Abstract
We report the case of long-term persisting rheumatoid arthritis (RA), treated with CD20-CD19 CAR-T when it became associated with diffuse large B cell lymphoma (DLBCL), resulting in a sustained drug-free remission of the preceding RA, as well as of the subsequent DLBCL that formed the indication of the CAR-T therapy using zamtocabtagene autoleucel, with a 1-year follow-up. According to our best knowledge, this is the first published clinical case report of long-term persisting RA treated with CAR-T cell therapy.
Keywords: arthritis, rheumatoid; B-lymphocytes; autoimmune diseases; therapeutics; hematology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Rheumatoid arthritis (RA) is a B cell-driven systemic autoimmune disease characterised by synovial inflammation of joints leading to destructive complications, of which long-term persisting and difficult-to-treat forms (D2T-RA) can lead to decades-long treatment beside remaining clinically active and symptomatic, for which B cell-depleting chimeric antigen receptor T cell (CAR-T) treatment, that is applied already for the treatment of B cell malignancies, may be a treatment option.
WHAT THIS STUDY ADDS
This is the first published case report of long-term persisting RA treated with CAR-T cell treatment after its association with diffuse large B cell lymphoma (DLBCL), showing the effectivity of this treatment against both RA and DLBCL with a 1-year follow-up.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our present case indicates that zamtocabtagene autoleucel could be an alternative efficacious curative treatment option for long-term persisting RA.
Introduction
Rheumatoid arthritis (RA) is a common systemic autoimmune disease that is associated with systemic complications (eg, cardiovascular, lung and central nervous system manifestations) in addition to joint involvement. The synovial inflammation of various joints, typically joints of the hands and feet, leads to bone erosions, cartilage destruction and joint deformities.
The pathogenesis of RA is largely unknown; genetic and environmental factors have significant contributions; cigarette smoking is a strong predisposing factor. Both rheumatoid factor (RF) and anticitrullinated protein antibody (ACPA) are unfavourable prognostic factors in RA. Although the current treatment strategy, especially the use of targeted synthetic and biological therapies, improved the outcome of the disease, many patients remain symptomatic and clinically active despite adequate treatment1 2 (difficult-to-treat-RA (D2T-RA)), and drug-free remission remains exceptionally rare,3 especially in the presence of bad prognostic factors.
Diffuse large B cell lymphoma (DLBCL) is the most common aggressive non-Hodgkin’s lymphoma (NHL), comprising 30%–40% of all NHL cases, originating from lymph nodes and presenting with lymphadenopathy and can be accompanied by systemic inflammatory symptoms (ie, B symptoms: fever, night sweat and weight loss) and tissue infiltrations that can cause organ obstruction and compression symptoms, with a rapid growth rate and rapidly fatal if untreated. Its current standard first-line treatment is cyclophosphamide, adriablastine, vincristine and prednisolone combination chemotherapy since the 1970s, and with added rituximab since the 2000s (R-CHOP) or its analogue variants. Especially patients not responding or relapsing after first-line treatment (relapsed/refractory (r/r) DLBCL) bear a challenge to the treating physician. The treatment of r/r DLBCL has recently undergone a revolution with the availability of chimeric antigen receptor T cell (CAR-T) treatments.4
Case report
We report here the use of autologous CD20-CD19 CAR-T cells in the treatment of r/r DLBCL that was associated with preceding and long-term persisting RA, resulting in a sustained drug-free remission of RA, as well as of DLBCL that formed the indication of CAR-T treatment.
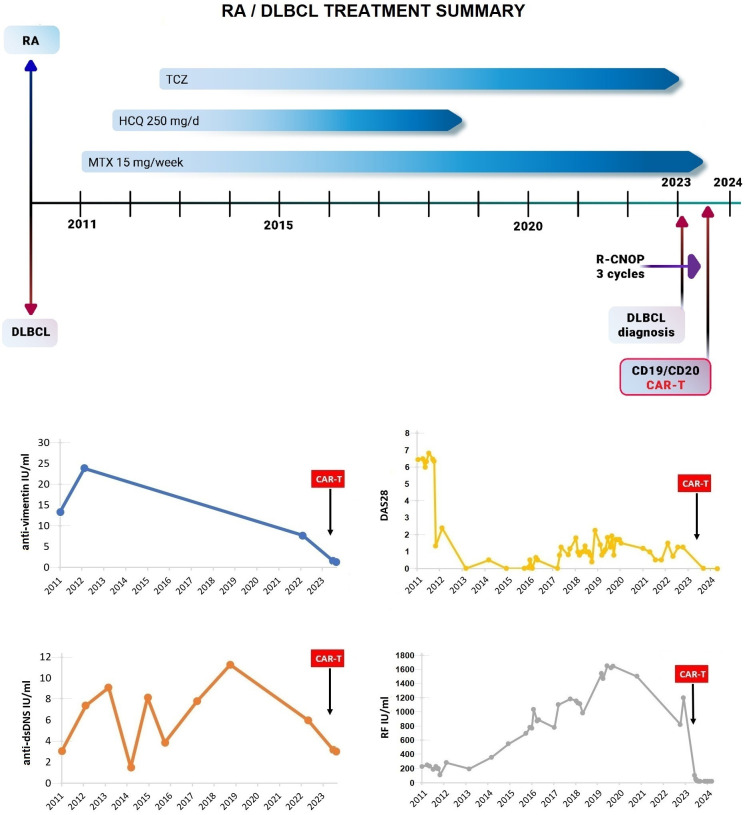
A 73-year-old male patient developed joint pain in 2010; 1 year later, the diagnosis of RA was established. The patient was and is still a heavy smoker up to August 2024 with 50 pack-years, currently still smoking one pack daily, and it is to be noted that in addition to its known association with RA, some studies also suggested a connection between heavy smoking and lymphoma.5 The patient had a highly active disease (Disease Activity Score in 28 joints (DAS28) at diagnosis: 6.43), associated with low titre ACPA positivity (31 IU/mL) and high titre RF positivity (250 IU/mL). Low-dose glucocorticoid and 15 mg/week methotrexate (MTX) were initiated in 2011, but no higher doses were possible due to increased liver enzyme levels. Hydroxychloroquine (HCQ) was also started (250 mg/day) due to high clinical disease activity. As clinical disease activity remained still high, thereafter the interleukin-6 inhibitor tocilizumab (TCZ) was started intravenously with the general dose of 8 mg/kg every 4 weeks (a total of 560 mg). Glucocorticoid treatment was tapered and discontinued; the patient had low/moderate disease activity with MTX+HCQ+TCZ therapy between 2011 and 2018, when HCQ was discontinued due to the ophthalmologist’s recommendation. The patient showed a good response to the applied therapy over time; however, the RF serum level continuously increased. In 2019, the RF level topped at 1645 IU/mL and remained constantly in that high range, accompanied with the fluctuating symptoms of RA and periods of severe pain. TCZ therapy was not switched due to patient preference in addition to the good clinical response.
In January 2023, a swollen cervical lymph node was observed and excised; histology confirmed Germinal Centre B cell-like-DLBCL,6 after which TCZ was stopped immediately, and the patient regularly visited the haematology department for tight control of DLBCL and discontinued rheumatology visits due to patient-related burden, while treatments for lymphoma were administered. Based on telemedicine, the patient had mild to moderate RA symptoms in this period. According to DLBCL staging examinations in February 2023, the initial disease stage was IV/B as per the Lugano classification system, with bulky right cervical lymphoid conglomerate, and rituximab, cyclophosphamide, mitoxantrone, vincristine and prednisolone (R-CNOP) chemoimmunotherapy protocol was initiated. After three cycles of treatment, interim positron emission tomography (PET)/CT restaging/analysis in May 2023 confirmed only a partial response, with strong suspicion of new mediastinal disease activity, due to which the patient was considered refractory to the applied treatment.
As a consequence, the patient was considered for second-line B cell depleting CAR-T treatment and was screened subsequently on 25 May 2023 in a pivotal phase II randomised, multicentre, open-label clinical trial (official short title: DALY 2-EU; original long title: A pivotal phase II randomised, multicentre, open-label study to evaluate the efficacy and safety of MB-CART2019.1 compared with standard of care therapy in participants with r/r DLBCL, who are not eligible for high-dose chemotherapy and autologous stem cell transplantation; EudraCT number: 2020-003908-14; sponsored by Miltenyi Biomedicine GmbH, Bergisch Gladbach, Germany.7 MTX treatment of RA was discontinued after the screening visit for CAR-T treatment.
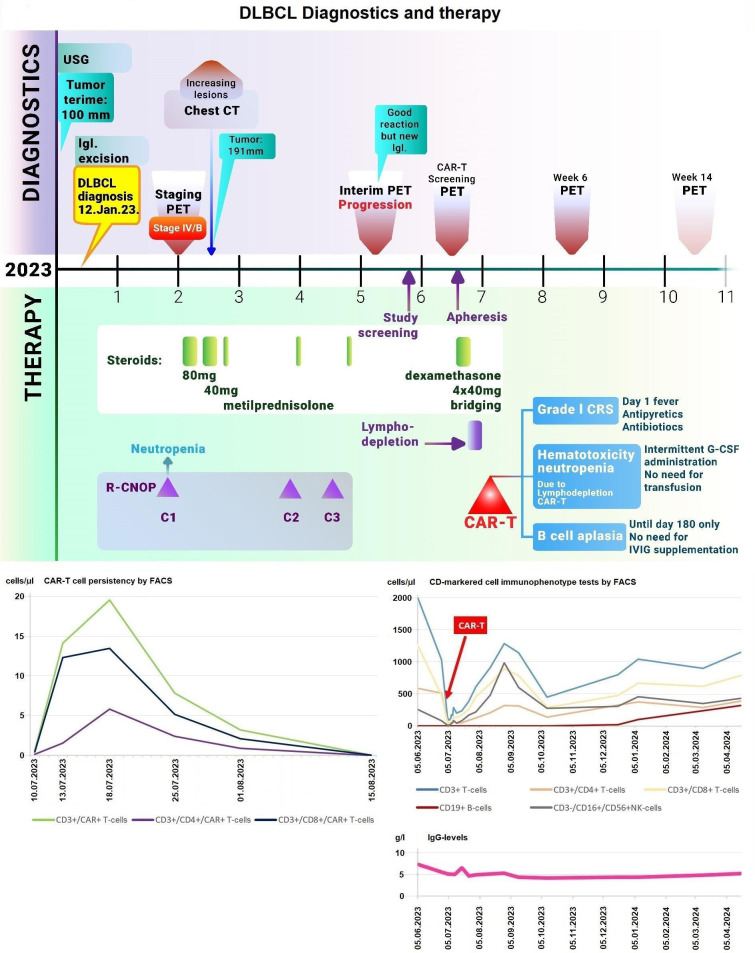
CD3+ cells were collected on 19 June 2023 by autologous fresh leukapheresis, from which tandem CD20-CD19-directed non-cryopreserved CAR-T cells (zamtocabtagene autoleucel, zamto-cel)8 were generated in Good Manufacturing Practice facility in the closed automated CliniMACS Prodigy system by transducing the autologous T-lymphocytes retrovirally with a CAR-containing intracellular CD3ζ signalling domain and a 4-1BB co-stimulatory domain with a CD8 transmembrane domain. Manufacturing took 12 days, with an additional 2 days for leukapheresis transport and 1 day for fresh non-cryopreserved CAR-T transport, resulting in an overall 15-day vein-to-vein time, during which dexamethasone bridging therapy was applied. After conditioning lymphodepletion with fludarabine and cyclophosphamide and transfusion of the manufactured fresh CAR-T cells were administered on day 0 (4 July 2023).
There was no severely immunocompromised state at the time of inclusion, no liver dysfunction presented, and there was no known history or presence of autoimmune central nervous system disease. CD19+ and CD20+ B cells were not detectable after the CAR-T treatment. After the administration of the CAR-T cell infusion, grade I cytokine release syndrome occurred, which was successfully treated with antipyretics. No immune effector cell-associated neurotoxicity syndrome, infection or tumour lysis syndrome was detectable. At haematological control on 25 July 2023, only a 1×1.5 cm long cervical lymph node was detectable by physical examination, and the patient had no complaints related to lymphoma.
Deep B cell aplasia developed and lasted until the day of +180 with only a mild decrease of IgG levels, not falling below 4.8 g/L, without the need for immunoglobulin replacement, indicating retained humoral immune memory and plasma cell functions. In response to recurrent neutropenia after the CAR-T cell therapy, the patient needed intermittent filgrastim injection until December 2023.
After the CAR-T treatment, CAR-T expansion was confirmed by flow cytometry (FC), followed by the consolidation phase. CAR-T cells were detected by FC between 10 July 2023 and 15 August 2023. A rise and subsequent decrease in white blood cell count also followed, also confirmed by FC immunophenotyping to different CD-markered cell subtypes (figure 1). Presently, no full set of detailed, specific laboratory analytics can be disclosed as the patient is still participating in the ongoing clinical study.
Figure 1. Patient with long-term persisting RA, associated with new-onset DLBCL treated by CAR-T. This panel summarises the treatments given for RA preceding DLBCL and subsequent treatments for DLBCL, as well as RA disease activity score DAS28, anti-vimentin and anti-double-stranded DNA antibody (anti-dsDNS) levels, and Rheumatoid Factor (RF) levels. CAR-T: chimeric antigen receptor T cell; DLBCL: diffuse large B cell lymphoma; R-CNOP: rituximab, cyclophosphamide, mitoxantrone, vincristine and prednisolone; RA: rheumatoid arthritis.
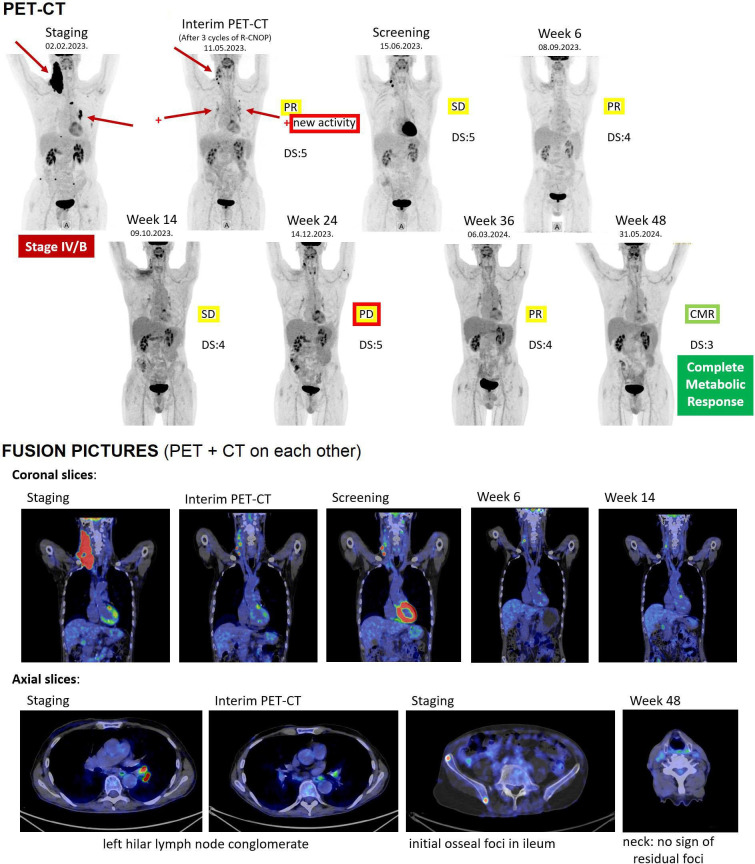
Subsequent rheumatology controls after the CAR-T therapy, from 26 July 2023, confirmed the patient staying in complete drug-free clinical remission of RA, which still lasts without interruption by 1-year follow-up of CAR-T treatment, resulting in a significant improvement of quality of life for the patient. The RF level showed a sharp decrease, from 1200 IU/mL right before diagnosis of DLBCL to 48 IU/mL after 3 weeks following CAR-T administration, further decreasing to around 13 IU/mL in upcoming months where it stagnates permanently (figure 2), in addition to low ACPA levels (indicating immunological remission as well). DLBCL remained in partial response (PR)/stable disease (SD) at radiology PET/CT controls, except at week 24, when the possibility of mild progressive disease (PD) was raised, which remained, however, unconfirmed by week 36 control that showed again PR, while finally, radiology control at week 48 demonstrated complete metabolic response (figure 3).
Figure 2. Upper graph summarises the main diagnostics and the therapy administered for DLBCL. The lower left graph shows the circulating CAR-T cell numbers following CAR-T administration. The right middle graph shows representative flow cytometric analysis results of circulating CD-markered cell numbers. The right lower graph shows the IgG levels. CAR-T: chimeric antigen receptor T cell; DLBCL: diffuse large B cell lymphoma; PET: positron emission tomography; R-CNOP: rituximab, cyclophosphamide, mitoxantrone, vincristine and prednisolone.
Figure 3. Pretreatment, post-R-CNOP and post-CAR-T PET-CT and CT scans. CAR-T: chimeric antigen receptor T cell; DS: Deauville Score; DLBCL: diffuse large B cell lymphoma; PD: progressive disease; PET: positron emission tomography; PR: partial response; R-CNOP: rituximab, cyclophosphamide, mitoxantrone, vincristine and prednisolone; SD: stable disease.
Discussion
According to our best knowledge, this is the first published case report of long-term persisting RA treated with CAR-T cell therapy.9 10 Reaching its 1-year follow-up, RA remains in complete drug-free remission, which is exceptionally rare with the current standard protocol.1,3 DLBCL, that formed the indication for CAR-T application, also did and does not require further treatment, only close monitoring. It is known that chemotherapy for lymphoma can temporarily alleviate RA symptoms11 and the applied treatment containing rituximab and dexamethasone bridging probably also contributed to it; however, long-lasting drug-free remission was achieved after CAR-T therapy in this case. Also notable is that the increasing reappearance of B cells after day 180 did not result in the reappearance of RA or DLBCL, indicating that the new B cell population no longer contained the defective clones causing RA or DLBCL. Moreover, the mild PD reported at week 24, followed by PR and then CMR at subsequent controls, may indicate that even if circulating CAR-T cells could no longer be detected, they might have remained active in tissues, continuing to eliminate the remainder of the malignant disease. These results suggest that besides effectivity against DLBCL, bispecific tandem CD20-CD19-directed non-cryopreserved CAR-T cell therapy with zamto-cel might be a therapeutic option in long-term persisting and possibly other severe forms of RA, such as D2T-RA.1 2 The therapy appears to be effective both in RA and DLBCL by the temporary elimination of the B cell line up to plasmablast state, including the malignant B cell clone as well as B cells participating in the maintenance of the autoimmune response.12
Our case has some limitations, as RA disease activity was measured in DAS-28 (calculated with erythrocyte sedimentation rate) instead of the Clinical Disease Activity Index score that would have been more appropriate to TCZ therapy.
In autoimmune diseases, the potential role of B cell depletion was recently raised by more publications,13,19 including RA,9 and similar efficacy was reported with CD19-directed cryopreserved CAR-T in a case of RA associated with myasthenia gravis.10 While presently no approved CAR-T therapy is indicated for them, the following have published results of successful experimental treatment with it besides the aforementioned ones: systemic lupus erythematosus,14 15 systemic sclerosis,17 antisynthetase syndrome13 and idiopathic inflammatory myositis,19 while more CAR-T trials are underway for further ones.20
Well-designed clinical trials at specialised centres are needed to study the potential place of CAR-T therapy in the treatment of RA and other autoimmune diseases. Given the role of B cells in a variety of immune-mediated diseases, the findings of this case underline that CAR-T therapy with zamto-cel that targets B cells may have future applications in its treatment.
Acknowledgements
We thank Miltenyi Biomedicine GmbH for the clinical trial sponsoring and Miltenyi Biotec B.V. & Co. KG for the manufacturing of zamtocabtagene autoleucel. It was the special condition of the patient at consenting to note in the publication that he thanks all those who had prayed for him, which had helped him.
Footnotes
Funding: The authors have not declared a specific grant for this case report from any funding agency in the public, commercial or not-for-profit sectors. The Article Processing Charge is covered and the referred clinical study has been sponsored by Miltenyi Biomedicine GmbH.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and was approved by the Hungarian Medical Research Council’s Ethics Committee for Clinical Pharmacology, Báthory u. 10., Budapest, Hungary, case file number BMEÜ2168-0/2022-EKL. EudraCT number: 2020-003908-14. Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
David Szabo, Email: david.szabo@miltenyi.com.
Alexandra Balogh, Email: alexa.balogh92@gmail.com.
Laszlo Gopcsa, Email: gopcsa.laszlo@dpckorhaz.hu.
Laura Giba-Kiss, Email: giba-kiss.laura@dpckorhaz.hu.
Gergely Lakatos, Email: gregory.lakatos@gmail.com.
Melinda Paksi, Email: drpakmel@gmail.com.
Marienn Reti, Email: reti.marienn@dpckorhaz.hu.
Peter Takacs, Email: peter.takacs@miltenyi.com.
Pearl van Heteren, Email: pearl.vanheteren@miltenyi.com.
Gregor Zadoyan, Email: gregor.zadoyan@miltenyi.com.
Silke Holtkamp, Email: silke.holtkamp@miltenyi.com.
Toon Overstijns, Email: toon.overstijns@miltenyi.com.
Stefan Miltenyi, Email: stefan@miltenyi.com.
Peter Remenyi, Email: premenyi@dpckorhaz.hu.
György Nagy, Email: gyorgyngy@gmail.com.
References
- 1.Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31–5. doi: 10.1136/annrheumdis-2020-217344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2022;81:20–33. doi: 10.1136/annrheumdis-2021-220973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy G, van Vollenhoven RF. Sustained biologic-free and drug-free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther. 2015;17:181. doi: 10.1186/s13075-015-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gergely L, Nagy Zs. Classification and Treatment of Diffuse Large B-Cell Lymphoma. Hematol Transzfuziol. 2024;57:11–8. doi: 10.1556/2068.2024.10004. [DOI] [Google Scholar]
- 5.Taborelli M, Montella M, Libra M, et al. The dose-response relationship between tobacco smoking and the risk of lymphomas: a case-control study. BMC Cancer. 2017;17:421. doi: 10.1186/s12885-017-3414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlynarczyk C, Fontán L, Melnick A. Germinal center‐derived lymphomas: The darkest side of humoral immunity. Immunol Rev. 2019;288:214–39. doi: 10.1111/imr.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borchmann P, Vandenberghe P, Urbano‐Ispizua A, et al. A Randomized Phase II study of MB‐CART2019.1 Compared To Standard of Care In Patients With Relapsed/Refractory DLBCL Ineligible For ASCT – DALY 2‐EU Trial. Hematol Oncol. 2023;41:840–1. doi: 10.1002/hon.3166_OT20. [DOI] [Google Scholar]
- 8.Borchmann P, Taylor J. Zamtocabtagene Autoleucel (MB-CART2019.1): An Investigational CAR-T Cell Product with Tandem Targeting of CD19 and CD20 as a Potential Treatment Option for Patients with Relapsed/ Refractory B Cell Non-Hodgkin Lymphoma. EMJ Hematol. 2022;10:29–31. doi: 10.33590/emjhematol/22C0248. [DOI] [Google Scholar]
- 9.Ohno R, Nakamura A. Advancing autoimmune Rheumatic disease treatment: CAR-T Cell Therapies - Evidence, Safety, and future directions. Semin Arthritis Rheum. 2024;67:152479. doi: 10.1016/j.semarthrit.2024.152479. [DOI] [PubMed] [Google Scholar]
- 10.Haghikia A, Hegelmaier T, Wolleschak D, et al. Clinical efficacy and autoantibody seroconversion with CD19-CAR T cell therapy in a patient with rheumatoid arthritis and coexisting myasthenia gravis. Ann Rheum Dis. 2024:ard-2024-226017. doi: 10.1136/ard-2024-226017. [DOI] [PubMed] [Google Scholar]
- 11.Wöhrer S, Troch M, Zwerina J, et al. Influence of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone on serologic parameters and clinical course in lymphoma patients with autoimmune diseases. Ann Oncol. 2007;18:647–51. doi: 10.1093/annonc/mdl467. [DOI] [PubMed] [Google Scholar]
- 12.Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20:179–99. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller F, Boeltz S, Knitza J, et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet. 2023;401:815–8. doi: 10.1016/S0140-6736(23)00023-5. [DOI] [PubMed] [Google Scholar]
- 14.Mougiakakos D, Krönke G, Völkl S, et al. CD19-Targeted CAR-T Cells in Refractory Systemic Lupus Erythematosus. N Engl J Med. 2021;385:567–9. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]
- 15.Mackensen A, Müller F, Mougiakakos D, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28:2124–32. doi: 10.1038/s41591-022-02017-5. [DOI] [PubMed] [Google Scholar]
- 16.Haghikia A, Hegelmaier T, Wolleschak D, et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 2023;22:1104–5. doi: 10.1016/S1474-4422(23)00375-7. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann C, Müller F, Jörg HWD, et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis. 2023:1117–20. doi: 10.1136/ard-2023-223952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schett G, Mielenz D, Nagy G, et al. B-cell depletion in autoimmune diseases. Ann Rheum Dis. 2024:ard-2024-225727. doi: 10.1136/ard-2024-225727. [DOI] [PubMed] [Google Scholar]
- 19.Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease — A Case Series with Follow-up. N Engl J Med. 2024;390:687–700. doi: 10.1056/NEJMoa2308917. [DOI] [PubMed] [Google Scholar]
- 20.Blache U, Tretbar S, Koehl U, et al. CAR T cells for treating autoimmune diseases. RMD Open. 2023;9:e002907. doi: 10.1136/rmdopen-2022-002907. [DOI] [PMC free article] [PubMed] [Google Scholar]