Abstract
Several amidated biologically active peptides such as pancreastatin, thyrotropin-releasing hormone, pancreatic polypeptide and amylin are produced in endocrine pancreatic tissue which contains the enzyme necessary for their final processing, i.e. peptidylglycine alpha-amidating mono-oxygenase (EC 1.14.17.3). The enzyme needs ascorbic acid for activity as well as copper and molecular oxygen. The present work shows that pancreatic islet cells prepared from overnight cultures of isolated islets from 5-7-day-old rats accumulate 14C-labelled ascorbic acid by a Na(+)-dependent active transport mechanism which involves a saturable process (estimated Km 17.6 microM). Transport was inhibited by ouabain, phloridzin, cytochalasin B, amiloride and probenecid. Glucose inhibited or stimulated uptake, depending on the length of incubation time of the cells. The uptake of dehydroascorbic acid was linearly dependent on concentration. Dehydroascorbic acid was converted to ascorbic acid by an unknown mechanism after uptake. The uptake of both ascorbic acid and dehydroascorbic acid was inhibited by tri-iodothyronine, and uptake of ascorbic acid, but not of dehydroascorbic acid, was inhibited by glucocorticoids. Isolated secretory granules contained a fairly low concentration of iron but a high concentration of copper.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsten P., Amitai G., Kehrl J., Dhariwal K. R., Klein H. G., Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem. 1990 Feb 15;265(5):2584–2587. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Cullen E. I., May V., Eipper B. A. Transport and stability of ascorbic acid in pituitary cultures. Mol Cell Endocrinol. 1986 Dec;48(2-3):239–250. doi: 10.1016/0303-7207(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Heckman G. D., Daniels A. J. Characterization of ascorbic acid transport by adrenomedullary chromaffin cells. Evidence for Na+-dependent co-transport. J Biol Chem. 1983 Nov 10;258(21):12886–12894. [PubMed] [Google Scholar]
- Eipper B. A., Glembotski C. C., Mains R. E. Bovine intermediate pituitary alpha-amidation enzyme: preliminary characterization. Peptides. 1983 Nov-Dec;4(6):921–928. doi: 10.1016/0196-9781(83)90091-8. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Park L. P., Dickerson I. M., Keutmann H. T., Thiele E. A., Rodriguez H., Schofield P. R., Mains R. E. Structure of the precursor to an enzyme mediating COOH-terminal amidation in peptide biosynthesis. Mol Endocrinol. 1987 Nov;1(11):777–790. doi: 10.1210/mend-1-11-777. [DOI] [PubMed] [Google Scholar]
- Emeson R. B. Hypothalamic peptidyl-glycine alpha-amidating monooxygenase: preliminary characterization. J Neurosci. 1984 Oct;4(10):2604–2613. doi: 10.1523/JNEUROSCI.04-10-02604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K., Marklund S. L., Täljedal I. B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981 Nov 1;199(2):393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Fink C. J., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. I. Isolation of a secretory granule fraction. J Cell Biol. 1969 Apr;41(1):154–161. doi: 10.1083/jcb.41.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizer J. S., Bateman R. C., Jr, Miller C. R., Humm J., Busby W. H., Jr, Youngblood W. W. Purification and characterization of a peptidyl glycine monooxygenase from porcine pituitary. Endocrinology. 1986 Jun;118(6):2262–2267. doi: 10.1210/endo-118-6-2262. [DOI] [PubMed] [Google Scholar]
- Kizer J. S., Busby W. H., Jr, Cottle C., Youngblood W. W. Glycine-directed peptide amidation: presence in rat brain of two enzymes that convert p-Glu-His-Pro-Gly-OH into p-Glu-His-Pro-NH2 (thyrotropin-releasing hormone). Proc Natl Acad Sci U S A. 1984 May;81(10):3228–3232. doi: 10.1073/pnas.81.10.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Knoth J., Viveros O. H., Diliberto E. J., Jr Evidence for the release of newly acquired ascorbate and alpha-aminoisobutyric acid from the cytosol of adrenomedullary chromaffin cells through specific transporter mechanisms. J Biol Chem. 1987 Oct 15;262(29):14036–14041. [PubMed] [Google Scholar]
- Kábrt J. Ascorbic acid in the pituitary gland. 3. Bound ascorbic acid in the adenohypophysis. Acta Univ Carol Med (Praha) 1982;28(5-6):345–368. [PubMed] [Google Scholar]
- Mackin R. B., Flacker J. M., Mackin J. A., Noe B. D. Peptidyl-glycine alpha-amidating monooxygenase is present in islet secretory granules of the anglerfish, Lophius americanus. Gen Comp Endocrinol. 1987 Aug;67(2):263–269. doi: 10.1016/0016-6480(87)90156-0. [DOI] [PubMed] [Google Scholar]
- Maltese J. Y., Giraud P., Kowalski C., Ouafik L. H., Salers P., Pelen F., Oliver C. Ontogenetic expression of peptidyl-glycine alpha-amidating monooxygenase mRNA in the rat pancreas. Biochem Biophys Res Commun. 1989 Jan 16;158(1):244–250. doi: 10.1016/s0006-291x(89)80204-9. [DOI] [PubMed] [Google Scholar]
- Moser U. Uptake of ascorbic acid by leukocytes. Ann N Y Acad Sci. 1987;498:200–215. doi: 10.1111/j.1749-6632.1987.tb23762.x. [DOI] [PubMed] [Google Scholar]
- Murthy A. S., Mains R. E., Eipper B. A. Purification and characterization of peptidylglycine alpha-amidating monooxygenase from bovine neurointermediate pituitary. J Biol Chem. 1986 Feb 5;261(4):1815–1822. [PubMed] [Google Scholar]
- Nishi M., Sanke T., Nagamatsu S., Bell G. I., Steiner D. F. Islet amyloid polypeptide. A new beta cell secretory product related to islet amyloid deposits. J Biol Chem. 1990 Mar 15;265(8):4173–4176. [PubMed] [Google Scholar]
- Ohsuye K., Kitano K., Wada Y., Fuchimura K., Tanaka S., Mizuno K., Matsuo H. Cloning of cDNA encoding a new peptide C-terminal alpha-amidating enzyme having a putative membrane-spanning domain from Xenopus laevis skin. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1275–1281. doi: 10.1016/0006-291x(88)90767-x. [DOI] [PubMed] [Google Scholar]
- Omaye S. T., Turnbull J. D., Sauberlich H. E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Ouafik L., Dutour A., Salers P., Giraud P., Boudouresque F., Castanas E., Oliver C. Evidence for high peptide alpha-amidation activity in the neonatal rat pancreas. Biochem Biophys Res Commun. 1986 Jul 16;138(1):179–184. doi: 10.1016/0006-291x(86)90263-9. [DOI] [PubMed] [Google Scholar]
- Paquette T. L., Gingerich R., Scharp D. Altered amidation of pancreatic polypeptide in cultured dog islet tissue. Biochemistry. 1981 Dec 22;20(26):7403–7408. doi: 10.1021/bi00529a013. [DOI] [PubMed] [Google Scholar]
- Rose R. C. Metabolism and transport of dehydroascorbic acid in erythrocytes of "spontaneous diabetic BB/W" Wistar rats. Metabolism. 1986 Jul;35(7):619–621. doi: 10.1016/0026-0495(86)90167-8. [DOI] [PubMed] [Google Scholar]
- Rose R. C. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta. 1988 Jun 9;947(2):335–366. doi: 10.1016/0304-4157(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Scharfmann R., Leduque P., Aratan-Spire S., Dubois P., Basmaciogullari A., Czernichow P. Persistence of peptidylglycine alpha-amidating monooxygenase activity and elevated thyrotropin-releasing hormone concentrations in fetal rat islets in culture. Endocrinology. 1988 Sep;123(3):1329–1334. doi: 10.1210/endo-123-3-1329. [DOI] [PubMed] [Google Scholar]
- Siliprandi L., Vanni P., Kessler M., Semenza G. Na+-dependent, electroneutral L-ascorbate transport across brush border membrane vesicles from guinea pig small intestine. Biochim Biophys Acta. 1979 Mar 23;552(1):129–142. doi: 10.1016/0005-2736(79)90252-9. [DOI] [PubMed] [Google Scholar]
- Som S., Basu S., Mukherjee D., Deb S., Choudhury P. R., Mukherjee S., Chatterjee S. N., Chatterjee I. B. Ascorbic acid metabolism in diabetes mellitus. Metabolism. 1981 Jun;30(6):572–577. doi: 10.1016/0026-0495(81)90133-5. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Thorens B., Weir G. C., Leahy J. L., Lodish H. F., Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn N. A., Nielsen F. S., Jeppesen C. K., Christensen B. L., Farver O. Uptake of dehydroascorbic acid and ascorbic acid to isolated nerve terminals and secretory granules from ox neurohypophyses. Acta Physiol Scand. 1986 Dec;128(4):629–638. doi: 10.1111/j.1748-1716.1986.tb08021.x. [DOI] [PubMed] [Google Scholar]
- Toggenburger G., Häusermann M., Mütsch B., Genoni G., Kessler M., Weber F., Hornig D., O'Neill B., Semenza G. Na+-dependent, potential-sensitive L-ascorbate transport across brush border membrane vesicles from kidney cortex. Biochim Biophys Acta. 1981 Sep 7;646(3):433–443. doi: 10.1016/0005-2736(81)90312-6. [DOI] [PubMed] [Google Scholar]
- Zhou A., Matsumoto T., Farver O., Thorn N. A. Uptake of ascorbic acid by freshly isolated cells and secretory granules from the intermediate lobe of ox hypophyses. Acta Physiol Scand. 1990 Feb;138(2):229–234. doi: 10.1111/j.1748-1716.1990.tb08837.x. [DOI] [PubMed] [Google Scholar]
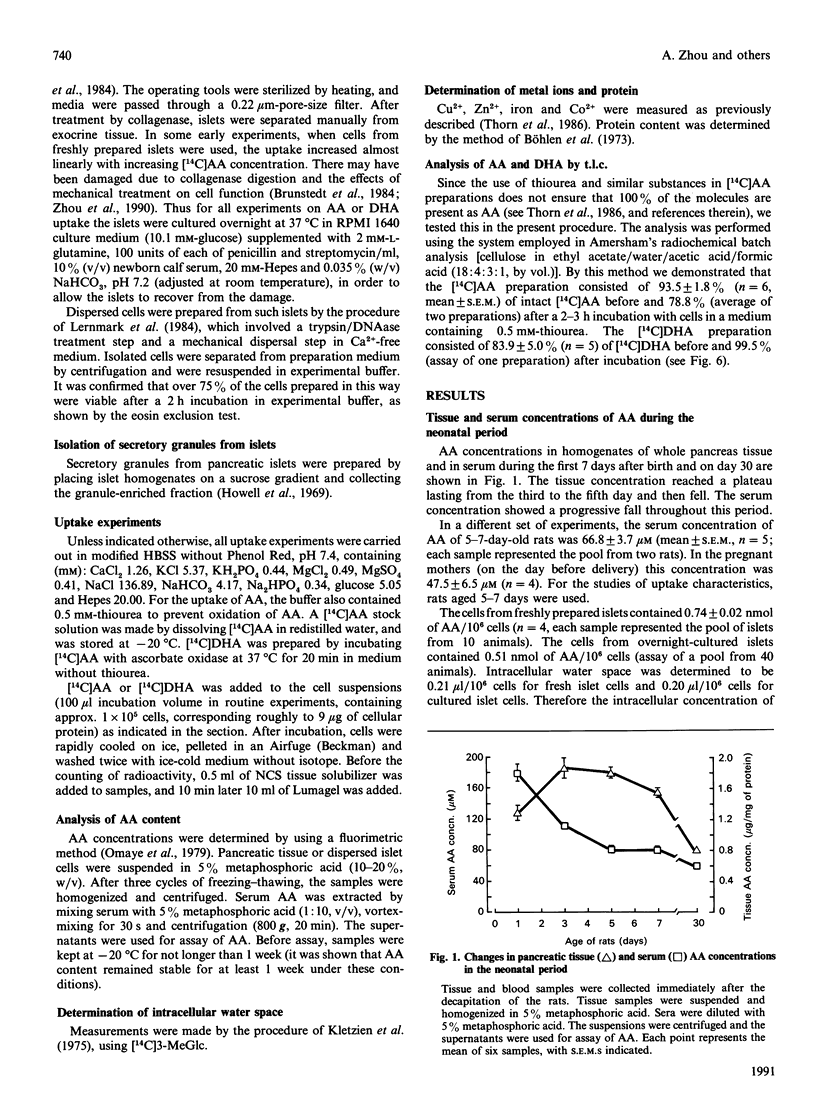
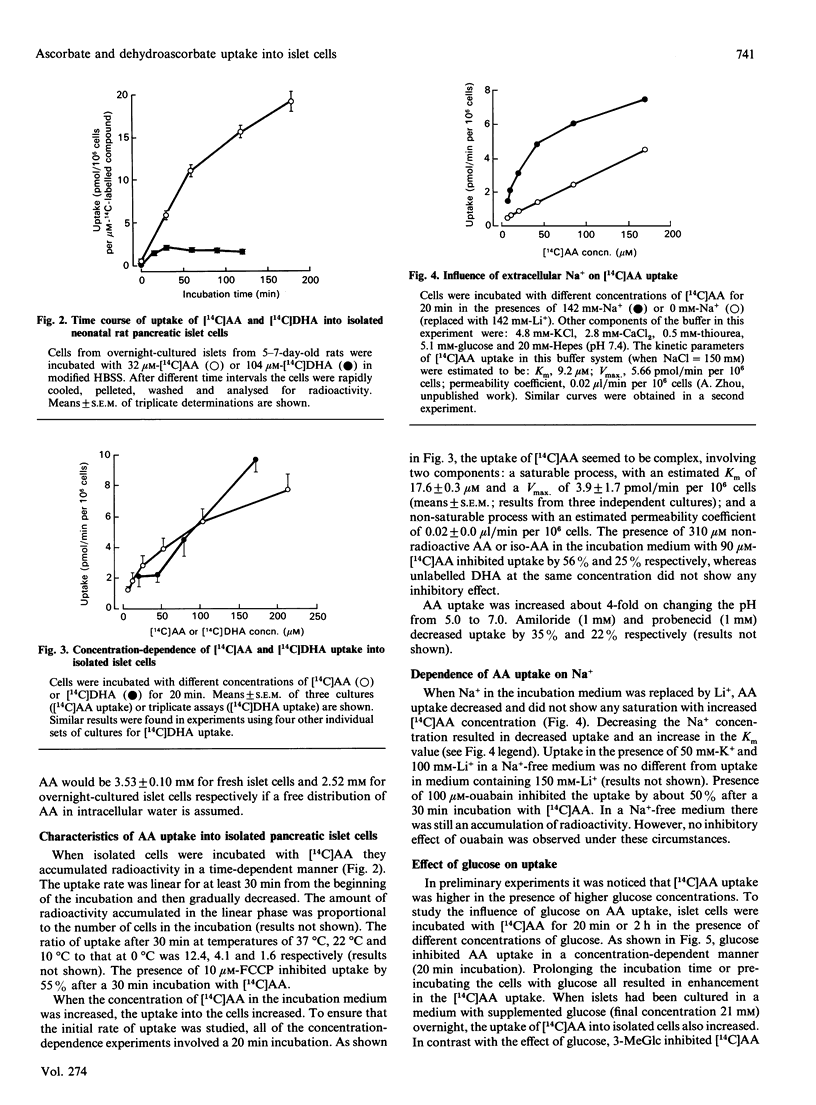
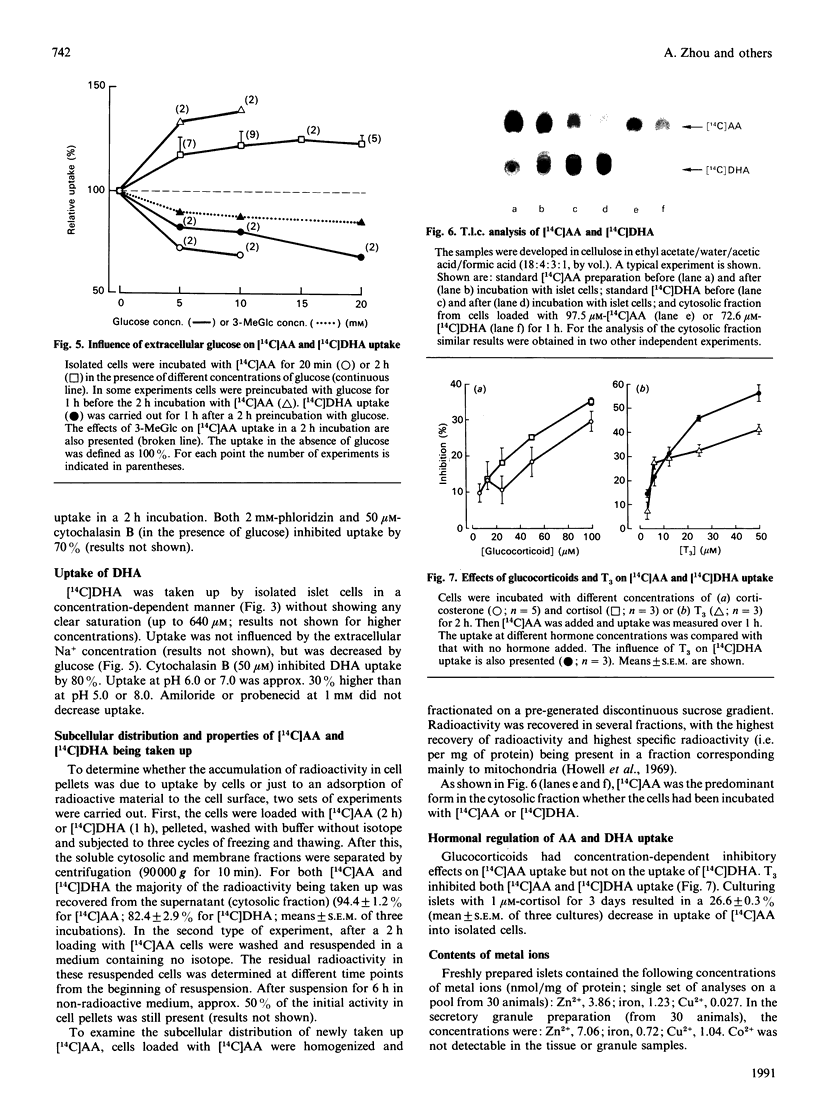
- Zhou A., Thorn N. A. Evidence for presence of peptide alpha-amidating activity in pancreatic islets from newborn rats. Biochem J. 1990 Apr 1;267(1):253–256. doi: 10.1042/bj2670253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M., Tritton T. R., Castle J. D. Exocrine secretion granules contain peptide amidation activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3297–3301. doi: 10.1073/pnas.83.10.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]



