Abstract
Abstract
Objective
Prior studies demonstrate that some untoward clinical outcomes vary by outdoor temperature. This is true of some endpoints common among persons with diabetes, a population vulnerable to climate change-associated health risks. Yet, prior work has been agnostic to the antidiabetes drugs taken by such persons. We examined whether relationships between ambient temperature and adverse health outcomes among persons with type 2 diabetes (T2D) varied by exposure to different antidiabetes drugs.
Design
Retrospective cohort.
Setting
Healthcare and meteorological data from five US states, 1999–2010.
Participants
US Medicaid beneficiaries with T2D categorised by use of antidiabetes drugs.
Exposure
Maximum daily ambient temperature (t-max).
Outcomes
Hospital presentation for serious hypoglycaemia, diabetic ketoacidosis (DKA) or sudden cardiac arrest (examined separately).
Methods
We linked US Medicaid to US Department of Commerce data that permitted us to follow individuals longitudinally and examine health plan enrolment, healthcare claims, and meteorological exposures—all at the person-day level. We mapped daily temperature from weather stations to Zone Improvement Plan (ZIP) codes, then assigned a t-max to each person-day based on the residential ZIP code. Among prespecified subcohorts of users of different pharmacologic classes of antidiabetes drugs, we calculated age and sex-adjusted occurrence rates for each outcome by t-max stratum. We used modified Poisson regression to assess relationships between linear and quadratic t-max terms and each outcome. We examined effect modification between t-max and a covariable for current exposure to a specific antidiabetes drug and assessed significance via Wald tests.
Results
We identified ∼3 million persons with T2D among whom 713 464 used sulfonylureas (SUs), dipeptidyl peptidase-4 inhibitors (DPP-4is), meglitinides, or glucagon-like peptide 1 receptor agonists (GLP1RAs). We identified a positive linear association between t-max and serious hypoglycaemia among non-insulin users of glimepiride and of glyburide but not glipizide (Wald p value for interaction among SUs=0.048). We identified an inverse linear association between t-max and DKA among users of the DPP-4i sitagliptin (p=0.016) but not the GLP1RA exenatide (p=0.080). We did not identify associations between t-max and sudden cardiac arrest among users of SUs, meglitinides, exenatide, or DPP-4is.
Conclusions
We identified some antidiabetes drug class-specific and agent-specific differences in the relationship between ambient temperature and untoward glycaemic but not arrhythmogenic, safety outcomes.
Keywords: diabetes & endocrinology, epidemiology, climate change, public health
Strengths and limitations of this study.
Strength: Links meteorological data to healthcare claims to answer a novel research question at the intersection of environmental epidemiology and pharmacoepidemiology.
Strength: Uses validated diagnosis-based algorithms for cohort building and for identifying health outcomes of interest.
Limitation: Relies on somewhat older data sets precluding the study of newer-to-market antidiabetes drugs and more contemporaneous extreme ambient temperature patterns.
Limitation: Uses residential Zone Improvement Plan codes as a proxy for individual exposure to ambient temperature.
Limitation: Potential residual confounding attributable to a lack of data on individual access to and use of heating, ventilation, and air conditioning as examples.
Introduction
The high and increasing prevalence of type 2 diabetes (T2D) and the untoward effects of anthropogenic climate change on health are both major global public health concerns. While these concerns have not been historically linked, recent reports1 2 suggest interconnections between diabetes and climate change given their shared global vectors (eg, urbanisation).3 Further, it is well understood that vulnerable populations4—such as minority and low-income communities—are disproportionately affected both by diabetes and climate change. Given this, there are numerous important areas of inquiry at the intersection of diabetes and climate change (and health disparities) including investigations of new-onset diabetes, morbidity in diabetes, food quality and security, and physical activity.1 3 A major imperative of the International Diabetes Federation1 includes investigations of the effects of ambient temperatures on health among persons with diabetes. While we5 and others6,8 have found such relationships, prior work has largely been agnostic to the antidiabetes drugs taken by study populations. This leaves an important knowledge gap because safety endpoints common in users of certain antidiabetes drugs may be affected by temperature. We therefore sought to examine whether relationships between ambient temperature and untoward health effects in vulnerable Americans with T2D differed by antidiabetes drug regimens. Our intent was to generate initial evidence about whether incorporating patient-experienced ambient temperatures might inform the personalisation of diabetes treatment to maximise patient safety.
Research design and methods
Overview and data sources
We conducted a retrospective cohort study using 1999–2010 US Medicaid enrolment and healthcare data from five states (online supplemental figure 1) linked to meteorological data from the US Department of Commerce National Oceanic and Atmospheric Administration (NOAA). Medicaid is a joint state-federal programme that provides health coverage to low-income Americans. This public programme is administered separately by states with eligibility rules that differ within the structure of federal guidelines.9 The programme covers approximately 20% of all Americans including many with complex and costly needs for care.10 It is one of the largest payers for healthcare in the US.11 NOAA is a US federal agency whose mission is to understand our natural world and help protect its resources including by monitoring global weather and climate.12 13 These linked data sources constituted a data set that permitted us to follow individuals longitudinally—examining health plan enrolment, healthcare claims and meteorological exposures, all at the person-day level.
Cohort construction
Our cohort consisted of persons with T2D having complete and valid information on sex and date of birth; by virtue of the data linkage, all subjects had valid Zone Improvement Plan (ZIP) codes of residence. T2D was determined by the presence of at least one any-claim type (eg, outpatient, emergency department, inpatient), any-position International Classification of Diseases Ninth Revision Clinical Modification (ICD-9-CM) diagnosis of 250.X0 or 250.X2 (indicative of T2D) but absence of an any-claim type, any-position ICD-9-CM diagnosis of 250.X1 or 250.X3 (indicative of type 1 diabetes)—where ‘Xs’ represented wildcards. Validation work by Klompas et al using a US electronic health record data set found this approach to have a positive predictive value (PPV)=91% (84–96%) and sensitivity=90% (83–96%).14 Observation time began on each person’s first observed T2D diagnosis and was censored on the earliest occurrence among disenrolment from Medicaid or death. Each person-day of observation was characterised by the exposure of interest, a stratification variable and outcome occurrence.
Exposure
The exposure of interest was the day-level maximum ambient temperature (t-max) at the persons’ ZIP code of residence (a surrogate for their geographical location on each observation day). Meteorological data from NOAA provided weather parameters (measured at weather stations) and station locations. For each individual, we linked their ZIP code of residence as ascertained from the Medicaid enrolment file to the population-weighted centroid of that ZIP code as estimated using ZIP code boundaries, census block group boundaries, and 2010 census block group-level population data. The daily t-max for each population-weighted ZIP code centroid was estimated from daily meteorological data, locations of weather stations, and spline interpolation. The use of spline interpolation to estimate properties (such as temperature) at unsampled sites based on data from sampled sites may enable more precise estimation than a simple averaging method.15,17 Additional detail on our approach is described in a prior issue of this journal.18
Stratification variable
We used time-varying exposure versus non-exposure to a specific antidiabetes drug within each pharmacologic drug class of interest using days’ supply values on day-level pharmacy dispensing claims (not permitting grace periods).19 This enabled us to examine relationships between t-max exposure (see above) and outcome (see below) stratified by the antidiabetes drug regimen in use.
We selected antidiabetes drugs within pharmacologic classes that were commonly used during the study period20 and had putative associations with one or more of the outcomes of interest. Therefore, we studied the following second-generation sulfonylureas (SUs) in analyses of serious hypoglycaemia21 22 and of sudden cardiac arrest:23,26 glyburide, glipizide and glimepiride. We studied the following meglitinides in analyses of sudden cardiac arrest:27 nateglinide and repaglinide. We studied the following dipeptidyl peptidase-4 inhibitors (DPP-4is) in analyses of diabetic ketoacidosis (DKA)28 and of sudden cardiac arrest:29 saxagliptin and sitagliptin. Linagliptin, for example, would also have been of interest but was not approved in the USA until 2011. We studied the following glucagon-like peptide 1 receptor agonists (GLP1RAs) in analyses of DKA30 and of sudden cardiac arrest:31 32 exenatide and liraglutide. Dulaglutide, for example, would also have been of interest but was not approved in the US until 2014. Sodium-glucose co-transporter 2 inhibitors (SGLT-2is) would also have been of interest especially for the DKA endpoint but the first agent in this class (canagliflozin) was not approved in the US until 2013. We did not prioritise the study of insulins since global estimates suggest that 7–16% of persons with T2D in 2030 will use insulin;33 as this still represents 2.6–5.9 million Americans, an examination of insulin is likely warranted in future work. That said, in analyses examining serious hypoglycaemia, we conducted prespecified sensitivity analyses in which we censored observation time when an individual began insulin therapy.
Outcomes
Outcomes of interest, each examined separately, were hospital presentation for: serious hypoglycaemia; DKA; and sudden cardiac arrest (consisting of sudden cardiac arrest or ventricular arrhythmia but termed sudden cardiac arrest for simplicity). We selected these endpoints because they are clinically relevant, major barriers to optimal diabetes care, commonly drug-induced, independently affected by ambient temperature, and of major interest to stakeholders.
Serious hypoglycaemia was defined by one of the following ICD-9-CM discharge diagnosis codes in any position on an emergency department claim or the principal position on an inpatient claim: (1) 251.0 (hypoglycaemic coma); (2) 251.1 (other specific hypoglycaemia); (3) 251.2 (hypoglycaemia, unspecified); or (4) 250.8X (diabetes with other specified manifestations), as long as not co-occurring with ≥1 exclusionary diagnosis suggesting manifestations other than hypoglycaemia (see detail in Leonard et al34). This algorithm has a PPV=89% for the emergency department component35 and 78% for the inpatient component.36 Performance measure values were derived from validation studies within the US Emergency Medicine Network and US Centers for Medicare and Medicaid Services data, respectively, using medical records as the gold standard.
DKA was defined by one of the following ICD-9-CM discharge diagnosis codes in any position on an inpatient claim: (1) 250.1 (diabetes with ketoacidosis); (2) 250.10 (diabetes mellitus (DM) with ketoacidosis, type 2 or unspecified type); (3) 250.11 (DM with ketoacidosis, type 1, not stated as uncontrolled); (4) 250.12 (DM with ketoacidosis, type 2 or unspecified type, uncontrolled); or (5) 250.13 (DM with ketoacidosis, type 1, uncontrolled). This algorithm has a PPV=89% (72–96%), although this US Medicaid-based validation study was limited to children and young adults with T2D.37 We decided to include components (3) and (5) of the DKA definition above since our study population was already limited to a population with T2D. We decided against an alternative method that restricts the DKA definition to principal diagnoses28 because such an approach has not been validated.
Sudden cardiac arrest was defined by one of the following ICD-9-CM discharge diagnosis codes in a first-listed position on an emergency department claim or principal position on an inpatient claim: (1) paroxysmal tachycardia (427.1); (2) ventricular fibrillation and/or flutter (427.4, 427.41 or 427.42); (3) cardiac arrest (427.5); (4) sudden death (798); (5) instantaneous death (798.1); or (6) death occurring in <24 hours from symptom onset not otherwise explained (798.2). This algorithm, with a PPV=85%,38 has been used by the US Food and Drug Administration’s Sentinel Initiative.
Analysis
Separately for each pharmacologic drug class (and drugs within classes), we calculated sex-adjusted and baseline age-adjusted occurrence rates for each outcome by prespecified categories of same-day t-max in degrees Celsius (°C) from which we then generated trendlines with 95% confidence bands via linear regression. We examined the statistical significance of beta coefficients for exposure (ie, t-max) terms from modified Poisson models (ie, generalised estimating equation Poisson using first-order autoregressive correlations structures). Put simply, such a model is a statistical method for analysing count data in the presence of correlation whereby correlation is assumed to be highest between adjacent time points yet decreases as the time horizon increases. All models included linear terms for t-max. We also included quadratic t-max terms if all antidiabetes drug-specific beta coefficients had p values of <0.05. Furthermore, models accounted for sex and baseline age as potential confounders but not for time-varying patient factors. Within each pharmacologic drug class, we performed a Wald test for interaction between the terms for ambient temperature and individual antidiabetes drug. Put simply, this test examines if the effect of one variable on an outcome differs by the level of a second variable. Analyses were conducted using Stata/MP V.17 and SAS V.9.4. This research was approved by the University of Pennsylvania’s institutional review board.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
We identified 2 955 110 persons with T2D followed for a median of 2.3 years. Among these persons, we identified 713 464 users of SUs, DPP-4is, meglitinides and/or GLP1RAs (see online supplemental table 1, Panel A); we included 697 678 (98%) with complete data on sex and date of birth. These persons contributed up to 1 120 522, 90 284, 43 564 and 11 279 person-years of observation, respectively. Individuals were predominantly white (35%), non-Hispanic/non-Latino (77%), women (59%) and California residents (43%) with a median (1st, 25th, 75th, 99th percentile) age of 60.7 (24.9, 48.8, 71.5, 92.3) years and follow-up time of 0.9 (0.01, 0.26, 2.41, 10.0) years. The median (1st, 25th, 75th, 99th percentile) t-max was 22.2°C (−3.6°C, 15.3°C, 28.0°C, 37.7°C). See additional baseline demographics in online supplemental table 1, Panel B.
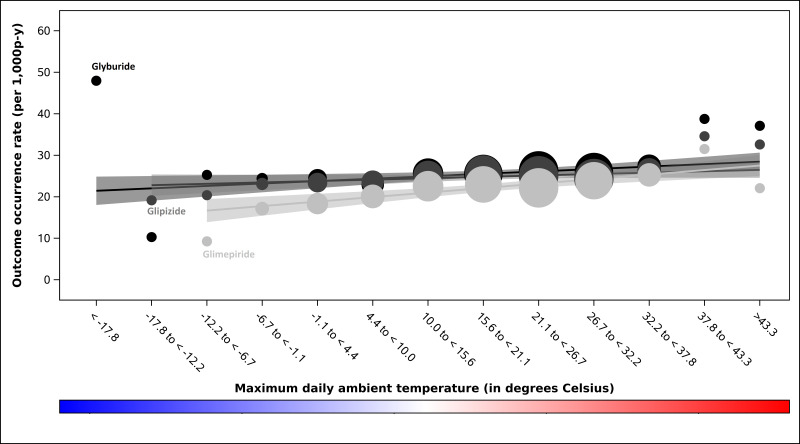
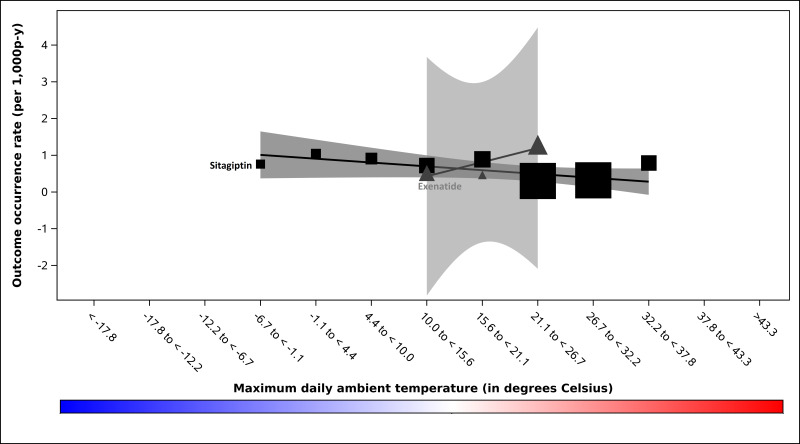
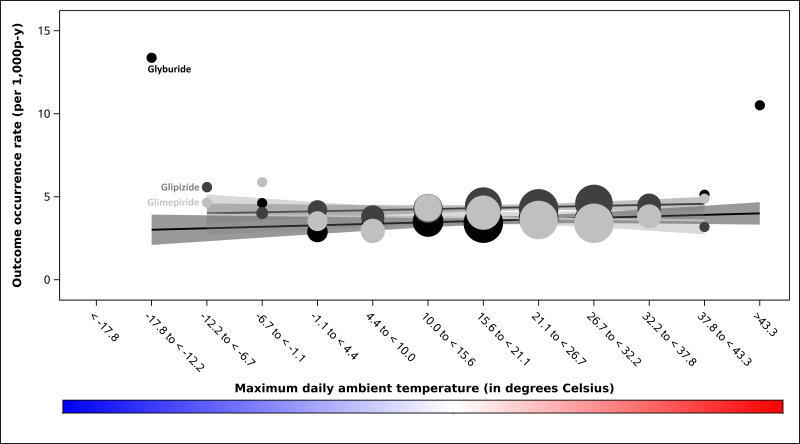
Outcome occurrence rates are presented in table 1 and online supplemental table 2. All modified Poisson models included linear t-max terms but none met the prespecified criterion for the inclusion of quadratic t-max terms. We identified a positive linear association between t-max and serious hypoglycaemia occurrence among users of glimepiride (p<0.001) and of glyburide (p<0.001) but not glipizide (p=0.300; Wald p value for interaction among SUs=0.054); see figure 1. In the preplanned sensitivity analysis in which SU observation time was censored on insulin initiation, this interaction was statistically significant (Wald p value for interaction among SUs=0.048). We identified an inverse linear association between t-max and DKA rate among users of the DPP-4i sitagliptin (p=0.016) but not the GLP1RA exenatide (p=0.080); see figure 2. We did not identify differential associations between t-max and sudden cardiac arrest rate among users of SUs (Wald p value for interaction among SUs=0.141; figure 3), meglitinides (Wald p value for interaction among meglitinides=0.410, online supplemental figure 2) or DPP-4is (Wald p value for interaction among DPP-4is=0.438, see online supplemental figure 3). We also did not identify an association between t-max and sudden cardiac arrest rate for the GLP1RA exenatide (linear temperature term, p=0.074, see online supplemental figure 4.
Table 1. Outcome counts and occurrence rates, by categories of maximum ambient temperature exposure, by antidiabetes drugs forming the subcohorts of interest.
| Serious hypoglycaemia* | Diabetic ketoacidosis | Sudden cardiac arrest/ventricular arrhythmia | |||||
| Antidiabetes drug class | tmax category, in degrees C | Outcomes, n | Rate†, per 1000 p-y | Outcomes, n | Rate†, per 1000 p-y | Outcomes, n | Rate†, per 1000 p-y |
| Sulfonylureas | <−17.8 | ‡ | 20.6 | NA | – | – | |
| −17.8 to <−12.2 | ‡ | 13.9 | ‡ | 7.8 | |||
| −12.2 to <−6.7 | 78 | 20.8 | 20 | 5.4 | |||
| −6.7 to <−1.1 | 460 | 22.6 | 91 | 4.5 | |||
| −1.1 to <4.4 | 1321 | 23.0 | 197 | 3.4 | |||
| 4.4 to <10.0 | 1752 | 22.8 | 270 | 3.4 | |||
| 10.0 to <15.6 | 3947 | 25.0 | 624 | 3.8 | |||
| 15.6 to <21.1 | 6514 | 25.3 | 1004 | 3.8 | |||
| 21.1 to <26.7 | 7346 | 25.3 | 1139 | 3.8 | |||
| 26.7 to <32.2 | 7101 | 25.1 | 1101 | 3.8 | |||
| 32.2 to <37.8 | 2891 | 26.8 | 456 | 4.2 | |||
| 37.8 to <43.3 | 406 | 36.3 | 54 | 4.6 | |||
| ≥43.3 | 28 | 32.9 | ‡ | 4.7 | |||
| Overall | 31 852 | 26.8 | 4964 | 4.0 | |||
| Meglitinides | <−17.8 | NA | NA | – | – | ||
| −17.8 to <−12.2 | – | – | |||||
| −12.2 to <−6.7 | ‡ | 6.7 | |||||
| −6.7 to <−1.1 | ‡ | 8.1 | |||||
| −1.1 to <4.4 | 13 | 6.1 | |||||
| 4.4 to <10.0 | 20 | 6.7 | |||||
| 10.0 to <15.6 | 35 | 5.9 | |||||
| 15.6 to <21.1 | 62 | 6.4 | |||||
| 21.1 to <26.7 | 63 | 5.7 | |||||
| 26.7 to <32.2 | 68 | 6.7 | |||||
| 32.2 to <37.8 | 28 | 8.2 | |||||
| 37.8 to <43.3 | ‡ | 8.7 | |||||
| ≥43.3 | – | – | |||||
| Overall | 299 | 5.9 | |||||
| DPP-4is | <−17.8 | NA | – | – | – | – | |
| −17.8 to <−12.2 | – | – | – | – | |||
| −12.2 to <−6.7 | – | – | ‡ | 9.4 | |||
| −6.7 to <−1.1 | ‡ | 0.8 | ‡ | 4.3 | |||
| −1.1 to <4.4 | ‡ | 1.0 | 16 | 2.4 | |||
| 4.4 to <10.0 | ‡ | 0.9 | 19 | 2.6 | |||
| 10.0 to <15.6 | ‡ | 0.7 | 38 | 2.9 | |||
| 15.6 to <21.1 | 16 | 0.9 | 53 | 2.8 | |||
| 21.1 to <26.7 | ‡ | 0.3 | 69 | 3.0 | |||
| 26.7 to <32.2 | ‡ | 0.3 | 53 | 2.4 | |||
| 32.2 to <37.8 | ‡ | 0.8 | 29 | 3.3 | |||
| 37.8 to <43.3 | – | – | ‡ | 2.5 | |||
| ≥43.3 | – | – | NA | NA | |||
| Overall | 65 | 0.6 | 292 | 2.8 | |||
| GLP1RAs | <−17.8 | NA | – | – | – | – | |
| −17.8 to <−12.2 | – | – | – | – | |||
| −12.2 to <−6.7 | – | – | – | – | |||
| −6.7 to <−1.1 | – | – | – | – | |||
| −1.1 to <4.4 | – | – | – | – | |||
| 4.4 to <10.0 | – | – | – | – | |||
| 10.0 to <15.6 | ‡ | 0.5 | – | – | |||
| 15.6 to <21.1 | ‡ | 0.4 | ‡ | 1.0 | |||
| 21.1 to <26.7 | ‡ | 1.2 | ‡ | 0.9 | |||
| 26.7 to <32.2 | – | – | ‡ | 2.5 | |||
| 32.2 to <37.8 | – | – | ‡ | 2.3 | |||
| 37.8 to <43.3 | – | – | – | – | |||
| ≥43.3 | – | – | – | – | |||
| Overall | 13 | 0.8 | 21 | 1.4 | |||
The preplanned sensitivity analysis excluding follow-up time uponon initiation of insulin included the study of <11, <11, 73, 400, 1,162, 1,498, 3,289, 5,396, 6,156, 5,968, 2,376, 329, and 26 3 ,899, 9374 and 13 405 outcomes and had occurrence rates of 12.7, 10.0, 21.2, 21.6, 22.2, 21.3, 23.0, 23.1, 23.4, 23.5, 24.7, 33.3, and 35.2 per 1000 p-y for t-max categories <−17.8, −17.8 to <−12.2, −12.2 to <−6.7, −6.7 to <−1.1, −1.1 to <4.4, 4.4 to <10.0, 10.0 to <15.6, 15.6 to <21.1, 21.1 to <26.7, 26.7 to <32.2, 32.2 to <37.8, 37.8 to <43.3, and ≥43.3 degrees C, respectively.
Age and sex adjusted occurrence rates; obtained from generalizedgeneralised estimating equation Poisson regression models and estimated at the mean age and sex. Rates were calculated as outcomes occurring during person-days exposed to the t-max category within each antidiabetes class of interest.
Cell count <11 or would enable back-calculation of a cell <11, therefore value suppressed to comply with the US Department of Health and Human Services CMS Cell Suppression Policy (HHS-0938–2020 F-7420).
C, Celsius; DPP-4is, dipeptidyl peptidase-4 inhibitors; GLP1RAs, glucagonlike peptide one receptor agonists; NA, not applicable, that is, drug-class-outcome pair was not prespecified as ‘of interest’; p-y, person years; tmax, maximum daily ambient temperature
Figure 1. Serious hypoglycaemia occurrence rates by prespecified strata of maximum daily ambient temperature, in degrees Celsius, among second-generation sulfonylurea users enrolled in five US state Medicaid programmes. Occurrence rates (ie, outcomes among exposed person-days) for sulfonylureas are represented by circles. We scaled the size of each data point to reflect its weight using the inverse of the variance estimate. Black-to-grey tones distinguish the sulfonylureas. Black circles=glyburide. Dark grey circles=glipizide. Light grey circles=glimepiride. Corresponding confidence bands are dark grey, medium grey and light grey, respectively. P values for quadratic terms for maximum daily ambient temperature were 0.015, 0.988 and 0.341 for glyburide, glipizide and glimepiride, respectively. P values for linear terms for maximum daily ambient temperature were<0.001, 0.300 and<0.001 for glyburide, glipizide and glimepiride, respectively; Wald test for interaction among these sulfonylureas: p=0.054. P-y, person-years.
Figure 2. Diabetic ketoacidosis occurrence rates by prespecified strata of maximum daily ambient temperature, in degrees Celsius, among sitagliptin (DPP-4i) and among exenatide (GLP1RA) users enrolled in five US state Medicaid programmes. Occurrence rates (ie, outcomes among exposed person-days) for dipeptidyl peptidase-4 inhibitors are represented by squares. Occurrence rates for glucagon-like peptide 1 receptor agonists are represented by triangles. We scaled the size of each data point to reflect its weight using the inverse of the variance estimate. Black-to-grey tones distinguish the antidiabetes agents. Black squares=sitagliptin. Dark grey triangles=exenatide. Corresponding confidence bands are dark grey and medium grey, respectively. P values for quadratic terms for maximum daily ambient temperature were 0.803 and 0.294 for sitagliptin and exenatide, respectively. P values for linear terms for maximum daily ambient temperature were 0.016 and 0.080 for sitagliptin and exenatide, respectively. P-y, person-years.
Figure 3. Sudden cardiac arrest/ventricular arrhythmia occurrence rates by prespecified strata of maximum daily ambient temperature, in degrees Celsius, among second-generation sulfonylurea users enrolled in five US state Medicaid programmes. Occurrence rates (ie, outcomes among exposed person-days) for sulfonylureas are represented by circles. We scaled the size of each data point to reflect its weight using the inverse of the variance estimate. Black-to-grey tones distinguish the sulfonylureas. Black circles=glyburide. Dark grey circles=glipizide. Light grey circles=glimepiride. Corresponding confidence bands are dark grey, medium grey and light grey, respectively. P values for quadratic terms for maximum daily ambient temperature were 0.013, 0.968 and 0.973 for glyburide, glipizide and glimepiride, respectively. P values for linear terms for maximum daily ambient temperature were 0.129, 0.249 and 0.207 for glyburide, glipizide and glimepiride, respectively; Wald test for interaction among these sulfonylureas: p=0.141. P-y, person-years.
Discussion
Persons with diabetes are especially vulnerable to the health effects of extreme ambient temperatures which will be exacerbated by climate change. One approach to facilitate climate resilience for persons with diabetes includes generating evidence to underpin mitigation and/or adaptation strategies to reduce the effects of extreme ambient temperatures on personal health.2 Randomised trials to generate such evidence seem unlikely. We used real-world healthcare data of vulnerable Americans with T2D linked to day-level temperature data and identified some antidiabetes drug class-specific and agent-specific differences in the occurrence of untoward glycaemic but not arrhythmogenic outcomes during temperature extremes. Such findings suggest interesting hypotheses that should spur further investigation into the role of personalised strategies to manage and mitigate risks of heat, cold, and extreme weather events in persons with diabetes.
Our finding of a positive linear relationship between t-max and serious hypoglycaemia rate among non-insulin users of some (ie, glimepiride, glyburide) but not other (ie, glipizide) second-generation SUs may be mechanistically explainable. In the setting of heat-related dehydration, impaired renal function may result in drug metabolite accumulation.39 Glimepiride and glyburide (but not glipizide) have renally-cleared active metabolites,40 that is, cyclohexyl hydroxymethyl glimepiride derivative and 4-hydroxy glyburide, respectively. These active metabolites could accumulate, increasing the duration of hypoglycaemic effects.
Studies of drug-induced DKA in T2D have focused predominantly on SGLT-2is, although DPP-4is and GLP1RAs have also been implicated.28 30 Since our data set predated SGLT-2i use, we examined sitagliptin and exenatide in the latter classes finding increased DKA rates during colder (vs hotter) ambient temperatures among users of sitagliptin. This is consistent with previously reported, although drug-agnostic, ambient temperature-DKA relationships.5 6 A potential mechanism is that cold stress may reduce insulin secretion.41
We did not observe compelling findings for our arrhythmogenic endpoint despite a priori hypotheses based on differences in the pharmacodynamic and/or adverse effect profiles of antidiabetes medications. For example, we hypothesised that GLP1RA exenatide users may have an increased sudden cardiac arrest rate during hotter ambient temperatures given the drug’s effect inhibiting thirst42 that could compound heat-related dehydration and precipitate serious arrhythmias.
Our study was limited by: reliance on somewhat dated data (precluding the study of newer-to-market antidiabetes drugs and more contemporaneous extreme ambient temperature patterns); use of residential ZIP code as a proxy for exposure to temperature; lack of data on access to heating, ventilation, and air conditioning; lack of laboratory results (including blood glucose and haemoglobin A1C); and residual confounding. With respect to generalisability, our findings may not apply to non-US Medicaid populations. Finally, we did not seek public or patient involvement in the development of our research question, design and conduct of our study, or interpretation of our findings; doing so may have strengthened our study’s relevance and applicability.
Conclusion
This exploratory geoenvironmental diabetology43 study harnessing real-world data of vulnerable Americans with T2D identified some antidiabetes drug class-specific and agent-specific differences in the occurrence of untoward glycaemic safety outcomes during ambient temperature extremes. Future work should investigate roles for personalised strategies to manage and mitigate risks of heat, cold, and extreme weather events in persons with diabetes.
supplementary material
Footnotes
Funding: This work was supported by: (1) the University of Pennsylvania’s Population Aging Research Center (PARC); (2) the University of Pennsylvania’s Center for Real-World Effectiveness and Safety of Therapeutics; and (3) the following federal grants: R01AG060975 (Principal Investigator (PI): CEL), R01DA048001 (multiple PIs: SH and Lang Li), R01AG025152 (multiple PIs: SH and Lang Li), R01AG064589 (multiple PIs: SH and Dylan Small), R01MH130435 (PI: SH), R01CE003347 (PI: SH), R01AG061632 (PI: Dr Andrew Zullo), P30AG012836 (PI: Dr Hans-Peter Kohler) and R01AG079911 (PI: Dr Therese Bittermann). The study supporters/funders were not involved in the design of the study; the collection, analysis or interpretation of data; the writing of the report; and did not impose any restrictions regarding the publication of the report.
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-085139).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Data were used under a data use agreement signed between the Trustees of the University of Pennsylvania and the US Centers for Medicare and Medicaid Services (CMS). The data use agreement terms preclude us from sharing data. Persons interested in using CMS data for research should contact the Research Data Assistance Center (a CMS contractor, www.resdac.org) for additional information.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein) or of any geographical or locational reference does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: The University of Pennsylvania institutional review board issued waivers of informed consent and HIPAA authorisation for this research study. The University of Pennsylvania’s Federalwide Assurance number is FWA00004028 and expires on 5 April 2026. Additional info on IRB registration numbers can be found at https://irb.upenn.edu/homepage/about-the-irb/federalwide-assurance-info/.
Contributor Information
Charles E Leonard, Email: celeonar@pennmedicine.upenn.edu.
Kacie Bogar, Email: kacie.bogar@pennmedicine.upenn.edu.
Colleen M Brensinger, Email: cbrensin@upenn.edu.
Warren B Bilker, Email: WARREN@UPENN.EDU.
Michelle L Bell, Email: michelle.bell@yale.edu.
James H Flory, Email: floryj@mskcc.org.
Christopher Shi, Email: cs116@rice.edu.
Cheng Chen, Email: alicechencheng@gmail.com.
Sean Hennessy, Email: hennessy@upenn.edu.
Data availability statement
Data may be obtained from a third party and are not publicly available.
References
- 1.International Diabetes Federation Diabetes and climate change report. 2012. [9-Sep-2024]. https://idf.org/media/uploads/2023/05/attachments-15.pdf Available. Accessed.
- 2.Ratter-Rieck JM, Roden M, Herder C. Diabetes and climate change: current evidence and implications for people with diabetes, clinicians and policy stakeholders. Diabetologia. 2023;66:1003–15. doi: 10.1007/s00125-023-05901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Shihabi F, Moore A, Chowdhury TA. Diabetes and climate change. Diabet Med. 2023;40:e14971. doi: 10.1111/dme.14971. [DOI] [PubMed] [Google Scholar]
- 4.American Public Health Association Climate changes health: vulnerable populations. 2024. [9-Sep-2024]. https://www.apha.org/topics-and-issues/climate-change/vulnerable-populations Available. Accessed.
- 5.Bogar K, Brensinger CM, Hennessy S, et al. Climate Change and Ambient Temperature Extremes: Association With Serious Hypoglycemia, Diabetic Ketoacidosis, and Sudden Cardiac Arrest/Ventricular Arrhythmia in People With Type 2 Diabetes. Diabetes Care. 2022;45:e171–3. doi: 10.2337/dc22-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu C-L, Chang H-H, Chen H-F, et al. Inverse relationship between ambient temperature and admissions for diabetic ketoacidosis and hyperglycemic hyperosmolar state: A 14-year time-series analysis. Environ Int. 2016;94:642–8. doi: 10.1016/j.envint.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Hensel M, Stuhr M, Geppert D. Reduced frequency of severe hypoglycemia at mild ambient temperatures between 10 and 20 °C: A population-based study under marine west coast climate conditions. J Diabetes Complications. 2017;31:1212–4. doi: 10.1016/j.jdiacomp.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kang S-H, Oh I-Y, Heo J, et al. Heat, heat waves, and out-of-hospital cardiac arrest. Int J Cardiol. 2016;221:232–7. doi: 10.1016/j.ijcard.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard T, Moride Y, Pottegard A. In: Encounter databases. Strom BL, Kimmel SE, editors. Hoboken: Wiley Blackwell; 2022. Textbook of pharmacoepidemiology; pp. 142–73. [Google Scholar]
- 10.Rudowitz R, Burns A, Hinton E, et al. 10 things to know about medicaid. 2023. [9-Sep-2024]. https://www.kff.org/medicaid/issue-brief/10-things-to-know-about-medicaid Available. Accessed.
- 11.Centers for Medicare and Medicaid Services Medicaid.gov. 2024. [9-Sep-2024]. https://www.medicaid.gov/about-us/index.html Available. Accessed. [PubMed]
- 12.US department of commerce. NOAA: about our agency. 2024. [9-Sep-2024]. https://www.noaa.gov/about-our-agency Available. Accessed.
- 13.NOAA Office of Legislative and Intergovernmental Affairs What is NOAA? 2011. [9-Sep-2024]. https://www.noaa.gov/sites/default/files/2021-10/What-is-NOAA-2011.pdf Available. Accessed.
- 14.Klompas M, Eggleston E, McVetta J, et al. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36:914–21. doi: 10.2337/dc12-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnero G, Godone D. COMPARISONS BETWEEN DIFFERENT INTERPOLATION TECHNIQUES. Int Arch Photogramm Remote Sens Spatial Inf Sci. 2013;XL-5/W3:139–44. doi: 10.5194/isprsarchives-XL-5-W3-139-2013. [DOI] [Google Scholar]
- 16.Hartkamp AD, Beurs K, Stein A, et al. Interpolation techniques for climate variables. [9-Sep-2024]. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=3f3bfa9c629b0d6a809a813e36ce88613366af10 Available. Accessed.
- 17.Childs C. Interpolating surfaces in ArcGIS spatial analyst. Arc User. 2004:32–5. [Google Scholar]
- 18.Nam YH, Bilker WB, Leonard CE, et al. Outdoor temperature and survival benefit of empiric potassium in users of furosemide in US Medicaid enrollees: a cohort study. BMJ Open. 2019;9:e023809. doi: 10.1136/bmjopen-2018-023809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen LH, Løkkegaard E, Andreasen AH, et al. Using prescription registries to define continuous drug use: how to fill gaps between prescriptions. Pharmacoepidemiol Drug Saf. 2008;17:384–8. doi: 10.1002/pds.1549. [DOI] [PubMed] [Google Scholar]
- 20.Hampp C, Borders-Hemphill V, Moeny DG, et al. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014;37:1367–74. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 21.Douros A, Yin H, Yu OHY, et al. Pharmacologic Differences of Sulfonylureas and the Risk of Adverse Cardiovascular and Hypoglycemic Events. Diabetes Care. 2017;40:1506–13. doi: 10.2337/dc17-0595. [DOI] [PubMed] [Google Scholar]
- 22.Leonard CE, Bilker WB, Brensinger CM, et al. Severe hypoglycemia in users of sulfonylurea antidiabetic agents and antihyperlipidemics. Clin Pharmacol Ther. 2016;99:538–47. doi: 10.1002/cpt.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheen AJ. Glucose-lowering agents and risk of ventricular arrhythmias and sudden cardiac death: A comprehensive review ranging from sulphonylureas to SGLT2 inhibitors. Diabetes Metab. 2022;48:101405. doi: 10.1016/j.diabet.2022.101405. [DOI] [PubMed] [Google Scholar]
- 24.Dhopeshwarkar N, Brensinger CM, Bilker WB, et al. Risk of sudden cardiac arrest and ventricular arrhythmia with sulfonylureas: An experience with conceptual replication in two independent populations. Sci Rep. 2020;10:10070. doi: 10.1038/s41598-020-66668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard CE, Brensinger CM, Aquilante CL, et al. Comparative Safety of Sulfonylureas and the Risk of Sudden Cardiac Arrest and Ventricular Arrhythmia. Diabetes Care. 2018;41:713–22. doi: 10.2337/dc17-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard CE, Hennessy S, Han X, et al. Pro- and Antiarrhythmic Actions of Sulfonylureas: Mechanistic and Clinical Evidence. Trends Endocrinol Metab. 2017;28:561–86. doi: 10.1016/j.tem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zünkler BJ. Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol Ther. 2006;112:12–37. doi: 10.1016/j.pharmthera.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Dawwas GK, Flory JH, Hennessy S, et al. Comparative Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Dipeptidyl Peptidase 4 Inhibitors and Sulfonylureas on the Risk of Diabetic Ketoacidosis. Diabetes Care. 2022;45:919–27. doi: 10.2337/dc21-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawwas GK, Hennessy S, Brensinger CM, et al. Comparative Safety of Dipeptidyl Peptidase‐4 Inhibitors and Sudden Cardiac Arrest and Ventricular Arrhythmia: Population‐Based Cohort Studies. Clin Pharma and Therapeutics. 2022;111:227–42. doi: 10.1002/cpt.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Yu M, Mei M, et al. The association between GLP-1 receptor agonist and diabetic ketoacidosis in the FDA adverse event reporting system. Nutr Metab Cardiovasc Dis. 2022;32:504–10. doi: 10.1016/j.numecd.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz M, Lawson F, Owens D, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16:6. doi: 10.1186/s12933-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, Lu W, Chen Z, et al. Association of glucagon-like peptide-1 receptor agonists with cardiac arrhythmias in patients with type 2 diabetes or obesity: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2022;14:195. doi: 10.1186/s13098-022-00970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018-30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7:25–33. doi: 10.1016/S2213-8587(18)30303-6. [DOI] [PubMed] [Google Scholar]
- 34.Leonard CE, Han X, Brensinger CM, et al. Comparative risk of serious hypoglycemia with oral antidiabetic monotherapy: A retrospective cohort study. Pharmacoepidemiol Drug Saf. 2018;27:9–18. doi: 10.1002/pds.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schelleman H, Bilker WB, Brensinger CM, et al. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther. 2010;88:214–22. doi: 10.1038/clpt.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobo WV, Cooper WO, Epstein RA, Jr, et al. Positive predictive value of automated database records for diabetic ketoacidosis (DKA) in children and youth exposed to antipsychotic drugs or control medications: a Tennessee Medicaid Study. BMC Med Res Methodol. 2011;11:157. doi: 10.1186/1471-2288-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hennessy S, Leonard CE, Freeman CP, et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf. 2010;19:555–62. doi: 10.1002/pds.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puga AM, Lopez-Oliva S, Trives C, et al. Effects of Drugs and Excipients on Hydration Status. Nutrients. 2019;11:669. doi: 10.3390/nu11030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sola D, Rossi L, Schianca GPC, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840–8. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention Managing diabetes in cold weather. 2024. [9-Sep-2023]. https://www.cdc.gov/diabetes/articles/managing-diabetes-cold-weather.html#:~:text=Keep%20your%20medicines%2C%20supplies%2C%20and,pumps%20and%20continuous%20glucose%20monitors Available. Accessed.
- 42.McKay NJ, Kanoski SE, Hayes MR, et al. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1755–64. doi: 10.1152/ajpregu.00472.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook CB, Wellik KE, Fowke M. Geoenvironmental Diabetology. J Diabetes Sci Technol. 2011;5:834–42. doi: 10.1177/193229681100500402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available.