Abstract
Background
This study aimed to evaluate the effects of physical activity on the cognition of patients with stroke, comparing the effectiveness of using isolated or combined rehabilitation, as well as the duration and intensity of training, to identify the characteristics of optimal training programs for post-stroke cognitive rehabilitation.
Methods
For this systematic review, we followed PRISMA guidelines and searched Web of Science, Scopus, PEDRo, SPORTdiscus, PubMed, Trial Registries, and Google Scholar for studies published between 2013 and April 12, 2023. We included randomized controlled trials (RCTs) of interventions that measured the effect of physical activity on cognition in patients with stroke. We restricted our search to reports published in the English language. Evidence from the RCTs was synthesized. The risk of bias was assessed using the Cochrane risk of bias tool.
Results
Of the 1,755 records identified, 34 were eligible, and data were available, with mainly low to moderate risk of general bias. The combined rehabilitation training programs proved more efficient when compared with isolated rehabilitation training programs in several cognitive domains. A moderate-intensity, 12-week intervention duration, with three weekly sessions, proved adequate.
Discussion
It seems that combined rehabilitation training programs are efficient for patients with stroke as these alter neuroplastic conditions due to synergistic or additional onset of action. Future research should investigate combined rehabilitation training programs, with follow-up, to assess how long the recorded improvements last. The protocol of this study is registered in PROSPERO, number CRD42021248533.
Keywords: Stroke, Physical exercise, Cognitive rehabilitation, Intervention type, Intervention duration
Resumo
Introdução
Este estudo pretende avaliar os efeitos da atividade física na cognição de pessoas com acidente vascular cerebral (AVC). Aqui, pretendemos comparar a eficácia do uso de programas de reabilitação isolada ou combinada, bem como a duração e intensidade dos programas, para identificar as características de programas de treino ideais para reabilitação cognitiva pós-AVC.
Métodos
Usámos as diretrizes PRISMA para realizar esta revisão sistemática e pesquisámos as seguintes bases de dados: Web of Science, Scopus, PEDRo, SPORTdiscus, PubMed, Trial Registries e Google Scholar para estudos publicados entre 2013 e 12 de abril de 2023. Incluímos ensaios clínicos aleatorizados (ECA) de intervenções que mediram o efeito da atividade física na cognição em pacientes com AVC. Restringimos a nossa busca a estudos publicados no idioma inglês. As evidências dos ECA foram sintetizadas e o risco de viés foi avaliado usando a ferramenta de risco de viés Cochrane.
Resultados
Dos 1755 registos identificados, 34 eram elegíveis e os dados estavam disponíveis, maioritariamente com risco baixo a moderado de viés geral. Os programas de treino de reabilitação combinados revelaram-se mais eficientes quando comparados com programas de treino de reabilitação isolados, em vários domínios cognitivos. Uma intervenção de intensidade moderada, duração de 12 semanas, com três sessões semanais, mostrou-se adequada.
Discussão
Parece que os programas combinados de treino de reabilitação são eficientes para pessoas com AVC, já que alteram as condições neuroplásticas devido à ação sinérgica ou adicional. Investigações futuras devem investigar programas combinados de treino de reabilitação, com follow-up para avaliar quanto tempo duram as melhorias registadas. O protocolo deste estudo está registado no PROSPERO, número CRD42021248533.
Palavras Chave: Acidente vascular cerebral, Exercício físico, Reabilitação cognitiva, Tipo de intervenção, Duração da intervenção
Introduction
Stroke is classically characterized as a neurological deficit resulting from an acute focal lesion of the central nervous system from vascular causes, including cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage [1, 2]. Impairments in cognitive function usually accompany the occurrence of stroke in people’s lives with an impact on daily life [3, 4], which are manifested in the 3–6 months after stroke [5]. Post-stroke cognitive impairments are currently the most disabling after-effects [6], with a prevalence of 16–53%, with approximately 10–20% of strokes being hemorrhagic and the remainder ischemic [7]. A 2011 study reported that worldwide, stroke is responsible for about 5.5 million deaths annually [8]. Evidence shows that one of the ways to reduce cognitive impairment is through cognitive rehabilitation, as it impacts even patients with chronic conditions. Cognitive rehabilitation can be accomplished in several ways, including through physical activity [9], since the regeneration of motor and cognitive functions is based on the plastic properties of the central nervous system and compensatory strategies to recover lost functions [10]. Evidence shows that physical activity improves muscle function, motor skills, cardiorespiratory function, and metabolic regulation [11–14].
Further, it decreases anxiety and depression levels and increases self-esteem [15], promotes social integration and the learning of social rules [16], and improves global cognitive functions [17]. It is well known that blood flow and oxygenation in cortical regions are influenced by the intensity of the exercise session [18, 19]. Further, increased prefrontal cortex oxygenation may indicate greater cortical activity, leading to enhanced cognitive processes such as working memory and attention [18, 20]. There is a consensus that physical activity is associated with a lower risk of stroke and mortality, as it is a potential preventive measure against cognitive decline [21].
Although a growing body of literature addresses the effect of physical activity on cognition, few address the post-stroke physical activity prescription and the impact of physical activity on post-stroke cognitive performance [21]. In a review, Barker and Eickmeyer [22] present general guidelines for physical activity, where the recommendation is that adults perform aerobic physical activity, emphasizing resistance or strength training, twice a week. Another study by Gambassi et al. [23] addresses resistance training for motor recovery of patients with stroke. Further, in a recent study, Izquierdo et al. [24] explain that the ideal prescription of exercises for preventing or treating dementia has yet to be defined.
There needs to be more literature clearly describing which type of physical activity training program is adequate to reduce post-stroke cognitive impairment and optimize recovery. Should the training program be isolated or combined? What should its duration, intensity, and weekly frequency be? In this context, an isolated intervention program is considered when only one type of activity is used during all interventions; in this case, only aerobic activity can be used, or resistance activity, strength activity, or postural balance, among others. When it comes to combined physical activity, there is a combination of physical activity, which can be aerobic with resistance or strength activity, among others. For example, in this line, it is known that aerobic activity improves cardiorespiratory fitness, increases the expression of neurotrophic factors, and increases hippocampal size [4, 25]; resistance activity increases muscle mass, strength, and power and improves executive function and selective attention [4, 26]; and strength activity enhances bone mineral density and neuromuscular performance, increases proteins involved in neuronal survival and synaptic plasticity in the hippocampus, improves memory [27, 28]. Thus, considering the gains each type of activity could bring the participants, a program that combines different types of activity per session throughout an intervention program might be even more efficient. Concerning duration, we find a variation of 1 day to more than a year. As for exercise intensity, there is also a variation from low to moderate to high intensity [29–31]. This shows that there is still debate about the type of physical activity program, ideal duration, and exercise intensity to lead to therapeutic benefits after stroke. Thus, this review aimed to evaluate the effects of physical activity programs on the cognition of patients with stroke, comparing the effectiveness of isolated or combined rehabilitation training programs and the duration and intensity of training to identify the most effective training programs for post-stroke cognitive rehabilitation.
Methods
Eligibility Criteria
Study eligibility was determined using the following criteria.
Population
Men and women 18 years of age and older with acute or chronic stroke in various stages participated.
Intervention
All types of physical activity for patients with stroke rehabilitation, defined as any bodily movement produced by the skeletal muscles that results in energy expenditure above resting levels, are performed in isolation or combination with other activities. There is no restriction on the duration of intervention programs, intensity, and frequency.
Comparator
Any type of activity was used as a control, from usual care, recreational activities, cognitive training, or even physical activity.
Outcome
Studies that assessed cognition in its various domains (such as attention and concentration, executive functions, memory, language, visuospatial ability, abstract thinking, calculation, and orientation) using either neuropsychological testing or imaging were included.
Study Design
Only randomized controlled trials were included. Non-experimental studies, single experimental designs, review and opinion articles, studies not published in English, and those published before 2013 were excluded.
Search Strategy
On April 12, 2023, the first author searched five databases, namely PubMed, Web of Science, Scopus, and SPORTDiscus, using the following search words and Boolean operators: (“physical training” OR exercise OR “physical activity” OR “physical intervention”) OR (“physical training” OR exercise OR “physical activity” OR “physical intervention”) AND (“Cognitive Rehabilitation” OR Cognition) OR (“Cognitive Rehabilitation” OR Cognition) AND (CVA OR Stroke OR “cerebrovascular accident”) OR (CVA OR Stroke OR “cerebrovascular accident”). In the PEDro database only, we used the following search terms: cognitive*, aerobic*, and stroke*. We also searched the reference lists of all included studies to identify any other studies that our search strategy might have missed. We used a combination of medical subject title (MeSH) terms where available, free text search terms, and Boolean operators. MeSH search terms using “Exercise,” “Cognition,” and “Stroke” were combined with “physical training” OR exercise OR “physical activity” OR “physical intervention,” “Cognitive Rehabilitation” OR “Cognition,” and “CVA OR Stroke OR “cerebrovascular accident.” Additionally, a manual search of peer-reviewed articles on the cognitive benefits of physical activity after stroke, considering the type of intervention program (combined or isolated, i.e., a combination of different types of physical training or a single type of physical training activity) and duration, was also performed to locate potentially eligible studies for inclusion in the review [32].
Selection Process
All studies were imported into Excel using Zotero (reference management software), facilitating screening and eliminating duplicate records. The three investigators conducted a comprehensive search of studies, independently reviewed titles and abstracts of the imported registries, and discussed inconsistencies until a consensus was reached. Then, two researchers independently examined the titles and abstracts of all articles retrieved. In case of disagreement, consensus on which reports to display in full-text was reached through discussion. If necessary, the third researcher was consulted to make the final decision. Subsequently, two researchers independently screened the full-text articles for inclusion. Again, in case of disagreement, consensus on inclusion or exclusion was reached through discussion, and, if necessary, the third investigator was consulted [33, 34].
Data Collection and Extraction
Two reviewers independently collected information from eligible studies using the data extraction form based on Lumley et al. [35]. A third reviewer arbitrated the discussion to resolve discrepancies between the two review authors. For each study, information collected included descriptive details about the first author’s last name and year of publication, study design and period, study methods, study participants (age group, eligibility criteria), interventions (type, duration, intensity), and outcome data.
Study Risk of Bias Assessment
The risk of bias for each study was assessed using the Cochrane Risk of Bias (RoB 2.0) tool [33, 36]. Domains in this checklist include: (1) selection bias (including random sequence generation and allocation concealment); (2) performance bias (including participant concealment); (3) detection bias (including blind assessment); (4) attrition bias (including incomplete result data); (5) reporting bias (including reporting of selective results); and (6) other biases (any other bias observed). Two reviewers independently applied the tool to each included study and recorded supporting information and justifications for judgments of risk of bias for each domain (low, some concerns, and high). The lack of consensus on the risk of biased judgments was resolved by discussion to reach an agreement between the two review authors, with a third review author acting as arbiter when necessary.
Results
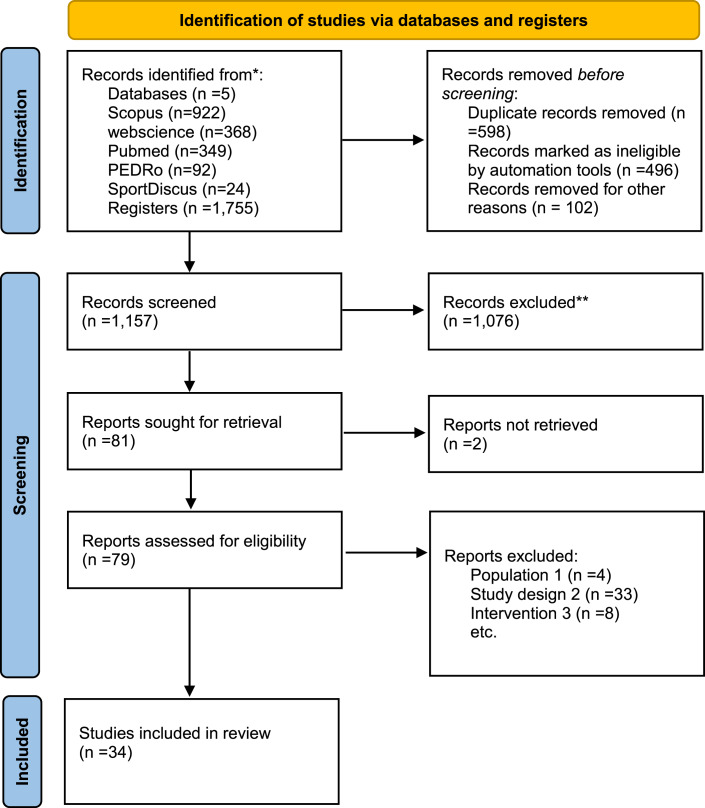
In the five databases searched, we found 1,755 records: 922 in Scopus, 368 in Web of Science, 349 in Pubmed, 92 in PEDRo, and 24 in SPORTDiscus. After removing duplicates, we examined 1,157 records, reviewing 79 full-text documents. Subsequently, we searched for documents that cited any of the initially included studies and the references of the included studies. However, no extra articles meeting the inclusion criteria were found in these searches, as shown in the flow diagram in Figure 1.
Fig. 1.
Study flow diagram.
Study Characteristics
The studies included in the review present data collected in thirteen countries on three continents (online suppl. Material 1; for all online suppl. material, see https://doi.org/10.1159/000535272). We found fourteen studies in Asia, twelve studies in Europe, and eight studies in America.
We included a total of 34 randomized controlled trials, of which three were double-blind [37–39], 22 were single-blind, and the remaining nine, although obeying randomization of participant recruitment, did not present any information about the concealment process, both for the participants and the evaluators. The sample size in the studies ranged from 18 to 362 participants. The mean age of the participants in the reviewed studies was 61.91 years. Concerning sex, 27 included men and women in their sample [29–31, 37, 40–62], four had a sample composed only of men [38, 39, 63, 64], two composed only of women [65, 66], and one did not specify the gender in its sample [67]. Regarding the types of exercises targeted at the intervention groups, the combined rehabilitation training programs involved: dual-task gait training [45, 58, 62], aerobic and cognitive training [29, 40, 54, 59, 60, 66], aerobic activity and strength activity [44, 46], Tai Chi [41, 56], aerobic and endurance training [30, 50, 63, 64]. The isolated aerobic training programs involved: aerobic training [31, 42, 48, 49], high-intensity training [37, 39, 53, 57], and yoga training [43, 67]. On the other hand, the control groups participated in programs involving single-task gait training with a treadmill [45, 62], performing conventional exercises [29, 31, 38, 39, 42, 43, 46, 48, 49, 55, 63, 64, 67], daily routines [30, 41, 61, 65], which included mobilization of upper and lower limbs, stretching and muscle strengthening with elastic bands and walking [51, 52]. An overview of the study and participant characteristics is listed in Table 1.
Table 1.
Characteristics of the included studies
| Author (year) | Study design | Country | Sample size | Age, mean | Gender | Intervention | Program types | Intensity and duration | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Baek et al. [62] (2021) | RCT | South Korea | 34 | T – 56.94 | Female/male | T– dual-task gait training with treadmill | Combined exercise | Speed ≥80%, 60 min, twice a week, for a period of 6 weeks | In the experimental group, the attentional capacity for dual-task processing improved |
| C – 56.13 | C – single-task gait training with treadmill | ||||||||
| Blanchet et al. [40] (2016) | RCT | Canada | 21 | All – 61.93 | Female/male | T – aerobic training alone | Combined exercise | 60–70% of the heart rate reserve and 20–30 min, twice weekly, 8 weeks | Attention improved after an 8-week intervention for both the aerobic training alone group and the combined aerobic and COG group. The results of the control group were suppressed from the final analysis. |
| T – combined aerobic and COG | |||||||||
| C – relaxation group | |||||||||
| Bo et al. [29] (2019) | RCT | China | 225 | T Comb – 66.68 | Female/male | T – combined intervention of physical exercise and cognitive | Combined exercise | Moderate intensity, and 50 min, 3 times/week, 12 weeks | Combined training produced significantly greater gains in cognitive function after the intervention compared to aerobic training alone or COG alone in stroke survivors with vascular cognitive impairment. |
| T – PE – 65.12 | T – physical exercise | ||||||||
| T – CT – 67.51 | T – COG | ||||||||
| C – 64.36 | C – usual care and watched video documentaries | ||||||||
| Boss et al. [46] (2017) | RCT | The Netherlands | 120 | 63.00 | Female/male | T – aerobic exercise and strength training | Combined exercise | Intensity will gradually be increased, and two 1 h exercise sessions per week, 12 weeks | There was no improvement in either group, as self-reported physical activity was not associated with cognitive performance. |
| C – usual care | |||||||||
| Chan and Tsang [68] (2017) | RCT | China | 26 | TC – 63.90 | Female/male | T – Tai Chi group | Combined exercise | Unspecified and two 1 h training sessions each week, 12 weeks | Improvement in the Tai Chi group from the pre-assessment to the post-assessment than that of the other groups |
| CE – 63.20 | T – conventional exercise | ||||||||
| C – 63.20 | C – regular activities | ||||||||
| Debreceni-Nagy et al. [42] (2019) | RCT | Hungary | 37 | T – 59.00 | Female/male | T – aerobic training by cycle ergometer | Isolated exercise | Low intensity and 30–60 min, 4 weeks | The low-intensity aerobic training group had an additive positive effect on improving special domains of cognitive function compared to the control group. |
| C – 62.00 | C – conventional physiotherapy | ||||||||
| Deijle et al. [64] (2022) | RCT | The Netherlands | 119 | T – 34.00 | Male | T – aerobic and strength training | Combined exercise | Started at 40%, and was then gradually increased to 80% heart rate, 1-h, 2–3 sessions per week, 12 weeks | No between-group differences were found on global cognitive functioning with the MoCA at 12 weeks |
| C – 36.00 | C – usual care | ||||||||
| Fernandez-Gonzalo et al. [30] (2016) | RCT | Spain | 32 | T – 61.20 | Female/male | T – resistance training | Combined exercise | High intensity and 2 times/week, 12 weeks | The resistance training group recorded better results in attention, working memory, speed of information processing, executive functions, and no improvement in the control group. |
| C – 65.70 | C – daily routines | ||||||||
| Fischbacher et al. [65] (2020) | RCT | Switzerland | 18 | 76.40 | Female | T – Dalcroze eurhythmics program | Combined exercise | Low intensity, 3 × 30 min/week, 12-month | In the two intervention groups, there was no improvement in cognitive performance; on the contrary, there was a decline of −1.0 points for Dalcroze and −1.7 points for SHEP, while in the control group, there was a non-significant improvement of 0.6 points. |
| T – simple home exercise program | |||||||||
| C – non-exercise control group | |||||||||
| Gjellesvik et al. [48] (2021) | RCT | Norway | 70 | T – 57.60 | Female/male | T – treadmill training | Isolated exercise | High intensity, 3 times per week, 8 weeks. | No benefit found for high-intensity on cognitive function as measured by the MoCA. The intervention group showed significantly greater improvement on TMT-B. |
| C– 58.70 | C– usual care | ||||||||
| Hsu et al. [37] (2020) | RCT | Taiwan | 25 | T – 58.50 | Female/male | T – high-intensity interval training | Isolated exercise | 40% to 80% heart rate, 2 to 3 sessions/week, 36 sessions | The high-intensity interval training group showed an increase in cerebral O2 utilization, associated with an increase in serum BDNF levels compared to the moderate-intensity continuous training group. |
| C – 53.10 | C – moderate-intensity continuous training | ||||||||
| Hsu et al. [31] (2018) | RCT | Canada | 70 | T – 71.70 | Female/male | T – aerobic training | Isolated exercise | Moderate intensity, 60 min, 3 times per week, 6 months | The aerobic training group significantly improved reaction time performance compared to the control group. |
| C – 72.30 | C – usual care and educational materials | ||||||||
| Hung et al. [38] (2017) | RCT | Taiwan | 43 | T – W – 55.66 | Male | T– Wii fit | Combined exercise | Unspecified, 30 min, 2 times per week, 12 weeks | The Wii Fit group had greater gains in abstraction/judgment domain, and language domain than the other 2 groups after intervention |
| T – 60.90 | T – Tetrax | ||||||||
| C – 51.75 | C – conventional weight-shifting training | ||||||||
| Ihle-Hansen et al. [49] (2019 | RCT | Norway | 362 | T – 71.40 | Female/male | T – aerobic | Isolated exercise | Moderate to intensity, 30 to 45–60 min, 2–3 times per week, 18 months | No clinically relevant effect of this program was found on cognitive functioning after 18 months compared with usual care |
| C – 72.00 | C – usual care | ||||||||
| Ji and Yu [67] (2018) | RCT | China | 58 | T – 62.17 | Unspecified gender | T – yoga training | Isolated exercise | Unspecified, 30–60 min, 12 weeks | The mean and standard deviation of brain wave fluctuations in the experimental group were significantly higher than those in the control group. |
| C – 61.29 | C – conventional rehabilitation therapy | ||||||||
| Kashyap et al. [43] (2022) | RCT | India | 80 | T – 52.85 | Female/male | T – yoga training | Isolated exercise | Unspecified, 4–5 days/week, 3 months | Significant improvements in both groups were more pronounced in the yoga group. |
| C – 55.18 | C – usual care | ||||||||
| Kim and Yim [44] (2017) | RCT | South Korea | 29 | T – 50.71 | Female/male | T – handgrip strength and walking training | Combined exercise | Intensity increased according to the participant’s level, 30–60 min, 5 times per week, 6 weeks | Significant increase was found only in the exercise group on K-MoCA test. However, the difference between the two groups was not significant on TMT and Stroop test |
| C – 51.87 | C – neuro-developmental treatment | ||||||||
| Koch et al. [50] (2020) | RCT | USA | 131 | T – 59.00 | Female/male | T – combined aerobic and resistance training | Combined exercise | 50–65% of heart rate reserve, 40–60 min, 3 times per 12 weeks | Significantly improved in the intervention group, but not in the control group. |
| C – 58.00 | C – sham combined aerobic and resistance training | ||||||||
| Steen Krawcyk et al. [39] (2019) | Denmark | 71 | T – 63.70 | Male | T – high-intensity interval training | Isolated exercise | High intensity 3 × 3 min with 2 min of active recovery, 5 days per week for 12 weeks | No changes were detected between groups in cognitive performance. | |
| C – 63.70 | C – usual care | ||||||||
| Liu-Ambrose et al. [51] (2022) | RCT | Canada | 120 | T – 70.65 | Female/male | T – multicomponent exercise training | Combined exercise | 60 min, twice weekly, 6 months | Multicomponent exercises induced clinically important improvements in cognitive function. |
| T – 71.29 | T – cognitive and social enrichment activities | Stretching and toning exercises improved compared with cognitive and social enrichment activities | |||||||
| C – 70.42 | C – stretching and toning exercises | ||||||||
| Liu-Ambrose and Eng [63] (2015) | RCT | Canada | 25 | T – 62.90 | Male | T – resistance, aerobic exercise | Combined exercise | Unspecified, 60 min, 2 times per week, 9-month | The experimental group significantly improved selective attention and conflict resolution, working memory compared to the control group |
| C – 66.90 | C – usual care | ||||||||
| Meester et al. [45] (2019) | RCT | UK | 50 | T – 60.85 | Female/male | T – dual-task treadmill training | Combined exercise | 55−85% heart rate maximum, 30 min, 20 sessions divided over 10 weeks | Experimental group increased mean (SD) two-minute walking distance from 90.7 (8.2) to 103.5 (8.2) meters, compared with 86.7 (8.5) to 92.8 (8.6) in the control group. There were no differences in other measures |
| C – 62.25 | C – control treadmill training | ||||||||
| Moore et al. [52] (2015) | RCT | UK | 40 | T – 68.00 | Female/male | T – warm-up, stretching, functional strengthening | Combined exercise | 40–80% of their maximum heart rate, 45–60 min, 3 times/week, 19 weeks | Significant within-group improvements were only made in the exercise group in cognition and stroke recover. |
| C – 70.00 | C – stretching training | ||||||||
| Pallesen et al. [53] (2019) | RCT | Norway | 30 | T – 55.00 | Female/male | T – high-intensity exercise | Isolated exercise | 60–70% heart rate, 20 min, 2 times per week, 4 weeks, | The high-intensity group, compared to the low-intensity group, achieved significant improvements in cognitive performance. |
| C – 50.00 | C – low-intensity exercise | ||||||||
| Ploughman et al. [54] (2019) | RCT | Canada | 52 | 63.00 | Female/male | T – aerobic + COG | Combined exercise | Moderate to vigorous intensity, 20–30 min, 3 times, 10 weeks, | The aerobic + COG group was the only group in which the improvement was significantly greater than the active control group (activity + games) |
| T – aerobic + games | |||||||||
| T – activity + COG | |||||||||
| C – activity + games | |||||||||
| Rathnamala et al. [66] (2020) | RCT | India | 60 | Unspecified | Female | T – physical activity with COG group | Combined exercise | Moderate-high, 50 min, 5 days per week for 12 weeks | The experimental group significantly improved cognitive performance compared to the control group |
| C – COG group | |||||||||
| Rosenfeldt et al. [47] (2019) | RCT | USA | 40 | T – 51.00 | Female/male | T – FE + RTP | Combined exercise | High intensity, 90 min, 8 weeks | The FE + RTP group showed improvements in the cognitive domain of memory, and in the other two groups there was no improvement in any cognitive domain. |
| T – 60.00 | T – VE + RTP | ||||||||
| C – 58.00 | C – EDU+RTP | ||||||||
| Shang et al. [55] (2021) | RCT | China | 76 | T – 63.68 | Female/male | T – grip training + conventional physiotherapy | Isolated exercise | 50 min/5 sessions/12 weeks | The experimental group significantly improved their MoCA score compared to the control group. |
| C – 64.13 | C – conventional physiotherapy | ||||||||
| Song et al. [56] (2021) | RCT | Korea | 34 | T – 58.72 | Female/male | T – Tai Chi group | Combined exercise | Low-intensity, 50 min, twice a week for 6 months | Compared with control group, the participants in the Tai Chi showed significant improvements cognitive function |
| C – 57.18 | C – symptom management program. | ||||||||
| Tang et al. [57] (2016) | RCT | Canada | 50 | T – 66.00 | Female/male | T – high-intensity aerobic exercise | Isolated exercise | High versus low intensity, 60 min, 3 times/week, 6 months | There was no association between pre-exercise cognitive function and post-exercise improvement |
| C – 64.00 | C – balance and flexibility | ||||||||
| Timmermans et al. [58], (2021) | RCT | The Netherlands | 40 | T – 52.00 | Female/male | T – treadmill-based C-Mill therapy | Combined exercise | Unspecified, 90 min, twice a week, 5 weeks | Group showed a tendency to a greater improvement in cognitive with cognitive dual-task compared to the FP group |
| C – 59.00 | C – standard overground FALLS | ||||||||
| Yeh et al. [59] (2021) | RCT | Taiwan | 56 | Seq – 53.05 | Female/male | SEQ | Combined exercise | 40–50% gradually increased to 60–70% heart rate, 30–60 min, 12 weeks | The SEQ group improved significantly at the MoCA compared with the AE and COG groups, but no between-group difference was found for the AE and COG groups. |
| AE – 57.36 | AE | ||||||||
| Cog – 60.17 | COG | ||||||||
| Yeh et al. [60] (2019) | RCT | Taiwan | 30 | T – 50.63 | Female/male | T – SEQ | Combined exercise | 30 min and 40–70% heart rate, 12–18 weeks | The SEQ group had significantly improved cognitive performance after training compared to the control group |
| C – 60.21 | C – nonaerobic physical exercise | ||||||||
| Zheng et al. [61] (2020) | RCT | China | 48 | T – 61.63 | Female/male | T – Baduanjin training | Combined exercise | Low to high intensity, 3 days a week and 40 min, 24 weeks | Improvement significant in the Baduanjin group for MoCA. Mean between-group differences were significantly lower (p < 0.05) for TMT-A and TMT-B tests in the Baduanjin group compared with controls |
| C – 62.75 | C – any specific exercise training |
AE, aerobic exercise training; C, control; COG, cognitive training; TMT, Trail Making Test; T, treatment; RCT, randomized controlled trial; FE + RTP, forced exercise repetitive task practice; MoCA, Montreal Cognitive Assessment; SEQ, sequential training; VE + RTP, voluntary exercise + repetitive task practice; EDU + RTP, education + repetitive task practice.
Risk of Bias in Studies
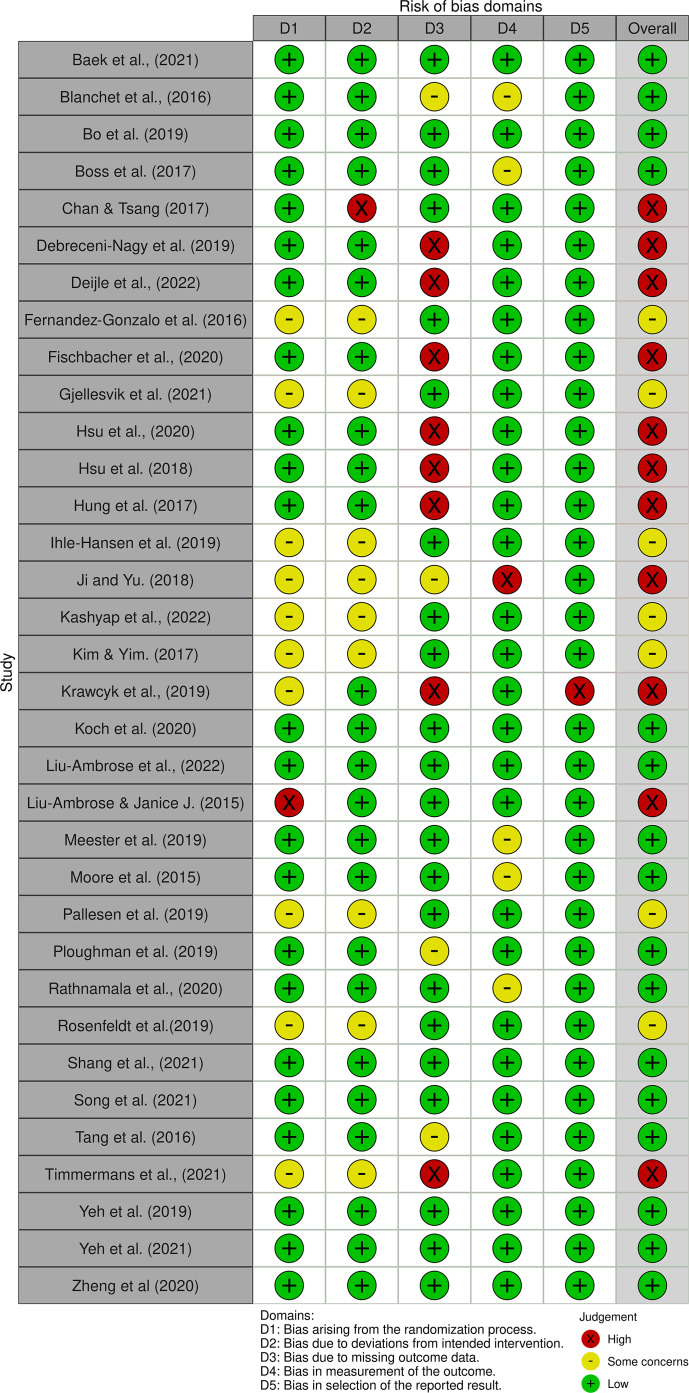
We note that of the 34 studies reviewed, sixteen had a low risk of bias, eleven had a high risk, and seven presented some concerns, as illustrated in Figure 2.
Fig. 2.
Risk of bias assessment.
Results of Syntheses
Of the 34 included studies, 23 used three types of combined rehabilitation training programs, essentially involving combinations of aerobic and cognitive training, aerobic exercise and strength training, and aerobic and resistance training. The remaining 11 studies used isolated rehabilitation training programs primarily involving aerobic exercise (online suppl. Material 2).
Cognitive Function Outcome Measures
Regarding the use of neuropsychological instruments at baseline, of the 23 studies that used a combined rehabilitation training program, only 13 assessed cognitive function as shown in online supplementary material 3, with cases using a single test [29, 41, 50, 61, 63, 65], two tests [38, 44, 46, 51, 59, 64], and three tests [52]. In the 11 studies using an isolated rehabilitation training program, 10 assessed cognitive function, either using a single test [37, 48, 53, 57, 67], two tests [39, 49, 55], three tests [31], or four tests [42].
Global Cognitive Function
Of the 23 studies that used combined rehabilitation training programs, only seven reported global cognitive functions assessed by neuropsychological tests [46, 50, 51, 60, 61, 63, 64], as detailed in online supplementary material 3. Of these, three reported significant improvements in the intervention group but not in the control group [50, 51, 60], and only three reported no between-group differences in global cognitive functioning [46, 61, 64]. One reported significant improvements in the control group but not in the intervention group [63]. Of the 11 studies that used an isolated rehabilitation training program, only three documented global functions [31, 48, 49]. One study reported significant improvements in the intervention group but not the control group [31]. In contrast, the other two reported no relevant benefit of this program in cognitive functioning [48, 49], as shown in Table 1.
Specific Domains of Cognition
Memory
Ten studies using a combined rehabilitation training program reported memory outcomes assessed by neuropsychological tests [29, 30, 40, 46, 47, 50, 52, 58, 63, 66], as shown in online supplementary material 3. Of these, eight reported significant improvements in the intervention group but not in the control group [29, 30, 47, 50, 52, 58, 63, 66], and only one [46] reported that self-reported physical activity was not associated with cognitive performance. However, one study reported no significant effects for short delays in working memory (Brown-Peterson paradigm: p = 0.92) or episodic memory (revised Hopkins Verbal Learning Test: p = 0.26) [40]. Of the 11 studies that used a rehabilitation training program alone, six reported on memory [39, 42, 48, 53, 55, 57]. Of these, only one reported improvements in the intervention group but not in the control group [55], two reported no significant effects of the intervention in both groups [42, 53], and three [39, 48, 57] showed no evidence of the impact of a rehabilitation training program alone on memory in patients with stroke, as shown in Table 1.
Attention
Ten studies that used a combined rehabilitation training program assessed attention using neuropsychological testing [30, 40, 44, 46, 51, 56, 59, 61, 63, 66], as illustrated in online supplementary material 3. Of these, eight reported improvements in the intervention group. Still, not in the control group [30, 40, 44, 51, 56, 59, 63, 66], one reported no significant difference between the Baduanjin group and the controls for attention [61], and a single one [46] did not report improvements. The seven studies that used a stand-alone rehabilitation training program also assessed attention using neuropsychological tests [31, 39, 43, 48, 53, 55, 57]. Of these, four showed improvement in the intervention group but not in the control group [31, 43, 53, 55], and three did not report any improvement [39, 48, 57], as shown in Table 1.
Visuospatial Ability
Four studies using a combined rehabilitation training program assessed visuospatial ability [56, 59–61], as depicted in online supplementary material 3. Three reported improvements in the intervention group but not in the control group in visuospatial ability [56, 59, 60], and one reported no significant difference between the Baduanjin group and the controls [61]. Three other studies that used a stand-alone rehabilitation training program also assessed visuospatial ability [39, 43, 48]. Of these, one reported improvements in the intervention group but not in the control group [43], and two reported no improvements at all [39, 48], as shown in Table 1.
Language and Processing Speed
Of the five studies that used a combined rehabilitation training program, one assessed language [56], and the remaining studies assessed processing speed [30, 50, 61, 66], as illustrated in online supplementary material 3. In five studies that assessed language and processing speed, four reported improvements in the intervention group but not in the control group [30, 50, 56, 66], and one reported no significant differences between the Baduanjin group and the controls for processing speed [61]. Of the five studies that used a stand-alone rehabilitation training program, two considered language [39, 48], and both did not show improvement. For the remaining three studies, which also used a rehabilitation training program alone, they assessed processing speed [42, 49, 53], with improvements in the intervention group but not in the control group [42, 53] and without improvements [49], as depicted in online supplementary material 3.
Executive Functions
Nine studies that used a combined rehabilitation training program assessed executive functions through neuropsychological tests [30, 41, 46, 50, 51, 56, 59, 61, 62], as shown in detail in online supplementary material Table 3. Eight of these nine studies reported improvements in the intervention group but not in the control group after the intervention; only one [46] did not report gains. Seven studies assessed executive functions [31, 39, 48, 49, 53, 55, 57] regarding the isolated rehabilitation training program. Of these studies, three showed improvements in the intervention group but not in the control group after the intervention [31, 53, 55], while four showed no improvements [39, 48, 49, 57], as shown in Table 1.
Type of Intervention Programs
Regarding the type of rehabilitation training program, of the 23 studies that used combined rehabilitation training programs, 20 recorded cognitive improvements after completing the recommended exercise sessions [29, 30, 38, 40, 41, 44, 45, 47, 50–52, 54, 56, 58–63, 66], while three did not record cognitive improvements [46, 64, 65]. Concerning the 11 studies that followed an isolated rehabilitation training recovery program, seven reported cognitive improvements [31, 37, 42, 43, 53, 55, 67], and four reported no cognitive improvements [39, 48, 49, 57] (online suppl. material 4).
Regarding the type of exercise most used in each rehabilitation training program, we note that combined rehabilitation training programs used a combination based on strength physical activity in nine studies [38, 41, 44, 47, 51, 52, 56, 61, 64]. In general, these studies included an active control group based on usual care [38, 41, 64], stretching and toning exercises [51, 52], education, and repetitive task practice [47]. One study included a passive control group, wherein the participants did not exercise or perform any other activity [61]. Further, ten combined rehabilitation training program studies used aerobic physical activity [29, 40, 45, 46, 54, 58, 59, 62, 65, 66] and included an active control group based on usual care [29, 46], relaxation or activity with games [54, 66], single-task gait training with treadmill [45, 62], standard overground FALLS [58], aerobic exercise training and cognitive training [59, 60]; and resistance physical activity [30, 50, 63], wherein the control group used usual care [63], sham combined aerobic and resistance training [50], and daily routines [30]. Concerning the isolated rehabilitation training programs, ten used aerobic physical activity [31, 37, 39, 42, 43, 48, 49, 53, 57, 67], and the control group used usual care [31, 39, 42, 43, 48, 49, 57, 67], moderate-intensity continuous training [37], low-intensity exercise [53], and only one study used strength exercise [55], and his control group received usual care. Most included studies compared an experimental exercise intervention to standard care or an alternative intervention, as illustrated in online supplementary material 5.
Duration of the Intervention Programs
The duration of the rehabilitative training programs varied from 4 weeks to a maximum duration of 18 months [49]. Specifically for the combined training programs, we noticed a variation from less than 11 weeks [40, 44, 45, 47, 54, 58, 62] to more than 6 months [29, 30, 46, 50–52, 56, 59–61, 64, 65]. Studies lasting up to 11 weeks all reported improvements [40, 44, 45, 47, 54, 58, 62], the studies up to 6 months or more, nine registered improvements [29, 30, 50–52, 56, 59–61], and three did not register improvements [46, 64, 65]. Concerning the isolated rehabilitation training programs, the duration ranged from less than 11 weeks [42, 48, 53, 57] to more than 1 year [31, 37, 39, 43, 49, 55, 67], as depicted in online supplementary material 6. In studies lasting up to 11 weeks, two showed improvements [42, 53], and two did not show improvements [48, 57]. Concerning studies lasting up to 1 year or more, five showed improvements [31, 37, 43, 55, 67], and two did not show improvements [39, 49]. As for the frequency with which rehabilitation training was carried out, we noted that there was a predominance of three times a week in 15 out of the 34 studies included, and the time spent for each session ranged from 20 min [40, 53, 54] to 90 min [47, 58].
Exercise Intensity in Interventions
Regarding the intensity with which the exercises were performed, in the studies reviewed here, three presented low-intensity physical activity [42, 56, 65], twelve presented moderate-intensity physical activity [29, 31, 40, 44, 46, 50, 53, 54, 59–61, 64], eleven presented high-intensity physical activity [30, 37, 39, 45, 47–49, 52, 57, 62, 66], and eight did not specify intensity [38, 41, 43, 51, 55, 58, 63, 67]. In studies with low-intensity physical activity, two recorded improvements [42, 56], and one did not show improvements [65]. In the studies using moderate-intensity physical activity, ten recorded improvements [29, 31, 40, 44, 50, 53, 54, 59–61], and two did not register improvements [46, 64]. Concerning the high-intensity physical activity studies, seven reported cognitive improvements [30, 37, 45, 47, 52, 62, 66], and four did not record improvements [39, 48, 49, 57]. Regarding the studies that did not specify the intensity, all presented improvements [38, 41, 43, 51, 55, 58, 63, 67].
Stroke Severity
Regarding stroke severity, in the studies reviewed here, 16 records involved participants with chronic stroke from 6 months to 1 year [30, 31, 43–46, 48, 51, 52, 54, 55, 57, 58, 60, 63, 65], 16 involved participants with subacute stroke [29, 37–42, 47, 49, 50, 53, 56, 59, 61, 62, 64], and two did not specify stroke severity of the involved participants [66, 67]. Regarding the studies in which the sample was composed of participants with chronic stroke, we noticed that 14 showed improvements [30, 31, 43–46, 48, 51, 54, 55, 57, 58, 63, 68], and only two showed no improvements [52, 65]. In studies in which the sample consisted of participants with subacute stroke, we noticed that 11 showed improvements [29, 37, 38, 41, 42, 47, 49, 50, 53, 56, 61] and five showed no improvement [39, 40, 59, 62, 64]. Regarding the studies that did not specify stroke severity, all showed improvements [66, 67].
Discussion
This review aimed to evaluate the effects of physical activity on the cognition of patients with stroke, comparing the effectiveness of isolated or combined rehabilitation training programs and the duration and intensity of training to identify the most effective training program for post-stroke cognitive rehabilitation. Thus, we systemized data that might allow for a recommendation for patients with stroke concerning the practice of physical activity safely and efficiently. Considering the potential benefits for cognition and mobility, since physical activity is linked to several physiological and neurological factors that promote longevity [69, 70], participating in physical activity post-stroke rehabilitation should benefit protective and regenerative functions [71]. However, considering the pre-defined eligibility criteria, there are still few published original studies with stroke rehabilitation programs using isolated and combined training, compared to a predominance of pharmacological therapies to lower blood pressure and conventional programs of muscle strength. Among the studies analyzed in this review, the combined rehabilitation training program appears to be more effective than the isolated rehabilitation training program, showing more significant improvements in several cognitive domains in the experimental group compared with the control group. One reason for this result is that the combined interventions offer favorable conditions for neuroplastic alterations due to the beginning of the synergy of action provided by the combination of physical activities [6] since each type of activity that intervenes in the combined training program has its specificity in terms of contribution. For example, it is known that aerobic exercise alone improves cardiorespiratory fitness and increases the expression of neurotrophic factors and hippocampal size [4, 25]. Resistance exercise, on the other hand, increases muscle mass, strength, and power and improves executive functions and selective attention [4, 26, 72]. Moreover, strength exercise improves bone mineral density and neuromuscular performance, increases proteins involved in neuronal survival and synaptic plasticity in the hippocampus, and improves memory [27, 28]. The combined benefits of at least two types of physical activity thus have the potential to increase the cognitive benefits compared to an isolated physical activity.
There are also differences between the two rehabilitation training programs regarding the type of physical activity that each rehabilitation training program uses. In the combined rehabilitation training programs, we identified a predominance of combinations involving physical activity, as with physical activity of strength. Of these, eight studies that performed strength activity recorded cognitive improvements in the experimental group compared to the control group [38, 41, 44, 47, 51, 52, 56, 61]. Despite these widespread improvements in various cognitive domains, we also note a study [61] that found no significant differences between the Baduanjin group and controls about processing speed. This study tries to create a cause-effect relationship between attention and processing speed by explaining that for attention, there was a significant effect on the response time of a Go/No Go test [61], as processing speed is usually described in relation to reaction time, i.e., the time elapsed between the relatively rapid presentation of a stimulus and the behavioral response [73]. This result is similar to those of a systematic review conducted by Wang et al. [74], as it also recognizes that Baduanjin training benefits people with stroke in several domains, such as global cognitive function, including memory and executive functions. However, it did not find any significant difference in attention. In combinations involving physical resistance activity, all three studies reported cognitive improvements in the experimental group compared to the control group [30, 50, 63]. For studies that performed aerobic physical activity combinations, six out of eight reported improvements in the experimental group compared to the control group [29, 54, 58, 59, 62, 66]. A similar situation to the one presented above is also noted for the memory domain, as neither group reports significant differences in memory [40]. This study rightly points to the lack of improvement seen in the memory domain, taking into account the duration of the intervention program (8 months), as it explains that cardiorespiratory fitness and memory require a more extended intervention, where fitness values must be high for exercise to induce physiological changes [40]. This finding is supported in the literature, as studies have shown that most motor recovery is almost completed within 10 weeks after stroke [75], while neuronal recovery can occur within weeks or even years [76]. Some studies did not record improvements in strength combinations. We believe this is due to the methodology chosen [46], as the rehabilitation program occurred at home and data were self-reported. One of the problems that negatively affects the practice of post-stroke physical activity has to do with the fear of falls, which generates a vicious cycle that leads to intolerance to physical activity, in addition to not helping to overcome post-stroke fatigue that is often experienced in patients with stroke [71, 75]. Therefore, the successful implementation of a physical activity program at home must be accompanied by motivational strategies that might help to increase the motivational rate [77–79]. As for another study [64], it is suggested that the lack of improvements results from the fact that the participants have mild cognitive impairment (MoCA scores of 24.9 ± 3.2 for the experimental group and 25.5 ± 2.9 for the control group), associated with the relatively young age of the participants (average age of participants of 64.30 years). Another study further confirms that the benefits resulting from rehabilitation for mild cognitive deficits may be low [70].
Regarding the study that involved the combination of aerobic physical activity [65], we believe that the lack of cognitive improvement verified after the rehabilitation training program that aimed to improve cognitive deficits is associated with adherence problems. Adherence was low since three in five participants did not attend the sessions. Evidence shows that the degree of commitment strongly influences clinical findings since adherence to a physical activity program improves post-stroke recovery [79, 80].
Regarding the isolated rehabilitation training program, where the predominance of aerobic physical activity was notorious, nine of the 11 studies registered cognitive improvements in the experimental group compared to the control group, and three did not report gains. The explanation for the lack of improvement is possibly linked to the heterogeneity of the study participants [49], as both patients with ischemic and hemorrhagic stroke were included, subacute to chronic patients were also included, and there was also a disparity between the ages of the participants (18–75 years). A change in the study methodology carried out at 6 months and then at 18 months after onset, associated with the type of intervention, i.e., high-intensity physical activity performed at home [39], might also explain the lack of improvements. It is possible that, in the face of high-intensity physical activity that occurs at home, stroke survivors become highly anxious about the risk of a new event. This fact can lead to fear or lack of desire to practice physical activity [75, 81]. Furthermore, the lack of improvements verified [57] might be due to the extensive duration of intervention, as the literature reports that the most significant gains in post-stroke recovery occur in the first 3 months [75, 76, 81].
Our results align with previous studies, demonstrating that combined training programs promote significant improvements in several domains, such as attention, memory, language, orientation, visuospatial ability, and executive functions, compared to isolated training programs [6, 80–82]. Since patients with stroke present cognitive problems [83], they should participate in combined rehabilitation programs.
Regarding the duration of rehabilitation training programs, it is essential to understand what predicts the effectiveness of exercise, as it can guide clinicians on how to better design exercise programs for people with stroke, as programs lasting less than 8 weeks are not long enough to elicit noticeable cognitive gains [84]. A moderator-effect analysis revealed that the 12–14-week exercise interventions are associated with the most significant magnitude of cognitive gains [85]. These results reinforce the need to use relatively long programs to rehabilitate people with stroke [85–88]. However, contradictions persist regarding the duration that best optimizes cognition, as seen in a recent review where the analysis showed no difference between using a program of more than 12 weeks or less [89]. This information is essential as it impacts doctors’ decisions. When relating the lack of improvements with intensity, the influence of low intensity is noted, as one study presented low-intensity physical activity [65]. In another, the intensity was gradually increased [64]. We suggest that the intensity of physical activity may have been too low, which failed to create physiological changes that led to the awakening of neuronal plasticity [78, 85].
Regarding the studies contemplating high-intensity physical activity [48, 57], we think that given the fear of falling and the risk of a new stroke event [75, 76], this fact may have led to adherence problems from the perspective of adequately taking advantage of physical activity sessions [80]. The study with the most extended duration used an isolated rehabilitation training program lasting 18 months. Interestingly, the two studies lasting longer than 1 year and the one lasting 18 months did not register improvements. As Yang and Wang [85] explain, when the exercise program goes beyond 14 weeks, the previously acquired cognitive benefits disappear again. This may indicate that longer-term rehabilitation training programs are poorly tolerated by patients with stroke since the time course after stroke is characterized by more significant improvements during the first few weeks after stroke. This might reflect the concomitant intrinsic pressure known as “neurological recovery” [90–93].
Two very recent meta-analyses by Yang and Wang [84, 85] also consolidate this idea of long-term exercise (exercise programs for 12 weeks) because it explains that the cycle of intervention with exercise after stroke is mainly controlled at 12 weeks since this period has good patient tolerance, as well as potentiation of long-term effects on physical and mental health, and further explains that 8 weeks of post-stroke aerobic training is more or less the ideal period to have a positive impact on cardiopulmonary and cognitive function [85].
Concerning exercise intensity, in the studies reviewed here, there is a predominance of moderate-intensity physical activity; this fact seems to result from the need to safeguard the health of the participants (patients with stroke), as post-stroke exercise recommendations include moderate-intensity continuous cardiovascular exercise with a suggested intensity of 40–70% maximum oxygen consumption or heart rate reserve of 50–80% of the maximum heart rate [94–96]. On the other hand, it is possible that higher-intensity exercises were not chosen because evidence indicates that high-intensity physical activity does not generate greater cognitive gain [85]. However, this is a controversial position, as studies show that intensity only mediates domains of gain [97].
It is important to emphasize that previous reviews offer relatively little information about the ideal prescription of physical exercises for cognitive health. There is still no clarity in most guidelines on the methodology, essentially in terms of the frequency with which physical activity should be performed, the time that the rehabilitation program should last as well as the activity that should be performed [98]; little is discussed about the need to adapt exercise based on an individual’s skill level, mobility, basic fitness level. However, a review [80, 99] states that at least 30 min of moderate-intensity physical exercise five times a week for at least 150 min can be efficient. However, it is essential not to forget that post-stroke physical activity is not risk-free [98, 100], so before starting physical activity, patients with stroke should go through a medical assessment to identify medical conditions that require special consideration or constitute a contraindication [100].
The results of this review further reinforce the underlying idea that physical activity benefits post-stroke physical and cognitive recovery, a fact that supports the need to prescribe physical activity as a therapy in the treatment of patients with stroke, as it can be safe, efficient, and allow the patient with stroke to adhere to a less sedentary lifestyle resulting from their pathology [75, 76]. Regarding the severity of the stroke, although the results of this review show that 16 of the 34 studies involved participants with chronic stroke, these data do not seem to have had a negative influence on cognitive performance because of the 34 studies included, of which 27 reached the purpose to reduce the post-stroke cognitive impairments after a rehabilitative training program. Of the seven studies that do not improve, four pertain to subacute stroke occurring within a month, and the lack of improvement might be associated with inadequacy of the intensity of the training programs, as exercise intensity should be monitored to ensure that it is strong enough to promote relevant physiological changes [81].
Although domain-level judgments provide the basis for a general assessment of the risk of bias [101, 102], and based on this rationale, we refer that, in general, there is no high risk of bias since most of the evaluated studies presented a low risk of bias; having given evidence on the effectiveness of a combined rehabilitation training program, moderate intensity, with a frequency of three times a week and duration of the program of 12 weeks, caution is needed when interpreting these results. The Cochrane tool for risk of bias led us to analyze the risk as not high. However, this must be interpreted cautiously because the ten included studies present concerns in the first and second domains, which denote problems with randomization [102]. We must also interpret these results cautiously since we systemized relatively few studies. These involve diverse neuropsychological instruments used to assess cognitive deficits since a variety of neuropsychological tests in use present one or more deficiencies linked to reliability or validity, standardization, lack of alternative forms, and lack of ecological validity [103].
Limitations of This Study
The present study provides an updated and extensive qualitative overview of the literature on the most effective rehabilitation training program to be used in the cognitive rehabilitation of patients with stroke. Our findings largely align with previous results that synthesized evidence on the effectiveness of post-stroke activity [78].
The major limitation of this study lies in the fact that it included methodologically heterogeneous studies, which made it difficult to compare the data, limiting the synthesis of the results. Heterogeneity was found concerning the sample, the duration of rehabilitation programs, the intensity of physical activity, the frequency with which sessions occurred, the severity of patients, and the diversity of neuropsychological tests used. In the future, these issues should be addressed, and given an array of more homogeneous studies, a meta-analysis might be an option, as it allows for transparent decisions supported by statistical analyses that help to limit biases [104].
Conclusion
This systematic review suggests that cognitive performance may improve more in people with stroke after combined rehabilitation training programs compared to rehabilitation training programs alone. This improvement seems to result from the synergistic effect of physical activity, which may be beneficial for the quality of life of this clinical group.
Recommendations
Physical activity for people with stroke should be preceded by a few precautions and guidelines:
For people with stroke, before starting physical activity, they should undergo a medical assessment to rule out or identify medical conditions that require special attention, as well as those that could be aggravated by physical activity.
The practice of physical activity for people with stroke should consider the principle of gradualism on an individual basis since cardiovascular fitness, essential in reducing the risk after stroke, is gradually achieved with regular physical activity.
The frequency, duration, and intensity of the physical activity session must be gradual to minimize adverse events and make it safe and motivating. A rehabilitation training program for people with stroke should combine different physical activities (e.g., aerobic and strength exercise, or aerobic, strength and endurance, or aerobic and cognitive training) to take more significant advantage of their synergistic action. The intensity with which each session is performed must be moderate enough to promote physiological changes that lead to cognitive optimization of physical activity.
Acknowledgments
We would like to kindly thank João Dias and Claúdia Catanho, Science Managers at UCP, for their collaboration in the protocol phase.
Statement of Ethics
This systematic review and its protocol were registered in the international prospective review registry (PROSPERO) under the registration number CRD42021248533.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was financially supported through a scholarship from Universidade Save – Mocambique and by National Funds through FCT – Fundação para a Ciência e a Tecnologia, I.P., under the project UIDP/04279/2020 to AMA. The authors have no relevant financial or non-financial interests to disclose.
Author Contributions
R.M. collected the data, developed the first draft of the manuscript, and extracted the studies. A.M.A. reviewed the first and subsequent versions and untied selection decisions. I.S.M. and R.M. decided which studies to include. I.S.M. and A.M.A. suggested improvements across all phases of the manuscript elaboration. All authors approved the final manuscript.
Funding Statement
This work was financially supported through a scholarship from Universidade Save – Mocambique and by National Funds through FCT – Fundação para a Ciência e a Tecnologia, I.P., under the project UIDP/04279/2020 to AMA. The authors have no relevant financial or non-financial interests to disclose.
Supplementary Material.
Supplementary Material.
References
- 1. Ojaghihaghighi S, Vahdati SS, Mikaeilpour A, Ramouz A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J Emerg Med. 2017;8(1):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44(7):2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo JW, Crawford JD, Desmond DW, Godefroy O, Jokinen H, Mahinrad S, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology. 2019;93(24):E2257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saunders DH, Sanderson M, Hayes S, Johnson L, Kramer S, Carter DD, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2020;3(3):CD003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, et al. Post-stroke cognitive impairment and dementia. Circ Res. 2022;130(8):1252–71. [DOI] [PubMed] [Google Scholar]
- 6. Kotov SV, Isakova EV, Zaitseva EV, Egorova YV. Multimodal stimulation in the neurorehabilitation of patients with poststroke cognitive impairments. Neurosci Behav Physiol. 2021;51(2):142–6. [DOI] [PubMed] [Google Scholar]
- 7. Aam S, Einstad MS, Munthe-Kaas R, Lydersen S, Ihle-Hansen H, Knapskog AB, et al. Post-stroke cognitive impairment: impact of follow-up time and stroke subtype on severity and cognitive profile: the Nor-COAST Study. Front Neurol. 2020;11:699–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 Suppl):S85–90. [DOI] [PubMed] [Google Scholar]
- 9. Saj A, Chen P, Perennou D, Azouvi P. A special issue on cognitive rehabilitation. Ann Phys Rehabil Med. 2021;64(5):101562. [DOI] [PubMed] [Google Scholar]
- 10. Ceanga M, Dahab M, Witte OW, Keiner S. Adult neurogenesis and stroke: a tale of two neurogenic niches. Front Neurosci. 2021;15:700297–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pang MYC, Charlesworth SA, Lau RWK, Chung RCK. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis. 2013;35(1):7–22. [DOI] [PubMed] [Google Scholar]
- 12. Bangsbo J, Hansen PR, Dvorak J, Krustrup P. Recreational football for disease prevention and treatment in untrained men: a narrative review examining cardiovascular health, lipid profile, body composition, muscle strength and functional capacity. Br J Sports Med. 2015;49(9):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng G, Zhou W, Xia R, Tao J, Chen L. Aerobic exercises for cognition rehabilitation following stroke: a systematic review. J Stroke Cerebrovasc Dis. 2016;25(11):2780–9. [DOI] [PubMed] [Google Scholar]
- 14. Shephard RJ. Physical activity, fitness, and health: the current consensus. Quest. 1995;47(3):288–303. [Google Scholar]
- 15. Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. 2018;9:509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schüller I, Demetriou Y. Physical activity interventions promoting social competence at school: a systematic review. Educ Res Rev. 2018;25:39–55. [Google Scholar]
- 17. Levin O, Netz Y, Ziv G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: a systematic review. Eur Rev Aging Phys Act. 2017;14(1):20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moriarty TA, Mermier C, Kravitz L, Gibson A, Beltz N, Zuhl M. Acute aerobic exercise based cognitive and motor priming: practical applications and mechanisms. Front Psychol. 2019;10:2790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rooks CR, Thom NJ, McCully KK, Dishman RK. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol. 2010;92(2):134–50. [DOI] [PubMed] [Google Scholar]
- 20. Herold F, Wiegel P, Scholkmann F, Müller NG. Applications of functional near-infrared spectroscopy (fNIRS) neuroimaging in exercise–cognition science: a systematic, methodology-focused review. J Clin Med. 2018;7(12):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Damsbo AG, Mortensen JK, Kraglund KL, Johnsen SP, Andersen G, Blauenfeldt RA. Prestroke physical activity and poststroke cognitive performance. Cerebrovasc Dis. 2020;49(6):632–8. [DOI] [PubMed] [Google Scholar]
- 22. Barker K, Eickmeyer S. Therapeutic exercise. Med Clin North Am. 2020;104(2):189–98. [DOI] [PubMed] [Google Scholar]
- 23. Gambassi BB, Coelho-Junior HJ, Schwingel PA, Almeida FDJF, Gaspar Novais TM, Lauande Oliveira Pd L, et al. Resistance training and stroke: a critical analysis of different training programs. Stroke Res Treat. 2017;2017:4830265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Heal Aging. 2021;25(7):824–53. [DOI] [PubMed] [Google Scholar]
- 25. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang YK, Pan CY, Chen FT, Tsai CL, Huang CC. Effect of resistance-exercise training on cognitive function in healthy older adults: a review. J Aging Phys Act. 2012;20(4):497–517. [DOI] [PubMed] [Google Scholar]
- 27. Mosti MP, Carlsen T, Aas E, Hoff J, Stunes AK, Syversen U. Maximal strength training improves bone mineral density and neuromuscular performance in young adult women. J Strength Cond Res. 2014;28(10):2935–45. [DOI] [PubMed] [Google Scholar]
- 28. Novaes Gomes FG, Fernandes J, Vannucci Campos D, Cassilhas RC, Viana GM, D’Almeida V, et al. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology. 2014;50:106–17. [DOI] [PubMed] [Google Scholar]
- 29. Bo W, Lei M, Tao S, Jie LT, Qian L, Lin FQ, et al. Effects of combined intervention of physical exercise and cognitive training on cognitive function in stroke survivors with vascular cognitive impairment: a randomized controlled trial. Clin Rehabil. 2019;33(1):54–63. [DOI] [PubMed] [Google Scholar]
- 30. Fernandez-Gonzalo R, Fernandez-Gonzalo S, Turon M, Prieto C, Tesch PA, García-Carreira C. Muscle, functional and cognitive adaptations after flywheel resistance training in stroke patients: a pilot randomized controlled trial. J NeuroEng Rehabil. 2016;13(1):37–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hsu CL, Best JR, Davis JC, Nagamatsu LS, Wang S, Boyd LA, et al. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br J Sports Med. 2018;52(3):184–91. [DOI] [PubMed] [Google Scholar]
- 32. Donato H, Donato M. Stages for undertaking a systematic review. Acta Med Port. 2019;32(3):227–35. [DOI] [PubMed] [Google Scholar]
- 33. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021:372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;74(9):790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009;3:CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. [DOI] [PubMed] [Google Scholar]
- 37. Hsu CC, Fu TC, Huang SC, Chen CPC, Wang JS. Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: a randomized controlled trial. Ann Phys Rehabil Med. 2021;64(4):101385. [DOI] [PubMed] [Google Scholar]
- 38. Hung JW, Chou CX, Chang HF, Wu WC, Hsieh YW, Chen PC, et al. Cognitive effects of weight-shifting controlled exergames in patients with chronic stroke: a pilot randomized comparison trial. Eur J Phys Rehabil Med. 2017;53(5):694–702. [DOI] [PubMed] [Google Scholar]
- 39. Steen Krawcyk R, Vinther A, Petersen NC, Faber J, Iversen HK, Christensen T, et al. Effect of home-based high-intensity interval training in patients with lacunar stroke: a randomized controlled trial. Front Neurol. 2019;10:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blanchet S, Richards CL, Leblond J, Olivier C, Maltais DB. Cardiorespiratory fitness and cognitive functioning following short-term interventions in chronic stroke survivors with cognitive impairment: a pilot study. Int J Rehabil Res. 2016;39(2):153–9. [DOI] [PubMed] [Google Scholar]
- 41. Chan WN, Tsang WWN. Effect of Tai Chi training on dual-tasking performance that involves stepping down among stroke survivors: a pilot study. Evid Based Complement Altern Med. 2017;2017:9134173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Debreceni-Nagy A, Horváth J, Bajuszné Kovács N, Fülöp P, Jenei Z. The effect of low-intensity aerobic training on cognitive functions of severely deconditioned subacute and chronic stroke patients: a randomized, controlled pilot study. Int J Rehabil Res. 2019;42(3):275–9. [DOI] [PubMed] [Google Scholar]
- 43. Kashyap M, Rai NK, Singh R, Joshi A, Rozatkar AR, Kashyap PV, et al. Effect of early yoga practice on post stroke cognitive impairment. Ann Indian Acad Neurol. 2023;26(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim J, Yim J. Effects of an exercise protocol for improving handgrip strength and walking speed on cognitive function in patients with chronic stroke. Med Sci Monit. 2017;23:5402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meester D, Al-Yahya E, Dennis A, Collett J, Wade DT, Ovington M, et al. A randomized controlled trial of a walking training with simultaneous cognitive demand (dual-task) in chronic stroke. Eur J Neurol. 2019;26(3):435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boss HM, Van Schaik SM, Witkamp TD, Geerlings MI, Weinstein HC, Van den Berg-Vos RM. Cardiorespiratory fitness, cognition and brain structure after TIA or minor ischemic stroke. Int J Stroke. 2017;12(7):724–31. [DOI] [PubMed] [Google Scholar]
- 47. Rosenfeldt AB, Linder SM, Davidson S, Clark C, Zimmerman NM, Lee JJ, et al. Combined aerobic exercise and task practice improve health-related quality of life poststroke: a preliminary analysis. Arch Phys Med Rehabil. 2019;100(5):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gjellesvik TI, Becker F, Tjønna AE, Indredavik B, Lundgaard E, Solbakken H, et al. Effects of High-Intensity Interval Training after Stroke (The HIIT Stroke Study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2021;102(9):1683–91. [DOI] [PubMed] [Google Scholar]
- 49. Ihle-Hansen H, Langhammer B, Lydersen S, Gunnes M, Indredavik B, Askim T. A physical activity intervention to prevent cognitive decline after stroke: secondary results from the Life after stroke study: an 18-month randomized controlled trial. J Rehabil Med. 2019;51(9):646–51. [DOI] [PubMed] [Google Scholar]
- 50. Koch S, Tiozzo E, Simonetto M, Loewenstein D, Wright CB, Dong C, et al. Randomized trial of combined aerobic, resistance, and cognitive training to improve recovery from stroke: feasibility and safety. J Am Heart Assoc. 2020;9(10):e015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu-Ambrose T, Falck RS, Dao E, Best JR, Davis JC, Bennett K, et al. Effect of exercise training or complex mental and social activities on cognitive function in adults with chronic stroke: a randomized clinical trial. JAMA Netw Open. 2022;5(10):E2236510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore SA, Hallsworth K, Jakovljevic DG, Blamire AM, He J, Ford GA, et al. Effects of community exercise therapy on metabolic, brain, physical, and cognitive function following stroke: a randomized controlled pilot trial. Neurorehabil Neural Repair. 2015;29(7):623–35. [DOI] [PubMed] [Google Scholar]
- 53. Pallesen H, Bjerk M, Pedersen AR, Nielsen JF, Evald L. The effects of high-intensity aerobic exercise on cognitive performance after stroke: a pilot randomised controlled trial. J Cent Nerv Syst Dis. 2019;11:1179573519843493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ploughman M, Eskes GA, Kelly LP, Kirkland MC, Devasahayam AJ, Wallack EM, et al. Synergistic benefits of combined aerobic and cognitive training on fluid intelligence and the role of IGF-1 in chronic stroke. Neurorehabil Neural Repair. 2019;33(3):199–212. [DOI] [PubMed] [Google Scholar]
- 55. Shang X, Meng X, Xiao X, Xie Z, Yuan X. Grip training improves handgrip strength, cognition, and brain white matter in minor acute ischemic stroke patients. Clin Neurol Neurosurg. 2021;209(67):106886. [DOI] [PubMed] [Google Scholar]
- 56. Song R, Park M, Jang T, Oh J, Sohn MK. Effects of a Tai Chi-based stroke rehabilitation program on symptom clusters, physical and cognitive functions, and quality of life: a randomized feasibility study. Int J Environ Res Public Health. 2021;18(10):5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang A, Eng JJ, Krassioukov AV, Tsang TSM, Liu-Ambrose T. High-and low-intensity exercise do not improve cognitive function after stroke: a randomized controlled trial. J Rehabil Med. 2016;48(10):841–6. [DOI] [PubMed] [Google Scholar]
- 58. Timmermans C, Roerdink M, Meskers CGM, Beek PJ, Janssen TWJ. Walking-adaptability therapy after stroke: results of a randomized controlled trial. Trials. 2021;22(1):923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yeh TT, Chang KC, Wu CY, Chen CJ, Chuang IC. Clinical efficacy of aerobic exercise combined with computer-based cognitive training in stroke: a multicenter randomized controlled trial. Top Stroke Rehabil. 2022;29(4):255–64. [DOI] [PubMed] [Google Scholar]
- 60. Yeh TTChang K, Wu CY. The Active ingredient of cognitive restoration: a multicenter randomized controlled trial of sequential combination of aerobic exercise and computer-based cognitive training in stroke survivors with cognitive decline. Arch Phys Med Rehabil. 2019;100(5):821–7. [DOI] [PubMed] [Google Scholar]
- 61. Zheng G, Zheng Y, Xiong Z, Ye B. Effect of Baduanjin exercise on cognitive function in patients with post-stroke cognitive impairment: a randomized controlled trial. Clin Rehabil. 2020;34(8):1028–39. [DOI] [PubMed] [Google Scholar]
- 62. Baek CY, Chang WN, Park BY, Lee KB, Kang KY, Choi MR. Effects of dual-task gait treadmill training on gait ability, dual-task interference, and fall efficacy in people with stroke: a randomized controlled trial. Phys Ther. 2021;101(6):pzab067. [DOI] [PubMed] [Google Scholar]
- 63. Liu-Ambrose T, Eng JJ. Exercise training and recreational activities to promote executive functions in chronic stroke: a proof-of-concept study. J Stroke Cerebrovasc Dis. 2015;24(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deijle IA, Hemmes R, Boss HM, de Melker EC, van den Berg BTJ, Kwakkel G, et al. Effect of an exercise intervention on global cognition after transient ischemic attack or minor stroke: the MoveIT randomized controlled trial. BMC Neurol. 2022;22(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fischbacher M, Chocano-Bedoya PO, Meyer U, Bopp I, Mattle M, Kressig RW, et al. Safety and feasibility of a Dalcroze eurhythmics and a simple home exercise program among older adults with mild cognitive impairment (MCI) or mild dementia: the MOVE for your MIND pilot trial. Pilot Feasibility Stud. 2020;6:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rathnamala D, Senthil P, Selvan C. Effectiveness of cognitive training exercises and physical activity in subjects with transient ischemic attack with cognitive impairment. Ann Trop Med Public Heal. 2020;23(13):1322. [Google Scholar]
- 67. Ji H, Yu L. Effect of yoga exercise on cognitive ability and motor function recovery in stroke patients. NeuroQuantology. 2018;16(6):822–7. [Google Scholar]
- 68. Chan WN, Tsang WWN. The effect of Tai Chi training on the dual-tasking performance of stroke survivors: a randomized controlled trial. Clin Rehabil. 2018;32(8):1076–85. [DOI] [PubMed] [Google Scholar]
- 69. Dhir S, Teo WP, Chamberlain SR, Tyler K, Yücel M, Segrave RA. The effects of combined physical and cognitive training on inhibitory control: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;128:735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han P, Zhang W, Kang L, Ma Y, Fu L, Jia L, et al. Clinical evidence of exercise benefits for stroke. Adv Exp Med Biol. 2017;1000:131–51. [DOI] [PubMed] [Google Scholar]
- 71. Zhang H, Liu G, Zhang L, Wei W. Personalized biomarkers and neuropsychological status can predict post-stroke fatigue. Brain Sci. 2023;13(2):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Haworth J, Phillips M, Newson M, Rogers PJ, Torrens-Burton A, Tales A. Measuring information processing speed in mild cognitive impairment: clinical versus research dichotomy. J Alzheimers Dis. 2016;51(1):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang X, Wu J, Ye M, Wang L, Zheng G. Effect of Baduanjin exercise on the cognitive function of middle-aged and older adults: a systematic review and meta-analysis. Complement Ther Med. 2021;59:102727. [DOI] [PubMed] [Google Scholar]
- 75. Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? Int J Stroke. 2013;8(1):25–32. [DOI] [PubMed] [Google Scholar]
- 76. Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12(5):444–50. [DOI] [PubMed] [Google Scholar]
- 77. Jette AM, Lachman M, Giorgetti MM, Assmann SF, Harris BA, Levenson C, et al. Exercise: it’s never too late: the Strong-For-Life program. Am J Public Health. 1999;89(1):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mackay-Lyons M, Billinger SA, Eng JJ, Dromerick A, Giacomantonio N, Hafer-Macko C, et al. Aerobic exercise recommendations to optimize best practices in care after stroke: AEROBICS 2019 update. Phys Ther. 2020;100(1):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kaufman S, Becker G. Stroke: health care on the periphery. Soc Sci Med. 1986;22(9):983–9. [DOI] [PubMed] [Google Scholar]
- 80. Gunnes M, Indredavik B, Langhammer B, Lydersen S, Ihle-Hansen H, Dahl AE, et al. Associations between adherence to the physical activity and exercise program applied in the LAST Study and functional recovery after stroke. Arch Phys Med Rehabil. 2019;100(12):2251–9. [DOI] [PubMed] [Google Scholar]
- 81. Mackay-Lyons M, Billinger SA, Eng JJ, Dromerick A, Giacomantonio N, Hafer-Macko C, et al. Aerobic exercise recommendations to optimize best practices in care after stroke: AEROBICS 2019 update. Phys Ther. 2020;100(1):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Amorós-Aguilar L, Rodríguez-Quiroga E, Sánchez-Santolaya S, Coll-Andreu M. Effects of combined interventions with aerobic physical exercise and cognitive training on cognitive function in stroke patients: a systematic review. Brain Sci. 2021;11(4):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8(1):38–45. [DOI] [PubMed] [Google Scholar]
- 84. Davison R, Brick N, Kennedy N, Simms V, Simpson L. The effect of exercise intensity and dose on cognitive function in a post-stroke population: a systematic review and meta-analysis. PsychArxiv. 2020. [Google Scholar]
- 85. Yang B, Wang S. Meta-analysis on cognitive benefit of exercise after stroke. Complexity. 2021;2021:1–12. [Google Scholar]
- 86. Marzolini S, Oh P, McIlroy W, Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair. 2013;27(5):392–402. [DOI] [PubMed] [Google Scholar]
- 87. Rand D, Eng JJ, Liu-Ambrose T, Tawashy AE. Feasibility of a 6-month exercise and recreation program to improve executive functioning and memory in individuals with chronic stroke. Neurorehabil Neural Repair. 2010;24(8):722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: review of multiple meaningful benefits. Stroke. 2014;45(12):3742–7. [DOI] [PubMed] [Google Scholar]
- 89. Gallego Hernández A, González-Gálvez N. Physical exercise and cognitive function in post-stroke patients: a systematic review with meta-analysis. Apunt Educ Fis y Deportes. 2021;146(4):1–10. [Google Scholar]
- 90. Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke: outcome assessment and sample size requirements. Stroke. 1992;23(8):1084–9. [DOI] [PubMed] [Google Scholar]
- 91. Gresham GE. Stroke outcome research. Stroke. 1986;17(3):358–60. [DOI] [PubMed] [Google Scholar]
- 92. Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25(6):1181–8. [DOI] [PubMed] [Google Scholar]
- 93. Newman M. The process of recovery after hemiplegia. Stroke. 1972;3(6):702–10. [DOI] [PubMed] [Google Scholar]
- 94. Boyne P, Billinger S, MacKay-Lyons M, Barney B, Khoury J, Dunning K. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther. 2017;41(2):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Crozier J, Roig M, Eng JJ, MacKay-Lyons M, Fung J, Ploughman M, et al. High-intensity interval training after stroke: an opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neurorehabil Neural Repair. 2018;32(6–7):543–56. [DOI] [PubMed] [Google Scholar]
- 96. Reynolds H, Steinfort S, Tillyard J, Ellis S, Hayes A, Hanson ED, et al. Feasibility and adherence to moderate intensity cardiovascular fitness training following stroke: a pilot randomized controlled trial. BMC Neurol. 2021;21(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schmitt A, Upadhyay N, Martin JA, Rojas S, Strüder HK, Boecker H. Modulation of distinct intrinsic resting state brain networks by acute exercise bouts of differing intensity. Brain Plast. 2019;5(1):39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–60. [DOI] [PubMed] [Google Scholar]
- 99. Caetano LCG, Pacheco BD, Samora GAR, Teixeira-Salmela LF, Scianni AA. Self-efficacy to engage in physical exercise and walking ability best predicted exercise adherence after stroke. Stroke Res Treat. 2020;2020:2957623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Inness EL, Salbach NM, Brown G, Tee A, Kelly L, Moller J, et al. The Canadian stroke community-based exercise recommendations update 2020: a resource for community-based exercise providers. Montreal: Canadian Partnership for Stroke Recovery; 2021. [Google Scholar]
- 101. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 102. Higgins JP, Savović J, Page MJ, Sterne JAC. RoB 2 guidance: parallel trial: revised Cochrane risk-of-bias tool for randomized trials (RoB 2). London: ROB2 Development Group. Cochrane Collaboration; 2019. [Google Scholar]
- 103. Howieson D. Current limitations of neuropsychological tests and assessment procedures. Clin Neuropsychol. 2019;33(2):200–8. [DOI] [PubMed] [Google Scholar]
- 104. Lee YH. Strengths and limitations of meta-analysis. Korean J Med. 2019;94(5):391–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.