Abstract
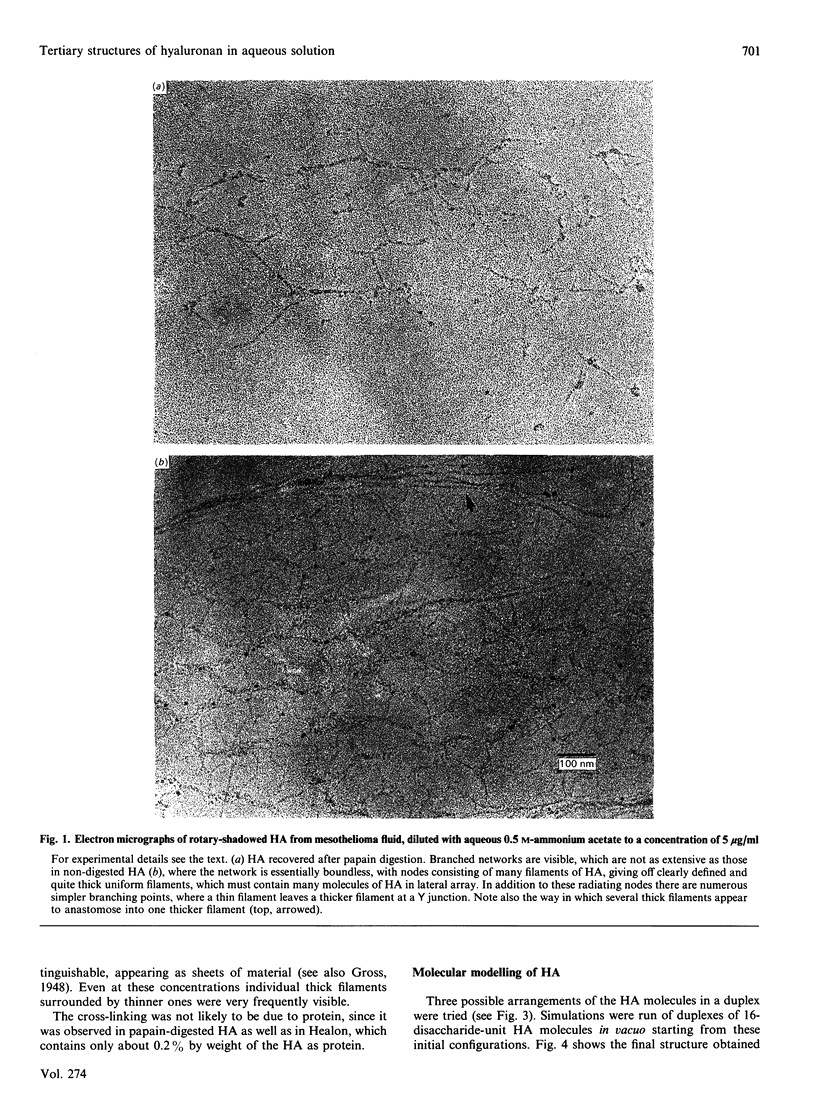
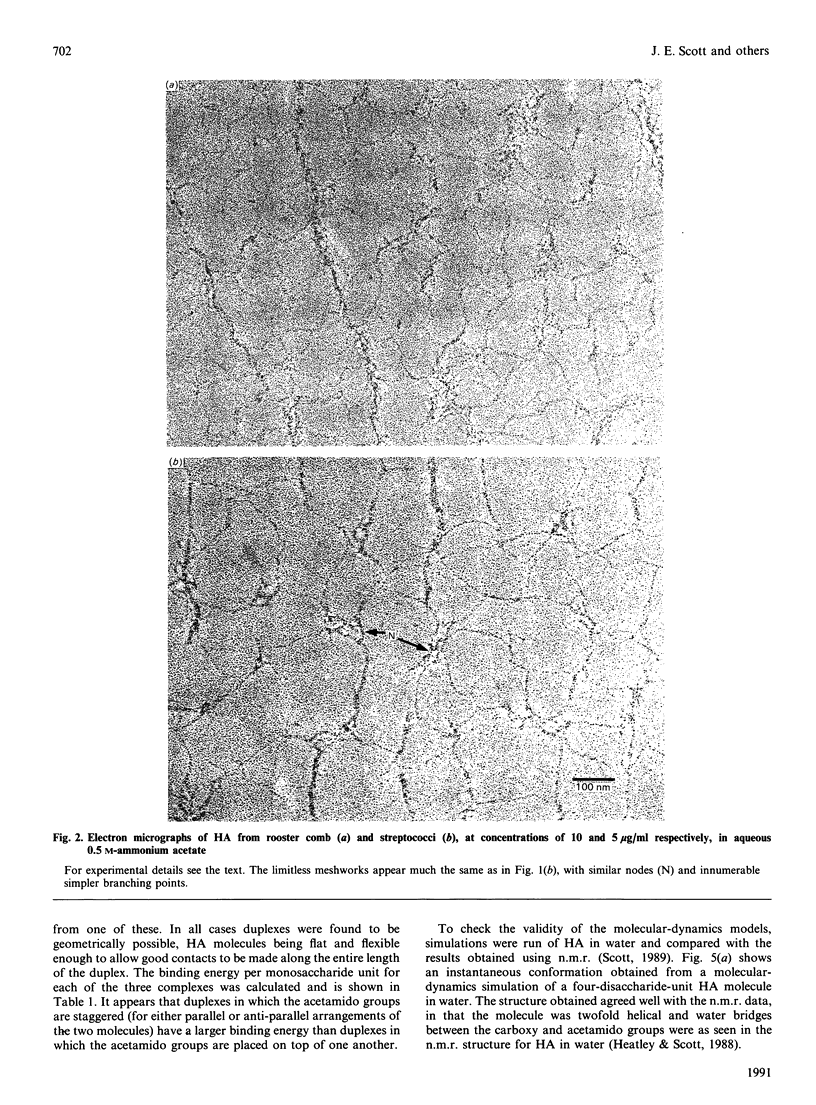
1. Hyaluronan from mesothelioma fluid, rooster comb and streptococci was examined by rotary shadowing and electron microscopy. All preparations showed extensive branched networks, but high-viscosity hyaluronan networks were essentially infinite, with no individual 'molecules' that were not integrated via multiple branched points into the meshwork. Low-viscosity hyaluronan, recovered after papain digestion of mesothelioma fluid, showed occasional single filaments that were independent of the main aggregates, some of which were themselves independent of other aggregates. 2. Hyaluronan is a polymer with a very marked capability to form meshworks at very low dilution (less than 1 microgram/ml). The longer the hyaluronan molecule, the more branching is potentially possible, and the more extensive and coherent is the network, with every hyaluronan molecule in contact with every other in the solution, via the network. This behaviour accounts for the mechanical properties of the soft tissues (e.g. vitreous humour) and fluids (e.g. synovial fluid) of which hyaluronan is a major component. 3. The hyaluronan twofold helix, previously demonstrated to be present in solution [Heatley & Scott (1988) Biochem. J. 254, 489-493] was shown by computer simulation and energy calculations to be sterically capable of extensive duplex formation, probably driven by interactions between the large hydrophobic patches on alternate sides of the tape-like polymer, forming stable aggregates at biological temperatures in water. This 'stickiness' is postulated to be the basis of the network-forming and laterally aggregating behaviour of hyaluronan. 4. The tertiary structures formed by hyaluronan may not be possible in the case of chondroitin 4-sulphate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fessler J. H., Fessler L. I. Electron microscopic visualization of the polysaccharide hyaluronic acid. Proc Natl Acad Sci U S A. 1966 Jul;56(1):141–147. doi: 10.1073/pnas.56.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatley F., Scott J. E. A water molecule participates in the secondary structure of hyaluronan. Biochem J. 1988 Sep 1;254(2):489–493. doi: 10.1042/bj2540489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., SHERMAN T. F. Degradation of the hyaluronic acid complex of synovial fluid by proteolytic enzymes and by ethylenediaminetetra-acetic acid. Biochem J. 1959 Jun;72(2):301–305. doi: 10.1042/bj0720301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. N., Davies M., Ogston A. G. The composition and physicochemical properties of hyaluronic acids prepared from ox synovial fluid and from a case of mesothelioma. Biochem J. 1965 Aug;96(2):449–474. doi: 10.1042/bj0960449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Cummings C., Greiling H., Stuhlsatz H. W., Gregory J. D., Damle S. P. Examination of corneal proteoglycans and glycosaminoglycans by rotary shadowing and electron microscopy. Int J Biol Macromol. 1990 Jun;12(3):180–184. doi: 10.1016/0141-8130(90)90029-a. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Hull W. E. Secondary structure of hyaluronate in solution. A 1H-n.m.r. investigation at 300 and 500 MHz in [2H6]dimethyl sulphoxide solution. Biochem J. 1984 May 15;220(1):197–205. doi: 10.1042/bj2200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Jones M. N., Wilkinson A., Olavesen A. H. Secondary structure of chondroitin sulphate in dimethyl sulphoxide. Eur J Biochem. 1983 Feb 15;130(3):491–495. doi: 10.1111/j.1432-1033.1983.tb07177.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Secondary structures in hyaluronan solutions: chemical and biological implications. Ciba Found Symp. 1989;143:6-15; discussion 15-20, 281-5. doi: 10.1002/9780470513774.ch2. [DOI] [PubMed] [Google Scholar]
- Swann D. A. Biochemistry of the vitreous. Bull Soc Belge Ophtalmol. 1987;223(Pt 1):59–72. doi: 10.1007/978-1-4757-1901-7_4. [DOI] [PubMed] [Google Scholar]






