Abstract
Abstract
Introduction
Physical activity (PA) is recommended in patients with type 2 diabetes mellitus (T2DM) to improve their glycaemic control. We aimed to assess PA levels among participants with controlled and uncontrolled T2DM.
Research design and methods
Three cross-sectional analyses of a prospective cohort conducted in Lausanne, Switzerland. PA levels (sedentary, light, moderate and vigorous) were either self-reported via questionnaire (first and second survey) or objectively assessed using accelerometry (second and third survey). T2DM control was defined as glycaemia <7.0 mmol/L or glycated haemoglobin <6.5% (48 mmol/mol).
Results
Data from 195 (30.3% women), 199 (30.1% women) and 151 (44.4% women) participants with T2DM were analysed in the first (2009–2012), second (2014–2017) and third (2018–2021) surveys. Approximately half of the participants did not have controlled glycaemia. Using subjective data, over 90% (first survey) and 75% (second survey) of participants reported moderate and vigorous PA >150 min/week. After multivariable adjustment, no differences were found regarding all types of self-reported PA levels between controlled and uncontrolled participants. Objective assessment of PA led to considerable differences according to the software used: 90% and 20% of participants with moderate and vigorous PA >150 min/week, respectively. After multivariable adjustment, no differences were found for all PA levels between controlled and uncontrolled participants, irrespective of the analytical procedure used. Using glycated haemoglobin, almost two-thirds of participants were considered as uncontrolled, and no differences were found for objectively assessed PA between controlled and uncontrolled participants.
Conclusions
No differences in PA levels were found between participants with controlled and uncontrolled T2DM.
Keywords: general diabetes, preventive medicine, epidemiologic studies
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Physical activity levels were assessed using accelerometer.
Diabetes control was assessed using both fasting plasma glucose and glycated haemoglobin.
Study was conducted in a single location; hence, generalisability might be an issue.
Possible selection bias exists as only motivated participants accepted to wear the accelerometer.
Introduction
Diabetes mellitus affects 537 million adults in the world, 90% of whom have type 2 diabetes mellitus (T2DM). It is predicted that this number of people with diabetes will increase to 783 million by 2045. Diabetes also has a financial cost estimated at US$966 billion, representing 9% of total adult health spending.1
Besides quitting smoking and adopting a healthy diet, physical activity (PA) is recommended in all patients with T2DM to improve their glycaemic control, insulin action, lipid levels and blood pressure,2 thus reducing the risk of cardiovascular disease. Simple activity such as walking 30 min/day can promote weight loss and improve glycaemic control.3 More structured exercise programmes are more effective to reduce insulin resistance in T2DM.4 Exercise programmes can be focused on aerobic, resistance or combined training, leading to significant improvements in haemoglobin A1c (HbA1c) levels.5 Still, it has been reported that patients with T2DM seldom adhere to the recommended amounts of PA. Indeed, barriers such as old age, female sex, lack of motivation, feeling of obligation, depression and fatigue can affect adhesion to recommended PA.6 For instance, in the EUROASPIRE IV and V studies, over half of patients with cardiovascular disease (CVD) and self-reported diabetes did not intend to do regular planned PA, and only one-quarter (26%) did.7
In Switzerland, it was estimated that, in 2021, 389 600 people aged between 20 and 79 years lived with diabetes, and 1 249 700 were affected by impaired glucose tolerance, with health costs amounting to US$4.9 billion.8 Still, the level of PA among people with T2DM and its impact on T2DM control have never been assessed.
Hence, we aimed to assess the effect of subjectively and objectively measured PA levels in subjects treated for T2DM according to diabetes control, using data from a population-based study.
Materials and methods
Participants
The CoLaus|PsyCoLaus study is a population-based prospective study assessing the clinical, biological and genetic determinants of CVD aged 35–75 years at baseline, living in the city of Lausanne, Switzerland.9 In each survey, participants answered questionnaires, underwent a clinical examination and blood samples were drawn for analyses. Recruitment began in June 2003 and ended in May 2006. The first follow-up was performed between April 2009 and September 2012; the second follow-up was performed between May 2014 and April 2017 and the third follow-up was performed between April 2018 and May 2021. For more details, see www.colaus-psycolaus.ch.
Self-reported physical activity
Subjective PA was assessed using the Physical Activity Frequency Questionnaire (PAFQ). This self-reported questionnaire has been validated in the population of Geneva, Switzerland, and assesses the type and duration of 70 kinds of (non)professional activities and sports during the previous week. Sedentary status was defined as spending >90% of daily energy in activities below moderate intensity and high intensity (defined as requiring at least four times the basal metabolic rate (BMR)).10 BMR multiples are close to metabolic equivalent of task (MET) multiples, although MET multiples do not consider participant sex, age or height.
For the purpose of this study, each type of activity was categorised into sedentary behaviour (SB, <2 METs), light PA (LPA, 2 to <3 METs), moderate PA (MPA, 3–6 METs) and vigorous PA (VPA, >6 METs) according to the compendium of physical activities.11 Total PA was defined as the sum of LPA, MPA and VPA. For each item of the PAFQ, the time spent per week was computed as average hours per day multiplied by the number of days when the activity was performed. For each item category (ie, corresponding to SB, LPA, MPA or VPA), the times were summed up and divided by 7 to estimate an average daily time.
We chose to include SB in the analysis as we have previously shown that it is associated with an increased risk of developing T2DM.12
Accelerometry-assessed physical activity
PA was objectively assessed using a wrist-worn triaxial accelerometer (GENEActiv, Activinsights, UK, www.activinsights.com). These devices are the same that have been used in the UK Biobank study,13 weigh 16 g and allow continuous monitoring of PA for a maximum of 45 days. The devices were preprogrammed with a 50 Hz sampling frequency and subsequently attached to the participants’ right wrist. Participants were requested to wear the device continuously for 14 days in their free-living conditions.
Raw accelerometry data were downloaded using the GENEActiv software V.2.9 (GENEActiv, Activinsights) and transformed into 1 min epoch files. Data were analysed using the GENEActiv Excel macro file ‘general PA’ V.1.9, which had been previously validated.14 A valid day was defined as ≥10 hours (ie, 600 min epoch) of diurnal wear time on weekdays and ≥8 hours (ie, 480 min epoch) on weekend days. The Excel macro file can be provided on request.
A second analysis was performed on the raw accelerometry data using the R-package GGIR V.1.5-9 (http://cran.r-project.org)15 with the thresholds defined by White et al,16 that is, an acceleration between 85 and 180 milli-g to define light PA, between 181 and 437 milli-g to define MPA and >437 milli-g to define VPA. The code used to analyse the data is provided in online supplemental file 1.
Participants were considered as complying with the recommendations if the weekly amount of MPA and VPA exceeded 150 min, as per European Society of Cardiology/European Association for the Study of Diabetes guidelines.2
Diabetes assessment
Participants were asked whether they had been told they had diabetes and, if the answer was positive, if they were taking any medication (including insulin) to treat their diabetes. Participants were considered as presenting with treated diabetes if they reported taking any antidiabetic drug. Diabetes control was defined as a fasting plasma glucose <7 mmol/L; a second analysis was conducted using diabetes control defined as a glycated haemoglobin <6.5% (48 mmol/mol).
Blood was drawn in the fasting state and biological assays were performed by the Centre Hospitalier Universitaire Vaudois Clinical Laboratory on fresh blood samples within 2 hours of blood collection. The following analytical procedures (with maximum interbatch and intrabatch coefficients of variation) were used: glucose was measured by hexokinase method (1.6%–0.8%). In the second and third follow-ups, glycated haemoglobin levels were also measured by high performance liquid chromatography with the Bio-Rad, D-10 system, which has a measurement range of 3.8% (18 mmol/mol) to 18.5% (179 mmol/mol).
Eligibility and exclusion criteria
All participants receiving treatment for diabetes were eligible for the study. Participants were excluded if they lacked PA data.
Covariates
Participants were queried regarding their personal and family history of cardiovascular risk factors, medical treatment and socio-economic status. Educational level was categorised into low (mandatory or apprenticeship), medium (high school) and high (university). Smoking status was categorised into never, former and current.
Body weight and height were measured with participants barefoot and in light indoor clothes. Body weight was measured in kilograms to the nearest 100 g using a Seca scale (Hamburg, Germany). Height was measured to the nearest 5 mm using a Seca height gauge. Body mass index (BMI) was computed and categorised into normal (<25 kg/m2), overweight (≥25 and <30 kg/m2) and obese (≥30 kg/m2).
Blood pressure was measured using an Omron HEM-907 automated oscillometric sphygmomanometer after at least a 10 min rest in a seated position, and the average of the last two measurements was used. Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, a diastolic blood pressure of ≥90 mm Hg or the presence of antihypertensive medication.
Patient and public involvement
It was not possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Statistical analysis
Statistical analyses were performed separately for each study period using Stata V.18.0 for windows (StataCorp, College Station, Texas, USA). Descriptive results were expressed as number of participants (percentage) for categorical variables and as average SD or median (IQR) for continuous variables. Bivariate analyses were performed using χ2 test or Fisher’s exact test for categorical variables and Student’s t-test, analysis of variance (ANOVA) or Kruskal-Wallis non-parametric test for continuous variables. Multivariable analysis of continuous data was performed using ANOVA and results were expressed as adjusted mean±SEM. Multivariable analysis of categorical data was performed using logistic regression and results were expressed as OR (95% CI). Multivariable analyses were conducted adjusting for sex (male, female), age (continuous), BMI categories (normal, overweight, obese), smoking status (never, former, current), educational level (low, medium, high).
A sensitivity analysis was conducted using multivariable linear regression adjusting for the same covariates to assess the association between PA and fasting plasma glucose or glycated haemoglobin. Results were expressed as standardised beta-coefficients.
Statistical significance was assessed for a two-sided test with p<0.05.
Results
Characteristics of participants
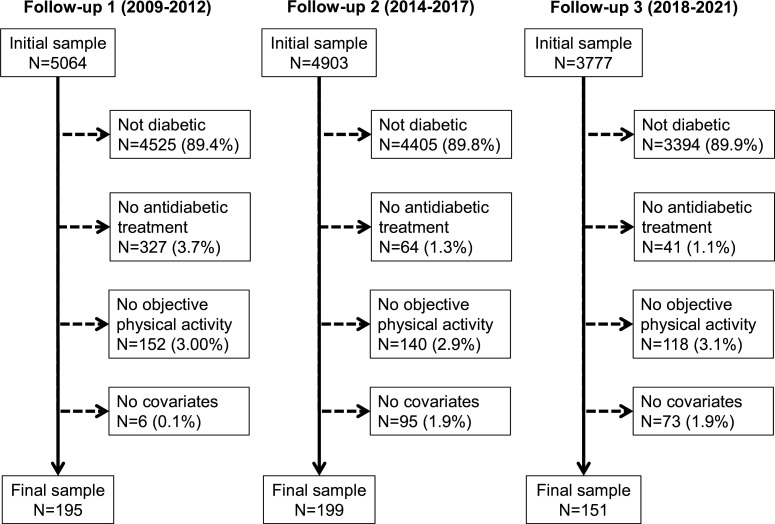
The selection procedure of the participants for the first, second and third follow-ups is summarised in figure 1 and the characteristics of the participants according to adequate or inadequate control of diabetes stratified by survey are provided in online supplemental table 1. Overall, one half of the participants treated for diabetes did not achieve adequate control. There were no consistent differences between controlled and uncontrolled participants, except that in the second follow-up, controlled participants were older and more frequently smokers.
Figure 1. Selection procedure for the first, second and third follow-ups, CoLaus|PsyColaus study, Lausanne, Switzerland.
Sedentary behaviour and physical activity levels according to diabetes control as per fasting plasma glucose
The bivariate analysis of reported PA levels between controlled and uncontrolled participants for the first and the second follow-ups are presented in online supplemental table 2. Overall, over 90% and 75% of participants were compliant with the 150 min/week of MPA+VPA in the first and second surveys, respectively. Participants spent half of their time in SB and very little in VPA. No differences in PA (in absolute time or as percentage of day) were found between controlled and uncontrolled participants, and similar findings were obtained after multivariable adjustment (table 1).
Table 1. Multivariable analysis of self-reported PA by control group, stratified by survey: CoLaus|PsyCoLaus study, Lausanne, Switzerland.
| First survey (2009–2012) | Second survey (2014–2017) | |||||
| Not controlled | Controlled | P value | Not controlled | Controlled | P value | |
| Sample size | 121 | 74 | 52 | 48 | ||
| Intensity of PA (min/day) | ||||||
| Sedentary | 527±15 | 542±19 | 0.543 | 525±25 | 556±26 | 0.395 |
| Light | 197±10 | 166±13 | 0.056 | 204±16 | 176±16 | 0.237 |
| Moderate | 186±11 | 191±15 | 0.819 | 181±17 | 185±18 | 0.864 |
| Vigorous | 32±8 | 46±10 | 0.250 | 43±10 | 26±11 | 0.262 |
| At least 150 min MVPA per week | 1 (ref) | NC | 1 (ref) | 0.85 (0.35–2.09) | 0.731* | |
| Intensity of PA (% of daily time) | ||||||
| Sedentary | 56±1.5 | 57.3±2 | 0.608 | 54.8±2.4 | 58.8±2.5 | 0.257 |
| Light | 20.9±1 | 17.7±1.3 | 0.057 | 21.4±1.6 | 18.8±1.7 | 0.275 |
| Moderate | 19.7±1.2 | 20.2±1.5 | 0.798 | 19.3±1.9 | 19.6±1.9 | 0.291 |
| Vigorous | 3.4±0.8 | 4.8±1 | 0.260 | 4.6±1 | 2.8±1.1 | 0.257 |
Results are expressed as mean±SEM for continuous variables and as OR and (95% CI) for categorical variables. Statistical analysis by analysis of variance for continuous variables and by logistic regression for categorical variables, adjusted for sex (male, female), age (continuous), BMI categories (normal, overweight, obese), smoking status (never, former, current), educational level (low, medium, high).
N=76 as several variables were dropped due to collinearity.
BMIbody mass indexMVPAmoderate and vigorous physical activityNCnot computablePA, physical activity
The results of the bivariate analysis of the objectively assessed PA levels using the GENEActiv macro between controlled and uncontrolled participants for the second and third follow-ups are presented in online supplemental table 3. Overall, over 90% of participants were compliant with the 150 min/week of MPA+VPA. Participants spent three quarters of their time in SB; conversely, they spent almost 2 hours/day on MPA. In the second survey, uncontrolled participants had higher levels and percentages of LPA and MPA, and a lower level and percentage of SB. In the third survey, no difference was found between controlled and uncontrolled participants. After multivariable adjustment, no significant differences were observed (table 2).
Table 2. Multivariable analysis, objectively assessed PA by control group as defined using fasting plasma glucose, stratified by survey: CoLaus|PsyCoLaus study, Lausanne, Switzerland.
| Second survey (2014–2017) | Third survey (2018–2021) | |||||
| Not controlled | Controlled | P value | Not controlled | Controlled | P value | |
| Sample size | 97 | 102 | 79 | 72 | ||
| Intensity of PA (min/day) | ||||||
| Sedentary | 609±12 | 634±11 | 0.131 | 601±14 | 591±15 | 0.634 |
| Light | 97±4 | 86±4 | 0.028 | 92±4 | 83±4 | 0.138 |
| Moderate | 122±7 | 106±7 | 0.124 | 122±9 | 119±9 | 0.798 |
| Vigorous | 1±1 | 1±1 | 0.684 | 1±1 | 1±1 | 0.476 |
| At least 150 min MVPA per week | 1 (ref) | 0.94 (0.23–3.77) | 0.925 | 1 (ref) | 1.45 (0.39–5.42) | 0.576 |
| Intensity of PA (% of daily time) | ||||||
| Sedentary | 73.8±1.1 | 77.1±1.1 | 0.035 | 74.3±1.2 | 74.7±1.3 | 0.812 |
| Light | 11.7±0.4 | 10.2±0.4 | 0.011 | 11.1±0.4 | 10.3±0.4 | 0.280 |
| Moderate | 14.4±0.8 | 12.5±0.8 | 0.105 | 14.5±1.1 | 14.7±1.1 | 0.897 |
| Vigorous | 0.2±0.1 | 0.1±0.1 | 0.679 | 0.1±0.1 | 0.2±0.1 | 0.232 |
Results are expressed as mean±SEM for continuous variables and as OR (95% CI) for categorical variables. Statistical analysis by analysis of variance for continuous variables and by logistic regression for categorical variables, adjusted for sex (male, female), age (continuous), BMI categories (normal, overweight, obese), smoking status (never, former, current), educational level (low, medium, high). PA data were assessed using the GENEActiv macro file ‘general physical activity’ V.1.9.
BMIbody mass indexMVPAmoderate and vigorous physical activityPA, physical activity
online supplemental table 4 shows the bivariate analysis between controlled and uncontrolled participants of follow-ups 2 and 3, for objectively assessed PA, using the R-package GGIR. Overall, less than 25% of participants were compliant with the 150 min/week of MPA+VPA. Participants spent approximately one-quarter of an hour per day in MPA, and 90% of their time in SB. In the second survey, uncontrolled participants had higher levels and percentages of LPA and MPA, and a lower level and percentage of SB. After multivariable adjustment, no significant differences were observed (online supplemental table 5).
The results of the sensitivity analysis using multivariable linear regression are provided in online supplemental tables 6–8. Besides a significant negative association between LPA and glucose levels in the second follow-up for PA assessed by the MACRO procedure, which was not confirmed in the third follow-up, no other association between PA levels and glucose levels was found.
Sedentary behaviour and physical activity levels according to diabetes control as per glycated haemoglobin
The results of the bivariate analysis of the objectively assessed PA levels using the GENEActiv macro between controlled and uncontrolled participants for the second and the third follow-ups are presented in online supplemental table 9. Almost two-thirds of participants were considered as uncontrolled. No differences were found between controlled and uncontrolled participants in bivariate and multivariable analyses (table 3).
Table 3. Multivariable analysis, objectively assessed PA by control group as defined by glycated haemoglobin, stratified by survey: CoLaus|PsyCoLaus study, Lausanne, Switzerland.
| Second survey (2014–2017) | Third survey (2018–2021) | |||||
| Not controlled | Controlled | P value | Not controlled | Controlled | P value | |
| Sample size | 123 | 76 | 95 | 56 | ||
| Intensity of PA (min/day) | ||||||
| Sedentary | 613±10 | 636±13 | 0.172 | 599±13 | 592±17 | 0.736 |
| Light | 94±3 | 87±4 | 0.216 | 89±4 | 85±5 | 0.543 |
| Moderate | 118±6 | 106±8 | 0.253 | 123±8 | 118±10 | 0.725 |
| Vigorous | 1±1 | 1±1 | 0.978 | 1±1 | 1±1 | 0.445 |
| At least 150 min MVPA per week | 1 (ref) | 1.54 (0.41–5.79) | 0.525 | 1 (ref) | 1.11 (0.29–4.33) | 0.879 |
| Intensity of PA (% of daily time) | ||||||
| Sedentary | 74.6±1.0 | 76.9±1.2 | 0.143 | 74.4±1.1 | 74.8±1.5 | 0.821 |
| Light | 11.3±0.3 | 10.4±0.4 | 0.108 | 10.8±0.4 | 10.5±0.5 | 0.798 |
| Moderate | 14.0±0.7 | 12.6±0.9 | 0.225 | 14.7±0.9 | 14.5±1.2 | 0.884 |
| Vigorous | 0.1±0.1 | 0.1±0.1 | 0.925 | 0.1±0.1 | 0.2±0.1 | 0.394 |
Results are expressed as mean±SEM for continuous variables and as OR (95% CI) for categorical variables. Statistical analysis by analysis of variance for continuous variables and by logistic regression for categorical variables, adjusted for sex (male, female), age (continuous), BMI categories (normal, overweight, obese), smoking status (never, former, current), educational level (low, medium, high). PA data assessed using the GENEActiv macro file ‘general physical activity’ V.1.9.
BMIbody mass indexMVPAmoderate and vigorous physical activityPA, physical activity
The results of the bivariate and multivariable analysis of the objectively assessed PA levels using the R-package GGIR between controlled and uncontrolled participants for the second and the third follow-ups are presented in online supplemental table 10 (bivariate) and online supplemental table 11 (multivariable). Almost two-thirds of participants were considered as uncontrolled. No differences were found between controlled and uncontrolled participants in bivariate and multivariable analyses.
The results of the sensitivity analysis using multivariable linear regression are provided in online supplemental tables 12 and 13. No significant association between PA levels and glycated haemoglobin was found.
Discussion
Our results show that over half of participants treated for type 2 diabetes are not controlled. They also show that neither self-reported nor objectively assessed PA levels differ according to diabetes control.
Characteristics of participants
Overall, participants with controlled T2DM represented less than half of the participants in each of the three follow-ups. These values are lower than those reported in most European countries.17 18 The reasons for such a low control are not easily identifiable: no differences were found between controlled and uncontrolled participants for almost all covariates analysed, and a previous study showed no differences in dietary intakes.19 Hence, the factors associated with T2DM control may be less effective healthcare or differences in PA levels, which will be detailed in the next section. Overall, our results indicate that over half of treated diabetics do not achieve adequate control in this Swiss population-based sample.
Sedentary behaviour and physical activity levels according to diabetes control
PA is a cost-saving treatment.20 21 Patients with T2DM who regularly participate in aerobic exercise have a better control of their disease.22 According to Swiss and international guidelines, it is recommended to spend 150 min/week doing moderate-to-vigorous PA.23
In our study, participants with T2DM reported over 150 min/day of MPA. Our findings suggest that most participants with T2DM comply with PA recommendations, although a reporting bias cannot be excluded. Conversely, the results of the objectively assessed PA differed considerably according to the analytical method applied. According to the GENEActiv macro, almost all participants treated for T2DM were compliant with current PA recommendations, while according to the R-package GGIR this percentage was less than 25%. These differences between analytical methods have been reported previously24 and raise the importance of standardisation of PA accelerometry measurements.25
After multivariable adjustment, no differences were found between controlled and uncontrolled participants regarding all PA levels, either as absolute time or as % of day. Our findings agree with a study conducted in Poland, where no differences in both subjectively and objectively assessed PA levels were found between controlled and uncontrolled participants.26 Conversely, our findings do not replicate those of two other studies, which showed significant improvement in glycaemic control in participants with T2DM when regular PA was part of a healthy lifestyle.27 28 Possible explanations include the methods used to categorise participants. For instance, both studies used self-filled questionnaire to categorise participants into active and inactive, while ours used both subjective and objective PA assessment. It is likely that the relatively small sample size of our study led to a low statistical power, and we cannot exclude an indication bias, with participants with uncontrolled T2DM being recommended to exercise more frequently than those who are controlled.
PA levels differed considerably according to the methodology used. The differences between reported and objectively assessed PA are known,29 and the differences in PA levels according to the software used to process the accelerometry data have also been detected previously.24 Overall, our results indicate that the method to assess PA might considerably impact the associations between PA and cardiometabolic risk factors. Hence, care should be taken when comparing findings from studies that used different software to assess PA.
Female sex, older age, comorbidities such as obesity and depression, lack of motivation and social influence have been suggested to decrease adherence to PA.30 It would thus be useful to consider these barriers in subjects with T2DM when prescribing regular PA6 and consider routine activities as domestic chores to increase PA.31
Implications for clinical practice
Overall, our results suggest that people with diabetes exhibit the same PA behaviour irrespective of their fasting glucose or HbA1c levels. As PA is part of the management of T2DM,2 more emphasis should be put by clinicians to motivate their patients to be more active, different types of PA being effective.5 Still, doctors might not have either the time or the knowledge32 to adequately advise their patients regarding PA. Hence, postgraduate training regarding PA prescription is advised.33
Strengths and limitations
The major strengths of this study is the use of both subjectively and objectively assessed PA. It used two different software to analyse PA and two different criteria (fasting plasma glucose and glycated haemoglobin) to define T2DM. The results were replicated in two time points and a population-based sample was used.
This study also has some limitations. First, the study was conducted in a single location, and results might not be extrapolated to other settings, although similar findings were obtained elsewhere.26 Second, a possible selection bias might have occurred, as more motivated participants may accept to wear the accelerometer more easily. Hence, it is likely that the amounts of PA might be overestimated, but not the comparisons between controlled and uncontrolled participants. Third, the cross-sectional design of this study cannot address the question whether effective PA levels can efficiently help manage diabetes. Still, our results are similar to those reported elsewhere,34 and suggest that PA levels should be implemented among people with diabetes. Finally, the amounts of LPA, MPA and VPA differed considerably according to the analytical procedure applied. This issue has already been discussed24 and recommendations have been issued.24 35 Furthermore, the results between controlled and uncontrolled participants were identical irrespective of the analytical procedure applied.
Conclusion
In this population-based study focusing on participants treated for T2DM, no differences were found between controlled and uncontrolled T2DM regarding self-reported or objectively assessed PA levels.
supplementary material
Footnotes
Funding: The CoLaus|PsyCoLaus study was supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, the Swiss National Science Foundation (grants 3200B0-105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468, 33CS30-148401, 33CS30_177535 and 3247730_204523) and the Swiss Personalized Health Network (project: Swiss Ageing Citizen Reference).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-078929).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: The institutional Ethics Committee of the University of Lausanne, which afterwards became the Ethics Commission of Canton Vaud (www.cer-vd.ch) approved the baseline CoLaus study (reference 16/03). The approval was renewed for the first (reference 33/09), the second (reference 26/14) and the third (reference PB_2018-00040) follow-ups. The approval for the entire CoLaus|PsyCoLaus study was confirmed in 2021 (reference PB_2018-00038, 239/09). Participants gave informed consent to participate in the study before taking part.
Data availability free text: The CoLaus|PsyCoLaus cohort data used in this study cannot be fully shared as they contain potentially sensitive patient information. As discussed with the competent authority, the Research Ethic Committee of the Canton of Vaud, transferring or directly sharing these data would be a violation of the Swiss legislation aiming to protect the personal rights of participants. Non-identifiable, individual-level data are available for interested researchers, who meet the criteria for access to confidential data sharing, from the CoLaus Datacenter (CHUV, Lausanne, Switzerland). Instructions for gaining access to the CoLaus data used in this study are available at https://www.colaus-psycolaus.ch/professionals/how-to-collaborate/
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Gaël VonLanthen, Email: Gael.vonlanthen@unil.ch.
Pedro Marques-Vidal, Email: pedro.marquesvidal@gmail.com.
Data availability statement
Data may be obtained from a third party and are not publicly available.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 3.Moghetti P, Balducci S, Guidetti L, et al. Walking for subjects with type 2 diabetes: a systematic review and joint AMD/SID/SISMES evidence-based practical guideline. Nutr Metab Cardiovasc Dis. 2020;30:1882–98. doi: 10.1016/j.numecd.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62:98–103. doi: 10.1016/j.rehab.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Pan B, Ge L, Xun Y-Q, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act . 2018;15:72. doi: 10.1186/s12966-018-0703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilafranca Cartagena M, Tort-Nasarre G, Rubinat Arnaldo E. Barriers and facilitators for physical activity in adults with type 2 diabetes mellitus: a scoping review. Int J Environ Res Public Health. 2021;18:5359. doi: 10.3390/ijerph18105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bacquer D, Astin F, Kotseva K, et al. Poor adherence to lifestyle recommendations in patients with coronary heart disease: results from the EUROASPIRE surveys. Eur J Prev Cardiol. 2022;29:383–95. doi: 10.1093/eurjpc/zwab115. [DOI] [PubMed] [Google Scholar]
- 8.International Diabetes Federation . IDF Diabetes Atlas. Tenth Edition. Brussels, Belgium: 2021. [Google Scholar]
- 9.Firmann M, Mayor V, Vidal PM, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guessous I, Gaspoz J-M, Theler J-M, et al. Eleven-year physical activity trends in a Swiss urban area. Prev Med. 2014;59:25–30. doi: 10.1016/j.ypmed.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of Physical Activities: an update of activity codes and MET intensities. Medicine & Science in Sports & Exercise. 2000;32:S498–516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Marques-Vidal P. Sleep well, but be active. Effect of sleep and sedentariness on incidence of diabetes. Prim Care Diabetes. 2023;17:454–9. doi: 10.1016/j.pcd.2023.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy S, Fuller H, Chau J, et al. Accelerometer-derived physical activity in those with cardio-metabolic disease compared to healthy adults: a UK Biobank study of 52,556 participants. Acta Diabetol. 2018;55:975–9. doi: 10.1007/s00592-018-1161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esliger DW, Rowlands AV, Hurst TL, et al. Validation of the GENEA Accelerometer. Med Sci Sports Exerc. 2011;43:1085–93. doi: 10.1249/MSS.0b013e31820513be. [DOI] [PubMed] [Google Scholar]
- 15.van Hees VT, Sabia S, Anderson KN, et al. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLoS One. 2015;10:e0142533. doi: 10.1371/journal.pone.0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White T, Westgate K, Wareham NJ, et al. Estimation of Physical Activity Energy Expenditure during Free-Living from Wrist Accelerometry in UK Adults. PLoS One. 2016;11:e0167472. doi: 10.1371/journal.pone.0167472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barreto M, Kislaya I, Gaio V, et al. Prevalence, awareness, treatment and control of diabetes in Portugal: Results from the first National Health examination Survey (INSEF 2015) Diabetes Res Clin Pract. 2018;140:271–8. doi: 10.1016/j.diabres.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Kotseva K, De Backer G, De Bacquer D, et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: A report from the European Society of Cardiology EURObservational Research Programme EUROASPIRE V survey in 16 European countries. Eur J Prev Cardiol. 2021;28:370–9. doi: 10.1177/2047487320908698. [DOI] [PubMed] [Google Scholar]
- 19.Pauli A, Marques-Vidal P. Impact of diet on the management of cardiovascular risk factors. Clin Nutr Open Sci. 2021;40:50–68. doi: 10.1016/j.nutos.2021.10.004. [DOI] [Google Scholar]
- 20.Barbosa A, Whiting S, Ding D, et al. Economic evaluation of physical activity interventions for type 2 diabetes management: a systematic review. Eur J Public Health. 2022;32:i56–66. doi: 10.1093/eurpub/ckac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuti-Koyama KA, Monteiro HL, Ribeiro Lemes Í, et al. Impact of type 2 diabetes mellitus and physical activity on medication costs in older adults. Int J Health Plann Manage. 2019;34:e1774–82. doi: 10.1002/hpm.2892. [DOI] [PubMed] [Google Scholar]
- 22.Gao S, Tang J, Yi G, et al. The Therapeutic Effects of Mild to Moderate Intensity Aerobic Exercise on Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Trials. Diabetes Ther. 2021;12:2767–81. doi: 10.1007/s13300-021-01149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Rev Esp Cardiol (Engl Ed) 2020;73:404. doi: 10.1016/j.rec.2020.04.007. [DOI] [Google Scholar]
- 24.Verhoog S, Gubelmann C, Bano A, et al. Comparison of different software for processing physical activity measurements with accelerometry. Sci Rep. 2023;13:2879. doi: 10.1038/s41598-023-29872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee IM, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. 2014;48:197–201. doi: 10.1136/bjsports-2013-093154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mynarski W, Psurek A, Borek Z, et al. Declared and real physical activity in patients with type 2 diabetes mellitus as assessed by the International Physical Activity Questionnaire and Caltrac accelerometer monitor: a potential tool for physical activity assessment in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2012;98:46–50. doi: 10.1016/j.diabres.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Duclos M, Dejager S, Postel-Vinay N, et al. Physical activity in patients with type 2 diabetes and hypertension--insights into motivations and barriers from the MOBILE study. Vasc Health Risk Manag. 2015;11:361–71. doi: 10.2147/VHRM.S84832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Hong X, Wang C, et al. Joint associations of fresh fruit intake and physical activity with glycaemic control among adult patients with diabetes: a cross-sectional study. BMJ Open. 2022;12:e056776. doi: 10.1136/bmjopen-2021-056776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verhoog S, Gubelmann C, Guessous I, et al. Comparison of the Physical Activity Frequency Questionnaire (PAFQ) with accelerometry in a middle-aged and elderly population: The CoLaus study. Maturitas. 2019;129:68–75. doi: 10.1016/j.maturitas.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Alghafri TS, Alharthi SM, Al-Farsi Y, et al. Correlates of physical activity and sitting time in adults with type 2 diabetes attending primary health care in Oman. BMC Public Health. 2017;18:85. doi: 10.1186/s12889-017-4643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cloix L, Caille A, Helmer C. Physical activity at home, at leisure, during transportation and at work in French adults with type 2 diabetes: the ENTRED physical activity study. Diabetes Metab. 2015;41:37–44. doi: 10.1016/j.diabet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien MW, Shields CA, Oh PI, et al. Health care provider confidence and exercise prescription practices of Exercise is Medicine Canada workshop attendees. Appl Physiol Nutr Metab. 2017;42:384–90. doi: 10.1139/apnm-2016-0413. [DOI] [PubMed] [Google Scholar]
- 33.Windt J, Windt A, Davis J, et al. Can a 3-hour educational workshop and the provision of practical tools encourage family physicians to prescribe physical activity as medicine? A pre-post study. BMJ Open. 2015;5:e007920. doi: 10.1136/bmjopen-2015-007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low J, Hesketh K, Little J, et al. In: 28th Annual Congress of the European College of Sports Science. Guilhem G, Rabita G, Brocherie F, editors. Paris, France: 2023. Physical activity levels and cardiometabolic markers in indviduals with newly diagnosed type 2 diabetes: a baseline analysis of the motivate t2d randomized controlled trial. [Google Scholar]
- 35.Moldovan IA, Bragg A, Nidhiry AS, et al. The Physical Activity Assessment of Adults With Type 2 Diabetes Using Accelerometer-Based Cut Points: Scoping Review. Interact J Med Res. 2022;11:e34433. doi: 10.2196/34433. [DOI] [PMC free article] [PubMed] [Google Scholar]