Abstract
Background
Neoadjuvant immune checkpoint inhibitors (ICIs) have improved survival outcomes compared with chemotherapy in resectable non-small cell lung cancer (NSCLC). However, the impact of actionable genomic alterations (AGAs) on the efficacy of neoadjuvant ICIs remains unclear. We report the influence of AGAs on treatment failure (TF) in patients with resectable NSCLC treated with neoadjuvant ICIs.
Methods
Tumor molecular profiles were obtained from patients with stage I–IIIA resectable NSCLC (American Joint Committee on Cancer seventh edition) treated with either neoadjuvant nivolumab (N, n=23) or nivolumab+ipilimumab (NI, n=21) followed by surgery in a previously reported phase-2 randomized study (NCT03158129). TF was defined as any progression of primary lung cancer after neoadjuvant ICI therapy in patients without surgery, radiographic and/or biopsy-proven primary lung cancer recurrence after surgery, or death from possibly treatment-related complications or from primary lung cancer since randomization. Tumors with AGAs (n=12) were compared with tumors without AGAs and non-profiled squamous cell carcinomas (non-AGAs+NP SCC, n=20).
Results
With a median follow-up of 60.2 months, the overall TF rate was 34.1% (15/44). Tumor molecular profiling was retrospectively obtained in 47.7% (21/44) of patients and select AGAs were identified in 12 patients: 5 epidermal growth factor receptor (EGFR), 2 KRAS, 1 ERBB2, and 1 BRAF mutations, 2 anaplastic lymphoma kinase (ALK) and 1 RET fusions. The median time to TF in patients with AGAs was 24.7 months (95% CI: 12.6 to 40.4), compared with not reached (95% CI: not evaluable (NE)–NE) in the non-AGAs+NP SCC group. The TF risk was higher in AGAs (HR: 5.51, 95% CI: 1.68 to 18.1), and lower in former/current smokers (HR: 0.24, 95% CI: 0.08 to 0.75). The odds of major pathological response were 4.71 (95% CI: 0.49 to 45.2) times higher in the non-AGAs+NP SCC group, and the median percentage of residual viable tumor was 72.5% in AGAs compared with 33.0% in non-AGS+NP SCC tumors.
Conclusions
Patients with NSCLC harboring select AGAs, including EGFR and ALK alterations, have a higher risk for TF, shorter median time to TF, and diminished pathological regression after neoadjuvant ICIs. The suboptimal efficacy of neoadjuvant chemotherapy-sparing, ICI-based regimens in this patient subset underscores the importance of tumor molecular testing prior to initiation of neoadjuvant ICI therapy in patients with resectable NSCLC.
Keywords: Immunotherapy, Lung Cancer, Nivolumab, Ipilimumab, Recurrence
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
We assessed the impact of select AGAs on the efficacy of neoadjuvant ICIs in patients with NSCLC treated with neoadjuvant nivolumab or nivolumab plus ipilimumab followed by surgery. Patients with tumors harboring AGAs exhibited higher rates of ICI treatment failure (TF), higher median percentage of residual viable tumor and shorter median time to TF.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results suggest that certain AGAs may adversely impact the benefit of neoadjuvant ICIs in resectable NSCLC and underscore the importance of tumor molecular testing prior to neoadjuvant immunotherapy.
Introduction
In operable non-small cell lung cancer (NSCLC), neoadjuvant immune checkpoint inhibitor (ICI) therapy is feasible and improves the rate of pathologic response when compared with standard-of-care chemotherapy.1,4 Recently, the combination of chemoimmunotherapy received regulatory approval in the neoadjuvant and perioperative settings for a subset of patients with operable NSCLC based on the results of CheckMate-816, KEYNOTE-671, and AEGEAN trials.5,7 Major and complete pathologic response rates have been used as emerging candidate surrogate endpoints of clinical benefit after neoadjuvant therapies.8 However, little is known about the impact of genomic alterations on pathological responses and treatment failure (TF) after neoadjuvant ICIs.
The randomized NEOSTAR trial3 was a phase 2 study that reported on the outcomes of neoadjuvant ICI therapy using the programmed cell death protein-1 inhibitor nivolumab or the combination of nivolumab and the cytotoxic T-lymphocytes associated protein 4 inhibitor ipilimumab followed by surgery. Since the completion of the NEOSTAR trial, tumor molecular profiling is a highly recommended practice in the early-stage, operable setting for NSCLC. Adjuvant osimertinib, a treatment targeting epidermal growth factor receptor (EGFR) sensitizing mutations, is now the standard of care for patients with resected NSCLCs harboring these mutations.9 10 Likewise, in patients with anaplastic lymphoma kinase (ALK)-rearrangement positive NSCLC who have undergone surgical resection, adjuvant alectinib is now recommended.11 Select phase 3 randomized controlled trials evaluating neoadjuvant and perioperative ICI therapy excluded patients whose tumors harbor EGFR and ALK alterations,5 7 12 13 and several ongoing trials are evaluating the role of perioperative targeted therapies for patients whose tumors harbor actionable genomic alterations (AGAs).14,16 This highlights the importance of tumor molecular profiling as an essential component of the decision-making process for neoadjuvant and perioperative immune-based treatment.
Patients treated on the randomized NEOSTAR trial received neoadjuvant nivolumab or nivolumab plus ipilimumab, irrespective of the presence of AGAs in their tumors, as testing of tumor molecular profiling was not mandated prior to initiation of neoadjuvant therapy in this study. With the availability of long-term follow-up data, we identified patients who experienced TF and explored the clinicopathological and molecular factors associated with TF. This exploratory analysis includes assessing the impact of AGAs on TF.
Methods
Patients and treatment
Patients with resectable stage I–IIIA (American Joint Committee on Cancer seventh edition staging system) NSCLC were randomized to treatment with either neoadjuvant nivolumab or nivolumab plus ipilimumab3 followed by surgical resection. The primary endpoint of the trial was the rate of major pathological response (MPR), defined as ≤10% residual viable tumor in the original resected tumor after neoadjuvant therapy on the study.3 17 The clinical results have been previously reported.3 18 Patients underwent long-term follow-up for evaluation of survival outcomes.
Tumor molecular profiling
Molecular profiling prior to the initiation of treatment was not required for trial enrollment. Post-hoc analysis of available tumor molecular profiling was performed for the current report. The specific profiling method depended on the availability and quality of appropriate tumor and/or blood samples. Tumor samples, when available, underwent in-house next-generation sequencing (NGS assay, DNA-based panel), capable of detecting 134 gene single nucleotide variants (SNVs) and 47 gene copy number gains, totaling 146 genes performed by the institutional Clinical Laboratory Improvement Amendments-certified Molecular Diagnostic Laboratory at MD Anderson Cancer Center, as previously described.19 20 Additionally, targeted intergenic and intragenic fusions in 51 genes were also identified using an NGS assay (RNA-based panel). By clinician discretion and tissue availability, cytogenetic analyses by fluorescent in-situ hybridization (FISH) were performed to identify MET amplification and rearrangements in RET, ALK, and ROS1. In certain cases, in-house NGS-based circulating cell-free DNA analysis via institutional liquid biopsy was performed using a blood sample for genomic profiling, capable of detecting SNVs in 70 genes, 19 gene copy number gains, and six gene fusions, as described previously.19 21 Tumor cell programmed death-ligand 1 (PD-L1) expression was evaluated on available baseline tumor samples as part of the research correlative analyses, when possible, using single chromogenic immunohistochemistry analysis (clone 28–8, ab205921, 1:100 dilution, Abcam).3 The details of the tissue preparation process have been previously described.22 PD-L1-stained slides underwent scoring by two independent and trained pathologists following the guidelines recommended by the International Association for the Study of Lung Cancer.23 Standard microscopy was used to identify any positive membrane staining on malignant tumor cells and determine the tumor proportion score.23 Tumor tissue molecular profiling reported here, involving NGS-based and/or cytogenetic (FISH-based) testing, was conducted on samples obtained from the primary tumor and/or surgical resection and/or metastatic lesion through bronchoscopic biopsy and/or radiographically guided percutaneous biopsy. Institutional liquid biopsy was obtained, where possible, through blood samples at the discretion of treating physicians at the time of suspected or evident radiographic disease recurrence/progression during surveillance or on tissue confirmation of the presence of disease.
Current study objectives
The purpose of this exploratory report was to describe the impact of select AGAs on TF, MPR and tumor pathological regression in patients treated with neoadjuvant ICIs. Additionally, the study explored the impact of ICI-based therapy, targeted therapy, or chemotherapy, as a subsequent treatment option after failure from neoadjuvant ICIs. TF was defined as the progression of primary lung cancer after neoadjuvant therapy in patients without surgery, radiographic and/or biopsy-proven primary lung cancer recurrence after surgery, or death from possibly treatment-related complications or from primary lung cancer, from randomization on study. The database cut-off date was August 2, 2023.
Statistical methods
The association of MPR with molecular subgroups (AGAs vs non-AGAs+non-profiled squamous cell carcinomas (NP SCC)) was assessed by odds ratio in the univariable logistic regression model. The TF-free survival distributions were estimated by the Kaplan-Meier method. The univariable Cox proportional hazards (PH) regression model was used to evaluate the risk of TF for AGAs and non-AGAs+NP SCC groups, baseline clinicopathological characteristics and pathologic response in which the landmark method at surgery date was applied. The multivariable Cox PH regression model was also used to estimate the risk of TF for AGAs and non-AGAs+NP SCC groups. Comparison test was not performed as analyses were exploratory in nature. All statistics were performed in R (V.4.3.3), SAS (V.9.4), Excel (V.2016), and GraphPad Prism (V.10.0.3).
Results
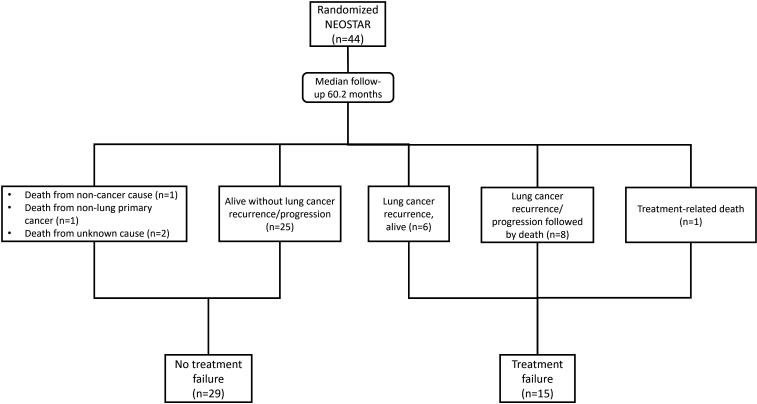
A total of 44 randomized patients who received at least one cycle of neoadjuvant ICI on trial were evaluated. With a median follow-up time of 60.2 months at the database cut-off, 15 patients (34.1%) had experienced TF. Among the 15 subjects with TF, there were a total of nine deaths, including 8 patients who had died after disease progression or recurrence, 1 patient died due to possibly treatment-related complications,3 1 patient experienced surgery-precluding disease progression after neoadjuvant treatment and died of progressing disease after other therapies. Out of the 15 patients with TFs, there were 13 patients with documented recurrences, of which, 4 were locoregional and 9 were distant. Out of 15 TFs, 8 patients had received adjuvant chemotherapy and 6 had received postoperative radiotherapy. Among the 29 non-TF patients, 1 died from non-cancer cause, 1 from non-lung primary cancer, and 2 from unknown causes without a known recurrence of their primary lung cancer (figure 1).
Figure 1. Treatment failure was defined as any progression of primary lung cancer in patients without surgery, radiographic and/or biopsy proven lung cancer recurrence after surgery, or death from possibly treatment-related complications or from primary lung cancer since randomization. Lung cancer recurrence/progression-free survival was defined as no evidence of progression and/or recurrence from the primary lung cancer at last radiographic scan since randomization. n, number.
Characteristics of tumor AGAs and non-AGAs+NP SCC patients
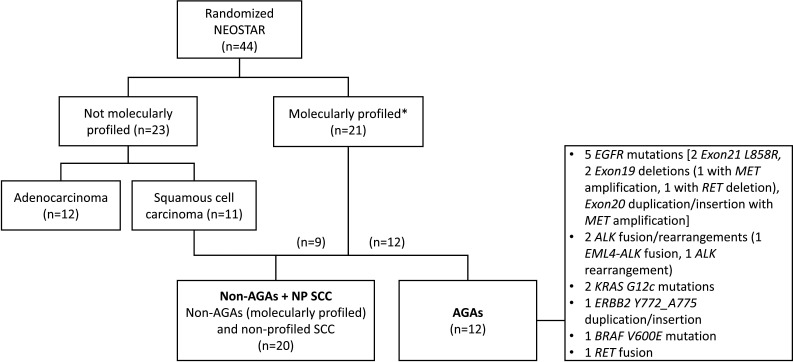
Among the 44 patients, 21 (47.7%) had undergone tumor and/or blood molecular profiling. Of the 23 patients without profiling, 12 had adenocarcinomas and 11 had squamous histology at baseline (figure 2). In an exploratory fashion, we compared patients with AGAs (n=12) to the non-AGAs+NP SCC group (n=20), which consisted of two patient cohorts: Patients who underwent tumor molecular profiling but were found to lack AGAs (non-AGAs, n=9) and patients with NP SCC (n=11) (figure 2).
Figure 2. Patients with tumors harboring actionable genomic alterations (AGAs, n=12) were compared to the non-AGAs + NP SCC group, which included profiled tumors without AGAs and non-profiled squamous cell carcinomas (n=20); Tumor histology is at baseline. *Molecular profiling methods included next-generation sequencing (NGS DNA panel and/or NGS RNA fusion panel, when available), and/or cytogenetic studies (when available), and/or circulating cell-free DNA (ccfDNA) (when available) via liquid biopsy. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; n, number; NP SCC, non-profiled squamous cell carcinomas.
AGAs were found in 12 (57.1%) profiled patients. These consisted of five mutations in EGFR (2 Exon 21 L858R mutations, 1 Exon 19 deletion with MET amplification, 1 Exon 19 deletion with RET deletion, 1 Exon 20 duplication with MET amplification), 2 KRAS G12C mutations, 1 ERBB2 Y772_A775dup mutation, 1 BRAF V600E mutation, 2 ALK (1 EML4-ALK fusion, 1 rearrangement) fusions and 1 KIF5B-RET fusion. TP53 was the most frequently altered gene, present in 57.1% (12/21) of sequenced samples. The most frequent AGAs were EGFR mutations, which were found in 23.8% (5/21) of sequenced samples, followed by KRAS G12C mutations found in 9.5% (2/21). Of note, there were four STK11 mutations, two of whom occurred with KRAS (1 G12C, 1 Q61H) co-mutation. Evaluation of clinicopathological characteristics in AGAs and non-AGAs+NP SCC patient groups revealed that the AGAs group had more patients younger than 65, and more never-smokers. Non-AGA+NP SCC group had a higher number of patients with squamous histology (table 1). Gender, race, clinical stage, and pretreatment tumor PD-L1 expression status were not different between the two groups (table 1).
Table 1. Clinicopathological characteristics of AGAs and non-AGAs+NP SCC patients.
| AGAs(n=12) | Non-AGAs + NP SCC(n=20) | |
| Age | ||
| < 65 | 7 (58%) | 5 (25%) |
| ≥ 65 | 5 (42%) | 15 (75%) |
| Gender | ||
| Female | 4 (33%) | 7 (35%) |
| Male | 8 (67%) | 13 (65%) |
| Smoking status | ||
| Never smoker | 5 (42%) | 2 (10%) |
| Former/current smoker | 7 (58%) | 18 (90%) |
| Race | ||
| White | 9 (75%) | 17 (85%) |
| Non-white | 3 (25%) | 3 (15%) |
| Stage | ||
| Stage I/II | 9 (75%) | 15 (75%) |
| Stage IIIA | 3 (25%) | 5 (25%) |
| Histology* | ||
| Squamous | 1 (8%) | 16 (80%) |
| Non-squamous | 11 (92%) | 4 (20%) |
| PD-L1† | ||
| <1 % | 6 (67%) | 8 (67%) |
| ≥1 % | 3 (33%) | 4 (33%) |
Demographic information for patients with tumors harboring actionable genomic alterations (AGAs) or patients with tumors not harboring AGAs or non-profiled squamous cell carcinomas (non-AGAs+NP SCC). The percentages for each characteristic are rounded to the nearest whole number to total 100%.
Tumor histology at baseline.
Pretreatment tumor PD-L1 expression status (n=21) on available samples by clone 28–8, Abcam3 23; the PD-L1 expression status of three patients in AGAs and eight patients in non-AGAs+NP SCC were unavailable.
PD-L1programmed death-ligand 1
Clinicopathological and molecular characteristics of patients with TF
Among the 37 patients who were resected on-trial, 27.0% (10/37) had TF, and among the 7 patients who did not undergo surgical resection or were resected off-trial, 71.4% (5/7) had TF. Out of 15 TFs, 60% (9/15) had identifiable AGAs.
TF was more common in patients with AGAs than in the non-AGAs+NP SCC group (75% (9/12) vs 20% (4/20)). The most common AGAs in the TF group were EGFR mutations (2 Exon 21 L858R mutations, 1 Exon 19 deletion with MET amplification, 1 Exon 19 deletion with RET deletion, 1 Exon 20 duplication with MET amplification), followed by one EML4-ALK fusion, one KIF5B-RET fusion, one KRAS G12C mutation and one BRAF V600E mutation. Six squamous cell carcinomas were profiled, one had an ALK rearrangement, and five did not harbor an AGA.
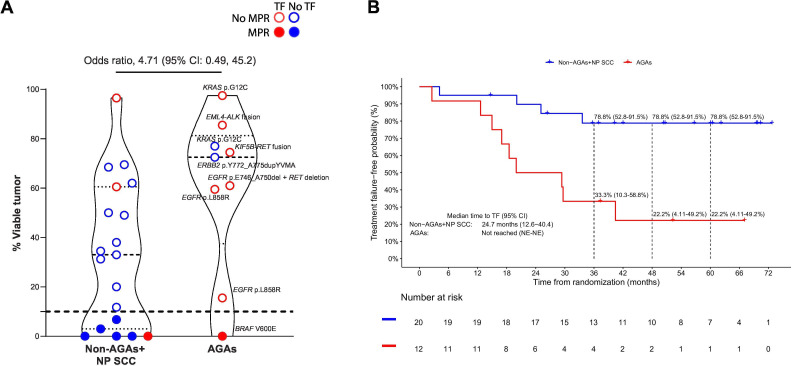
Among the 32 patients in AGAs and non-AGAs+NP SCC groups, 28 had pathologic response assessment of residual viable tumor in the resected tumor specimen. The odds of achieving MPR in the non-AGAs+NP SCC group were 4.71 (95% CI: 0.49 to 45.2) times higher than in the AGAs group (figure 3A). The AGAs group had a higher median percentage of residual viable tumor than the non-AGAs+NP SCC group (72.5% vs 33.0%) (figure 3A).
Figure 3. (A) Violin plot showing the pathological response in the resected tumor specimens from patients who underwent curative-intent surgery on trial in AGAs group (patients with tumors harboring an actionable genomic alterations, n=9) versus the non-AGAs+NP SCC group (patients with genomic profiling without AGAs or with non-profiled squamous cell carcinomas, n=19). The dds ratio of MPR was calculated in univariable logistic regression comparing the odds of achieving MPR in non-AGAs+NP SCC group with AGAs group. The dashed line indicates the median; the dotted lines indicate the lower quartile and upper quartile values; the top and bottom of the violin plots indicate the minima and maxima. (B) Kaplan-Meier curves of treatment failure-free survival probability for patients in the non-AGAs+NP SCC (n=20) and AGAs (n=12) groups. The 95% CIs are shown in parentheses by each treatment failure-free rate at 36, 48, and 60 months. The median time to TF with 95% CI was estimated using Kaplan-Meier method. Treatment failure was defined as any progression of primary lung cancer in patients without surgery, radiographic and/or biopsy-proven lung cancer recurrence after surgery, or death from possibly treatment-related complications or from primary lung cancer, since randomization. AGAs, actionable genomic alterations; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; MPR; major pathologic response (residual viable tumor ≤ 10% in the resected tumor specimen); NE, not evaluable; NP SCC, non-profiled squamous cell carcinomas; TF, treatment failure.
16.7% (2/12) of patients whose tumors had a TP53 mutation had an MPR, and none (0/4) of the tumors harboring an STK11 mutation achieved MPR. Three of the four patients with STK11 mutations were in the non-AGAs+NP SCC group, and one (33.3%) had TF, this patient had a KRAS Q61H co-mutation. The only AGAs patients with MPR harbored a BRAF V600E mutation and developed recurrence in the brain 40.4 months after randomization and was treated with metastasectomy and postoperative radiation and remained disease-free at the time of dataset cut-off.
Patients with tumors harboring AGAs had a shorter median time to TF than those without AGAs. The median time to TF for AGAs patients was 24.7 months (95% CI: 12.6 to 40.4), and was not reached (95% CI: not evaluable (NE)–NE) in the non-AGAs+NP SCC group (figure 3B). TF-free rates at 36, 48, and 60 months were 33.3% (95% CI: 10.3% to 58.8%), 22.2% (95% CI: 4.11% to 49.2%), and 22.2% (95% CI: 4.11% to 49.2%) for the AGAs group, and 78.8% (95% CI: 52.8% to 91.5%), 78.8% (95% CI: 52.8% to 91.5%), and 78.8% (95% CI: 52.8% to 91.5%) for the non-AGAs+NP SCC group.
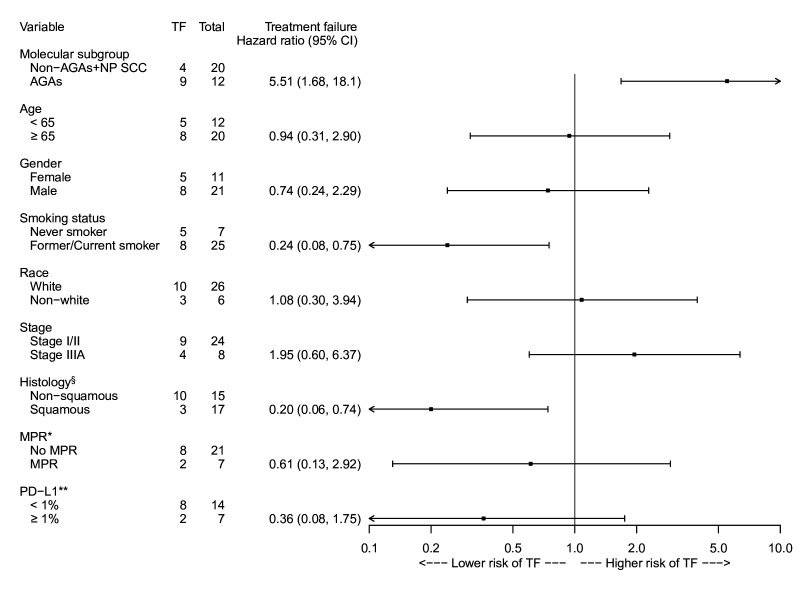
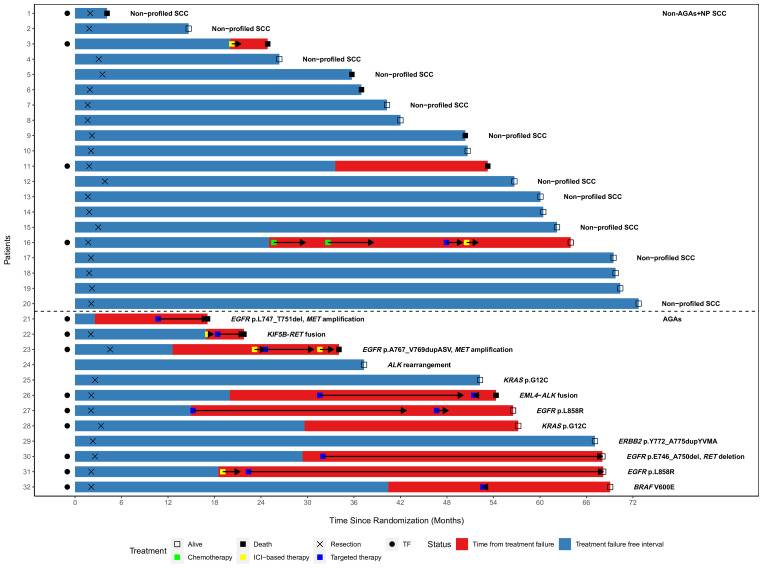
The risk for TF was also assessed for association with molecular subgroup, age, sex, smoking status, race, clinical stage, tumor histology, MPR status (for resected patients on-trial), and pretreatment tumor PD-L1 expression status (figure 4). The risk for TF was significantly higher for AGAs (HR: 5.51, 95% CI: 1.68 to 18.1), and lower in former/current smokers than never-smokers (HR: 0.24, 95% CI: 0.08 to 0.75). 80% (4/5) of never-smokers with TF also had AGAs (2 EGFR, 1 EML4-ALK fusion, and 1 KIF5B-RET fusion). Patients with squamous histology also had a lower risk of TF compared with those with non-squamous histology (HR: 0.20, 95% CI: 0.06 to 0.74). A multivariable Cox PH regression model was performed. However, since collinearity existed in histology and molecular subgroup, only smoking status and molecular subgroup were considered in the model. The AGAs had a higher risk for TF than the non-AGAs+NP SCC (HR: 4.35, 95% CI: 1.24 to 15.2) while the smoking status was no longer associated with TF (HR: 0.41, 95% CI: 0.12 to 1.37). The median time to first systemic retreatment was 20.2 months (range: 10.7–32.0) after randomization. The treatment course over time for non-AGAs+NP SCC (n=20, patients 1–20) and AGAs (n=12, patients 21–32) patients is displayed in figure 5. The specific genomic alterations for the available patients are detailed in online supplemental table 1).
Figure 4. Forest plot showing the treatment failure HR for each variable. The HR from the univariable Cox proportional hazard regression estimates the risk of TF in the interest group relative to the risk in the reference group. Treatment failure was defined as any progression of primary lung cancer in patients without surgery, radiographic and/or biopsy-proven lung cancer recurrence after surgery, or death from possibly treatment-related complications or from primary lung cancer, since randomization. * Data collected at time of surgery in resected tumors in patients who underwent surgery on trial (n=28), three patients in AGAs and one patient in non-AGAs+NP SCC did not undergo surgery; time from surgery until treatment failure was used. ** Pretreatment tumor PD-L1 expression status (n=21) on available samples by clone 28-8, Abcam3 23; the PD-L1 expression status of three patients in AGAs and eight patients in non-AGAs+NP SCC were unavailable. § Tumor histology at baseline. AGAs, patients with tumors harboring an actionable genomic alteration; MPR, major pathologic response (≤ 10% residual viable tumor in the resected tumor specimen); non-AGAs+NP SCC, patients with molecular profiling without AGAs or non-profiled squamous cell carcinomas; PD-L1, programmed death-ligand 1; TF, treatment failure; HR: hazard ratio.
Figure 5. Swimmer plot showing duration of time free from treatment failure (blue) and time since treatment failure (red) in patients with tumors without an actionable genomic alteration (AGAs) or a non-profiled squamous cell carcinomas (non-AGAs+NP SCC, patients 1–20) and patients with tumor harboring an AGA (AGAs, patients 21–32). Chemotherapy, ICI-based therapy, and targeted therapy were started after treatment failure (TF). Non-profiled adenocarcinomas (n=12) are not included. Type of treatments each patient received are displayed chronologically based on time since randomization. Patient 23 had surgery off-trial. Patient 24 did not have disease progression after neoadjuvant therapy, was offered curative-intent surgery but declined. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor.
A sensitivity analysis was performed by assigning the two patients with KRAS G12C to the non-AGAs+NP SCC group. The odds of achieving MPR in the non-AGAs+NP SCC + KRAS G12C group were 3.38 (95% CI: 0.35 to 32.6) times higher than in the AGAs group (online supplemental figure 1A). The AGAs group had a higher median percentage of residual viable tumor than the non-AGAs+NP SCC + KRAS G12C group (61.0% vs 34.5%) (online supplemental figure 1A), AGAs had a median time to TF of 19.2 months (95% CI: 2.57 to 40.4), and was not reached (95% CI: NE–NE) in non-AGAs+NP SCC + KRAS G12C group (online supplemental figure 1B). The TF risk in the AGAs group was 5.96 (95% CI: 1.92 to 18.5) times higher than the non-AGAs+NP SCC + KRAS G12C group.
Retreatment with ICI-based therapy
Among the 13 patients with TF and retreatment, 4 were retreated with ICI-based therapy, and 1 patient had disease control. This patient experienced disease recurrence 18.5 months after randomization and was treated with a chemoimmunotherapy regimen with stable disease for 2.1 months. Tumor profiling revealed TP53 and EGFR L858R Exon 21 mutations while on ICI-based therapy and the treatment was subsequently changed to osimertinib with disease control persisting 38.5 months after the first disease recurrence. The remaining three patients treated with ICI-based therapy did not achieve disease control. One patient recurred at 12.6 months and was treated with chemoimmunotherapy without response, while molecular profiling was pending. Molecular testing showed EGFR Exon 20 duplication with MET amplification and this patient was subsequently treated with targeted therapy with disease control for 6.20 months before radiographic disease progression. At the time of disease progression, treatment was changed to immunotherapy, but the patient died from lung cancer-related complications 2.47 months later. Another patient underwent definitive chemoradiation after neoadjuvant therapy (no surgery) and later experienced locoregional disease progression. Molecular profiling failed to reveal any AGAs and the patient started single-agent immunotherapy, but unfortunately died 4.80 months after treatment from cancer-related complications. Finally, one patient with recurrence 17.0 months after randomization was treated with one cycle of chemoimmunotherapy without response. The molecular profiling revealed a KIF5B-RET fusion, and the patient was enrolled in a RET TKI trial. However, the patient died from disease-related complications 4.67 months after retreatment initiation.
Treatment with targeted therapy alone
In addition to the patients treated with ICI-based therapy and targeted therapy described above, four additional patients were treated with targeted therapy alone. One patient with EML4-ALK fusion had metastatic recurrence at 20.0 months after randomization and was treated with targeted therapy with disease control at 18.5 months until disease progression. One patient with EGFR Exon 19 deletion was treated with targeted therapy with disease control at 22.8 months with ongoing treatment. One patient developed recurrence at 15 months after randomization and had EGFR L858R Exon 21 mutation, the patient was treated with targeted therapy with ongoing disease control at 27.5 months. Finally, one patient with EGFR Exon 19 deletion and MET amplification, had disease progression after neoadjuvant treatment, and was treated with definitive chemoradiation (no surgery). The disease eventually metastasized, and the patient was treated with targeted therapy, but ultimately died from complications secondary to lung cancer 6.37 months after targeted therapy initiation. In total, disease control was achieved with targeted therapy in 71.4% (5/7) of patients with TF who received targeted therapy alone or targeted therapy given in sequence with other systemic therapies.
KRAS and STK11 co-mutations
Among two patients with tumors harboring KRAS G12C mutations, one with STK11 co-mutations had locoregional recurrence 29.6 months after randomization and was treated with stereotactic body radiation therapy and the patient has had disease control for 14.4 months. The other patient with a tumor harboring KRAS G12C completed neoadjuvant ICI therapy followed by surgical resection on-trial and showed no evidence of disease recurrence at 52.3 months.
There were three additional patients who had tumors harboring an STK11 mutation. One patient with STK11 and KRAS Q61H mutations experienced disease recurrence 25.1 months after randomization. The patient was treated with chemotherapy regimens with disease control for 4.03 and 5.87 months but eventually experienced disease progression. The patient was then treated with a PARP inhibitor due to BRCA2 mutation for 2.03 months, but the disease progressed and chemoimmunotherapy plus bevacizumab was started with the completion of 2 cycles with stable disease at the time of data cut-off. Two other patients with STK11 mutation alone did not experience disease recurrence.
Discussion
In this study, we retrospectively evaluated the impact of select tumor molecular alterations on TF after neoadjuvant ICIs in patients with resectable NSCLC treated in a randomized phase 2 study. We found that patients with tumors harboring select AGAs had a greater median percentage of residual viable tumor after neoadjuvant ICI (chemotherapy-sparing) treatment when compared with those patients without AGAs in their disease. Patients in the AGAs group had a shorter median time to TF compared with those without these alterations. In aggregate, our results underscore the critical importance of tumor molecular profiling in patients with resectable NSCLC to guide the treatment decision-making process in order to maximize clinical outcomes.
Our findings suggest that neoadjuvant ICIs alone may not represent the most effective treatment approach for all patients. Most of the TFs were driven by patients with actionable tumor alterations including EGFR, RET, KRAS G12C, and ALK, among others, highlighting the need for pre-neoadjuvant therapy tumor molecular testing and appropriate targeted therapy-based approaches for select AGAs (including non-EGFR AGAs). Indeed, our study reveals that patients who experienced disease recurrence and were treated with appropriate targeted therapy achieved durable treatment response. In previous experiences with advanced and metastatic NSCLC, tumors harboring select driver alterations, including EGFR mutations and ALK fusions exhibited poor response to immunotherapy.24,26
The appropriate use of therapies at the right treatment stage can provide durable benefits to patients. The field has been rapidly changing, with evidence that targeted therapy should be incorporated into the treatment paradigm in appropriate patients. For patients with EGFR-mutant resected NSCLC, the ADAURA trial9 10 demonstrated improved disease-free survival (DFS) and overall survival (OS) benefit with adjuvant osimertinib in stage IB–IIIA patients when compared with placebo. The ALINA trial11 compared adjuvant alectinib to chemotherapy for stage IB–IIIA ALK+ NSCLC and demonstrated DFS benefit in both stage II–IIIA and intention-to-treat stage IB–IIIA groups, highlighting the benefit derived by tailoring targeted therapies to matched AGAs in the early-stage setting.
However, the effects of ICI therapy on patients with AGAs have been inconsistent in select trials in the early-stage setting. For example, the subgroup analysis of EGFR mutants from the AEGEAN trial evaluating perioperative durvalumab added to neoadjuvant chemotherapy versus chemotherapy plus placebo did not demonstrate event-free survival (EFS) benefit at 12 or 24 months for EGFR mutant population, and with a 3.8% pCR rate compared with 17.2% in the overall population.7 On the other hand, subgroup analysis in the IMpower010 study27 suggested a potential DFS benefit of adjuvant atezolizumab in EGFR-positive patients with PD-L1≥1%. EGFR and ALK-positive patients in KEYNOTE-671 also showed EFS benefit with perioperative pembrolizumab,6 however, it is worth noting that these observations were derived from a remarkably small sample size and should be interpreted with caution.
Furthermore, immunotherapy has been shown to improve OS in KRAS-mutants compared with chemotherapy,28 however, STK11 co-mutation in KRAS-mutants has been associated with resistance to immunotherapy.29 30 Due to the small sample size, our study does not allow for meaningful conclusions about the impact of KRAS co-mutations on the efficacy of neoadjuvant immunotherapy. Nonetheless, the present study extends the potential limited benefit from immunotherapy alone in patients harboring select (non-KRAS) AGAs in the neoadjuvant setting for early-stage resectable NSCLC. Integrating insights from other investigations, the optimal neoadjuvant treatment strategy for AGAs-associated NSCLC warrants further comprehensive exploration.
In the neoadjuvant setting, several efforts are ongoing to evaluate the role of targeted therapy with or without chemotherapy as compared with chemotherapy only. A phase 2 trial of neoadjuvant osimertinib (NCT03433469) demonstrated a 15% MPR rate and 48% partial response rate in appropriate stage I–IIIA operable patients.31 We await the results of the ongoing phase 3 NeoADAURA trial15 (NCT04351555), which is evaluating osimertinib with or without chemotherapy versus chemotherapy in patients with resectable EGFR-mutant NSCLC. The ongoing NAUTIKA-1 (NCT04302025) uses an umbrella design to screen for AGAs (ALK, ROS1, NTRK, BRAF, RET) and allocates patients to the appropriate neoadjuvant therapy trials prior to surgery.14 16 This will provide further evidence for appropriate treatment strategy based on tumor molecular profiles. As the field advances, the timing and role of immunotherapy and targeted therapy with or without chemotherapy in the perioperative setting will need to be further defined.
Comprehensive tumor molecular testing can provide vital information for the treatment of NSCLC. Some guidelines suggest screening for select molecular markers (EGFR, ALK, ROS1, BRAF, MET, RET, ERBB2, KRAS) and evaluation of PD-L1 expression.32 Long turnaround time and lack of adequate tissue can pose logistical barriers, but despite these challenges, tumor molecular profiling should be performed, whenever feasible. This testing allows providers to tailor appropriate therapy to tumors harboring molecular alterations for which targeted therapy has shown efficacy and is the standard of care, and to track progression of tumor biology for changes in driver alterations and development of therapeutic resistance.
Our study has several limitations. We recognize that not all patients underwent tumor molecular profiling, which was due to the era of the trial initiation and rapidly evolving field and knowledge of resectable lung cancer. Notwithstanding, every effort was made to profile patient tumors when appropriate and feasible. Also, the NP SCCs were used in the comparison group since targetable genomic alterations are less common in squamous histology, though it is possible that these tumors also contained targetable alterations in the context of standard of care. Finally, it is important to note that this study was performed based on a single-center experience and is limited to a small sample size. Therefore, further validation in larger cohorts is warranted to strengthen the reliability and generalizability of our results.
In conclusion, for patients presenting with resectable NSCLC and treated with neoadjuvant immunotherapy followed by surgery, tumors harboring AGAs portend a shorter median time to TF. This suggests that neoadjuvant immunotherapy alone may not be the most appropriate therapeutic option for select patients with targeted therapy-sensitive tumor alterations. The decision-making process leading to an evidence-based neoadjuvant treatment strategy should take several factors in consideration including the knowledge of molecular profiling of the otherwise operable NSCLC.
supplementary material
Acknowledgements
We thank the patients and their families for participating in this study. We thank all the members of our regulatory, clinical, data coordination and translational research teams in the Departments of Thoracic/Head and Neck Medical Oncology, and Thoracic Surgery at the MD Anderson Cancer Center for their support on this study. We thank the members of the Translational Molecular Pathology Department involved in the correlative analyses of this study and members of the strategic alliance teams at Bristol Myers Squibb and the MD Anderson Cancer Center for their support.
Footnotes
Funding: Funding support for the original clinical trial was provided by Bristol Myers Squibb. Support for the current study was also partially provided by the National Institutes of Health (NIH)/National Cancer Institute (NCI) through the University of Texas Lung Specialized Program of Research Excellence SPORE grant 5P50CA070907, the NIH/NCI P30 CA016672 Cancer Center Support Grant (to the Biostatistics Resource Group), the NIH/NCI grant R01CA262425, the NIH/NCI grant U01CA264583, the Conquer Cancer Foundation of the American Society of Clinical Oncology Career Development Award 2018. TC and HK are Andrew Sabin Family Foundation Fellows of the University of Texas MD Anderson Cancer Center. The study was also partially supported by the generous philanthropic contributions to the University of Texas MD Anderson Cancer Center Lung Cancer Moon Shot Program, the Physician Scientist Program, the 4 Khalifa Scholar Award from the Khalifa Bin Zayed Al Nahyan Foundation, the Rexanna’s Foundation for Fighting Lung Cancer and the Bob Mayberry Foundation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and was approved by MD Anderson Cancer Center Institutional Review Board 2016-0982 NEOSTAR clinical study protocol, and LAB10-0288, analysis protocol. Participants gave informed consent to participate in the study before taking part.
Contributor Information
Nicolas Zhou, Email: NZhou@mdanderson.org.
Cheuk H Leung, Email: CLeung@mdanderson.org.
William N William Jr., Email: william.william@medicos.oncoclinicas.com.
Annikka Weissferdt, Email: aweissferdt@mdanderson.org.
Apar Pataer, Email: apataer@mdanderson.org.
Myrna C B Godoy, Email: mgodoy@mdanderson.org.
Brett W Carter, Email: bcarter2@mdanderson.org.
Frank V Fossella, Email: ffossell@mdanderson.org.
Anne S Tsao, Email: astsao@mdanderson.org.
George R Blumenschein, Email: gblumens@mdanderson.org.
Xiuning Le, Email: XLe1@mdanderson.org.
Jianjun Zhang, Email: jzhang20@mdanderson.org.
Ferdinandos Skoulidis, Email: FSkoulidis@mdanderson.org.
Jonathan M Kurie, Email: jkurie@mdanderson.org.
Mehmet Altan, Email: maltan@mdanderson.org.
Charles Lu, Email: clu@mdanderson.org.
Bonnie S Glisson, Email: bglisson@mdanderson.org.
Lauren A Byers, Email: lbyers@mdanderson.org.
Yasir Y Elamin, Email: yyelamin@mdanderson.org.
Reza J Mehran, Email: rjmehran@mdanderson.org.
David C Rice, Email: drice@mdanderson.org.
Garrett L Walsh, Email: gwalsh@mdanderson.org.
Wayne L Hofstetter, Email: WHofstetter@mdanderson.org.
Jack A Roth, Email: jroth@mdanderson.org.
Hai T Tran, Email: htran@mdanderson.org.
Jia Wu, Email: JWu11@mdanderson.org.
Luisa M Solis Soto, Email: lmsolis@mdanderson.org.
Humam Kadara, Email: HKadara@mdanderson.org.
Stephen G Swisher, Email: SSwisher@MDAnderson.org.
Ara A Vaporciyan, Email: avaporci@MDAnderson.org.
Don L Gibbons, Email: dlgibbon@mdanderson.org.
Heather Y Lin, Email: hyanlin@mdanderson.org.
J Jack Lee, Email: jjlee@mdanderson.org.
John V Heymach, Email: JHeymach@MDAnderson.org.
Marcelo V Negrao, Email: mvnegrao@mdanderson.org.
Boris Sepesi, Email: boris.sepesi@hcahealthcare.com.
Tina Cascone, Email: TCascone@MDAnderson.org.
Data availability statement
Data are available upon reasonable request.
References
- 1.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976–86. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15:816–26. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Cascone T, William WN, Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27:504–14. doi: 10.1038/s41591-020-01224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad MM, Forde PM, Girard N, et al. 1261O Neoadjuvant nivolumab (N) + ipilimumab (I) vs chemotherapy (C) in the phase III CheckMate 816 trial. Ann Oncol. 2023;34 doi: 10.1016/j.annonc.2023.09.739. [DOI] [Google Scholar]
- 5.Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022;386:1973–85. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:491–503. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med. 2023;389:1672–84. doi: 10.1056/NEJMoa2304875. [DOI] [PubMed] [Google Scholar]
- 8.Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18:547–57. doi: 10.1038/s41571-021-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y-L, Tsuboi M, He J, et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N Engl J Med. 2020;383:1711–23. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med. 2023;389:137–47. doi: 10.1056/NEJMoa2304594. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y-L, Dziadziuszko R, Ahn JS, et al. Alectinib in Resected ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2024;390:1265–76. doi: 10.1056/NEJMoa2310532. [DOI] [PubMed] [Google Scholar]
- 12.Cascone T, Awad MM, Spicer JD, et al. Perioperative Nivolumab in Resectable Lung Cancer. N Engl J Med. 2024;390:1756–69. doi: 10.1056/NEJMoa2311926. [DOI] [PubMed] [Google Scholar]
- 13.Lu S, Zhang W, Wu L, et al. Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non-Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial. JAMA. 2024;331:201–11. doi: 10.1001/jama.2023.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Sepesi B, Toloza EM, et al. EP02.04-005 Phase II NAUTIKA1 Study of Targeted Therapies in Stage II-III NSCLC: Preliminary Data of Neoadjuvant Alectinib for ALK+ NSCLC. J Thorac Oncol. 2022;17:S233–4. doi: 10.1016/j.jtho.2022.07.390. [DOI] [Google Scholar]
- 15.Tsuboi M, Weder W, Escriu C, et al. P03.02 Neoadjuvant Osimertinib with/without Chemotherapy vs Chemotherapy for EGFR Mutated Resectable NSCLC: NeoADAURA. J Thorac Oncol. 2021;16 doi: 10.1016/j.jtho.2021.01.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sepesi B, Jones DR, Meyers BF, et al. LCMC LEADER neoadjuvant screening trial: LCMC4 evaluation of actionable drivers in early-stage lung cancers. J Clin Oncol. 2022;40:TPS8596. doi: 10.1200/JCO.2022.40.16_suppl.TPS8596. [DOI] [Google Scholar]
- 17.Pataer A, Kalhor N, Correa AM, et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. J Thorac Oncol. 2012;7:825–32. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sepesi B, Zhou N, William WN, Jr, et al. Surgical outcomes after neoadjuvant nivolumab or nivolumab with ipilimumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2022;164:1327–37. doi: 10.1016/j.jtcvs.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascone T, Leung CH, Weissferdt A, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med. 2023;29:593–604. doi: 10.1038/s41591-022-02189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong L, Aminu M, Li S, et al. Efficacy and clinicogenomic correlates of response to immune checkpoint inhibitors alone or with chemotherapy in non-small cell lung cancer. Nat Commun. 2023;14:695. doi: 10.1038/s41467-023-36328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam VK, Zhang J, Wu CC, et al. Genotype-Specific Differences in Circulating Tumor DNA Levels in Advanced NSCLC. J Thorac Oncol. 2021;16:601–9. doi: 10.1016/j.jtho.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra ER, Villalobos P, Mino B, et al. Comparison of Different Antibody Clones for Immunohistochemistry Detection of Programmed Cell Death Ligand 1 (PD-L1) on Non-Small Cell Lung Carcinoma. Appl Immunohistochem Mol Morphol. 2018;26:83–93. doi: 10.1097/PAI.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol. 2018;13:1302–11. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negrao MV, Skoulidis F, Montesion M, et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer. 2021;9:e002891. doi: 10.1136/jitc-2021-002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–8. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–57. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 28.Mok TSK, Lopes G, Cho BC, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann Oncol. 2023;34:377–88. doi: 10.1016/j.annonc.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Ricciuti B, Arbour KC, Lin JJ, et al. Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status. J Thorac Oncol. 2022;17:399–410. doi: 10.1016/j.jtho.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoulidis F, Goldberg ME, Greenawalt DM, et al. Stk11/Lkb1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8:822–35. doi: 10.1200/JCO.2017.35.15_suppl.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aredo JV, Urisman A, Gubens MA, et al. Phase II trial of neoadjuvant osimertinib for surgically resectable EGFR -mutated non-small cell lung cancer. J Clin Oncol. 2023;41:8508. doi: 10.1200/JCO.2023.41.16_suppl.8508. [DOI] [Google Scholar]
- 32.Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13:323–58. doi: 10.1016/j.jtho.2017.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.