS generated from [32P]Pi-labelled human Erythrocytes, [3H]Ins-labelled cultured rat fibroblasts and [3H]Ins-labelled smooth muscle fragments from guinea pig small intestine were compared with the h.p.l.c. elution profiles of their masses.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Quantitative changes in inositol 1,4,5-trisphosphate in chemoattractant-stimulated neutrophils. J Biol Chem. 1986 Nov 25;261(33):15644–15647. [PubMed] [Google Scholar]
- Bredt D. S., Mourey R. J., Snyder S. H. A simple, sensitive, and specific radioreceptor assay for inositol 1,4,5-trisphosphate in biological tissues. Biochem Biophys Res Commun. 1989 Mar 31;159(3):976–982. doi: 10.1016/0006-291x(89)92204-3. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Cantarow W., Stollar B. D. The use of bacterial luciferase and a liquid scintillation spectrometer to assay the enzymatic synthesis of NAD. Anal Biochem. 1976 Apr;71(2):333–340. doi: 10.1016/s0003-2697(76)80002-4. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Batty I. H., Nahorski S. R. Mass measurements of inositol(1,4,5)trisphosphate in rat cerebral cortex slices using a radioreceptor assay: effects of neurotransmitters and depolarization. Biochem Biophys Res Commun. 1988 Dec 15;157(2):684–691. doi: 10.1016/s0006-291x(88)80304-8. [DOI] [PubMed] [Google Scholar]
- Dangelmaier C. A., Daniel J. L., Smith J. B. Determination of basal and stimulated levels of inositol triphosphate in [32P]orthophosphate-labeled platelets. Anal Biochem. 1986 May 1;154(2):414–419. doi: 10.1016/0003-2697(86)90007-2. [DOI] [PubMed] [Google Scholar]
- Dean N. M., Beaven M. A. Methods for the analysis of inositol phosphates. Anal Biochem. 1989 Dec;183(2):199–209. doi: 10.1016/0003-2697(89)90468-5. [DOI] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Metabolism of inositol bis-, tris-, tetrakis- and pentakis-phosphates in GH3 cells. Biochem J. 1988 Mar 1;250(2):493–500. doi: 10.1042/bj2500493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascard P., Journet E., Sulpice J. C., Giraud F. Functional heterogeneity of polyphosphoinositides in human erythrocytes. Biochem J. 1989 Dec 1;264(2):547–553. doi: 10.1042/bj2640547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudermann T. W., Cooper T. G. A sensitive bioluminescence assay for myo-inositol. Anal Biochem. 1986 Oct;158(1):59–63. doi: 10.1016/0003-2697(86)90588-9. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Nealson K. H. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Heathers G. P., Juehne T., Rubin L. J., Corr P. B., Evers A. S. Anion exchange chromatographic separation of inositol phosphates and their quantification by gas chromatography. Anal Biochem. 1989 Jan;176(1):109–116. doi: 10.1016/0003-2697(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Takemura H., Putney J. W., Jr Formation and metabolism of [3H]inositol phosphates in AR42J pancreatoma cells. Substance P-induced Ca2+ mobilization in the apparent absence of inositol 1,4,5-trisphosphate 3-kinase activity. J Biol Chem. 1988 Oct 25;263(30):15297–15303. [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- King C. E., Stephens L. R., Hawkins P. T., Guy G. R., Michell R. H. Multiple metabolic pools of phosphoinositides and phosphatidate in human erythrocytes incubated in a medium that permits rapid transmembrane exchange of phosphate. Biochem J. 1987 May 15;244(1):209–217. doi: 10.1042/bj2440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavi J., Raunio R., Malkov Y., Lövgren T. The effect of luciferase and NADH:FMN oxidoreductase concentrations on the light kinetics of bacterial bioluminescence. Biochem Biophys Res Commun. 1983 Feb 28;111(1):266–273. doi: 10.1016/s0006-291x(83)80146-6. [DOI] [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. An enzymatic fluorimetric assay for myo-inositol. Anal Biochem. 1984 Sep;141(2):382–389. doi: 10.1016/0003-2697(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Mayr G. W. A novel metal-dye detection system permits picomolar-range h.p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988 Sep 1;254(2):585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J. L. Inositol bis-, tris-, and tetrakis(phosphate)s: analysis in tissues by HPLC. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4162–4166. doi: 10.1073/pnas.83.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen E. A., MacKenzie R. E. Flavine specificity of enzyme-substrate intermediates in the bacterial bioluminescent reaction. Structural requirements of the flavine side chain. Biochemistry. 1973 Apr 10;12(8):1482–1491. doi: 10.1021/bi00732a003. [DOI] [PubMed] [Google Scholar]
- Monaco M. E. Inositol metabolism in WRK-1 cells. Relationship of hormone-sensitive to -insensitive pools of phosphoinositides. J Biol Chem. 1987 Sep 25;262(27):13001–13006. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Palmer S., Wakelam M. J. The Ins(1,4,5)P3 binding site of bovine adrenocortical microsomes: function and regulation. Biochem J. 1989 Jun 1;260(2):593–596. doi: 10.1042/bj2600593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portilla D., Morrison A. R. Bradykinin-induced changes in inositol trisphosphate mass in MDCK cells. Biochem Biophys Res Commun. 1986 Oct 30;140(2):644–649. doi: 10.1016/0006-291x(86)90780-1. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Salmon D. M., Bolton T. B. Early events in inositol phosphate metabolism in longitudinal smooth muscle from guinea-pig intestine stimulated with carbachol. Biochem J. 1988 Sep 1;254(2):553–557. doi: 10.1042/bj2540553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayman J. A., Kirkwood M. T. Bradykinin-stimulated changes in inositol phosphate mass in renal papillary collecting tubule cells. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1119–1125. doi: 10.1016/0006-291x(87)91553-1. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Morrison A. R., Lowry O. H. Enzymatic fluorometric assay for myo-inositol trisphosphate. Anal Biochem. 1987 May 1;162(2):562–568. doi: 10.1016/0003-2697(87)90434-9. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Downes C. P. Product-precursor relationships amongst inositol polyphosphates. Incorporation of [32P]Pi into myo-inositol 1,3,4,6-tetrakisphosphate, myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol 3,4,5,6-tetrakisphosphate and myo-inositol 1,3,4,5,6-pentakisphosphate in intact avian erythrocytes. Biochem J. 1990 Jan 15;265(2):435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Downes C. P. An analysis of myo-[3H]inositol trisphosphates found in myo-[3H]inositol prelabelled avian erythrocytes. Biochem J. 1989 Sep 15;262(3):727–737. doi: 10.1042/bj2620727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Downes C. P. Metabolic and structural evidence for the existence of a third species of polyphosphoinositide in cells: D-phosphatidyl-myo-inositol 3-phosphate. Biochem J. 1989 Apr 1;259(1):267–276. doi: 10.1042/bj2590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarver A. P., King W. G., Rittenhouse S. E. Inositol 1,4,5-trisphosphate and inositol 1,2-cyclic 4,5-trisphosphate are minor components of total mass of inositol trisphosphate in thrombin-stimulated platelets. Rapid formation of inositol 1,3,4-trisphosphate. J Biol Chem. 1987 Dec 25;262(36):17268–17271. [PubMed] [Google Scholar]
- Vallejo M., Jackson T., Lightman S., Hanley M. R. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987 Dec 17;330(6149):656–658. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]


