Abstract
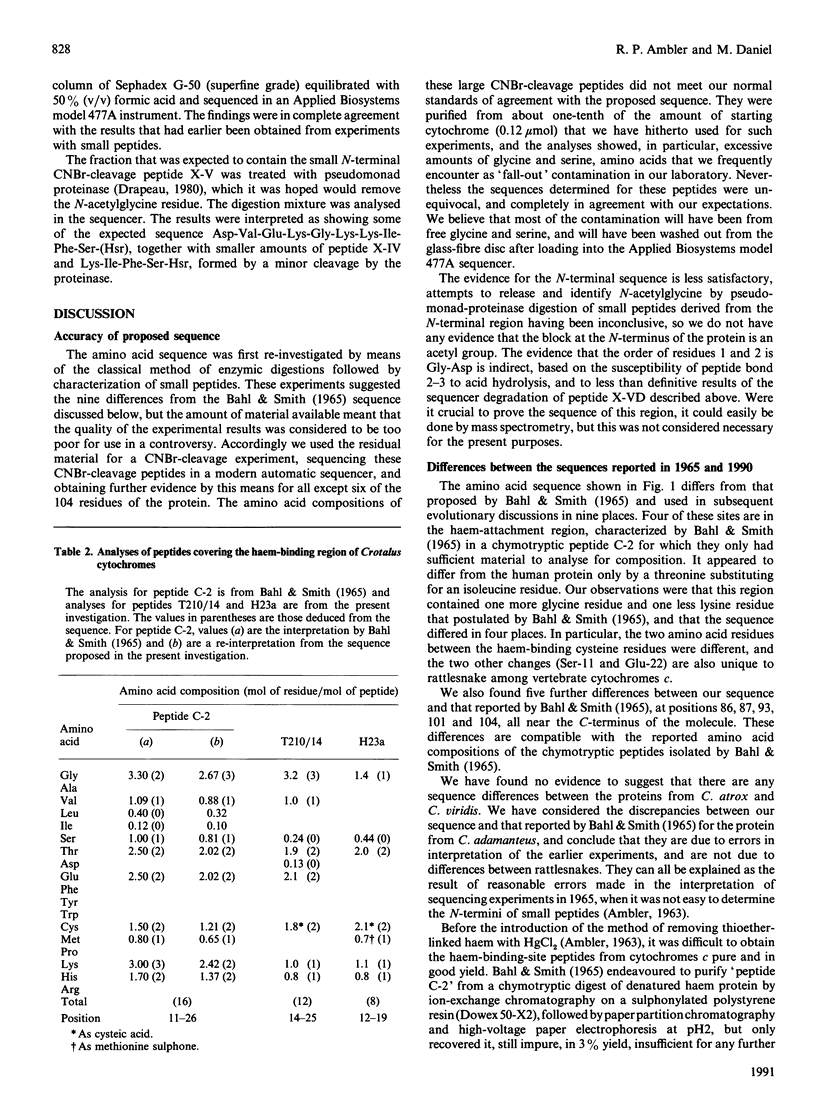
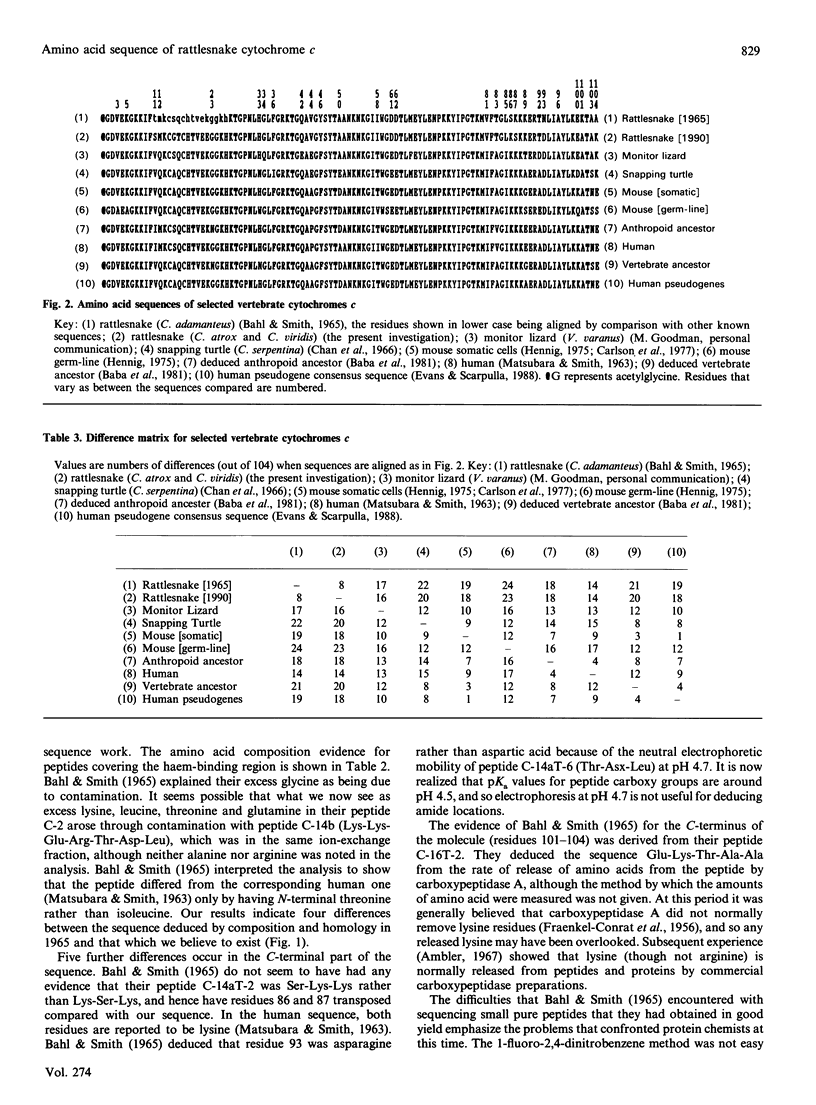
The amino acid sequence of rattlesnake cytochrome c was originally reported in 1965, and was one of the earlier sequences to be studied. When compared with other mitochondrial cytochromes c, the snake sequence was soon seen to be anomalous. There were several positions in which the snake protein resembled human cytochrome c, although comparable anomalies were not reported for the protein from other reptiles such as lizard and turtle. Explanations of these results have included accelerated evolution in the snake lineage, paralogy rather than orthology, and faulty determination of the sequence, and the rattlesnake is now often omitted from cytochrome c phylogenetic trees. We have re-investigated the sequence of the snake protein, and believe that the correct sequence differs in nine places from that used for evolutionary theorizing since 1965. Four of these differences are near the haem-attachment site, in a region that was only analysed for amino acid composition in the original investigation. The other five differences are towards the C-terminus of the molecule, and can be explained as being due to the wrong ordering of amino acids within peptides that had been satisfactorily purified. Despite these corrections, the rattlesnake cytochrome c sequence still more closely resembles human cytochrome c than it does that of any other protein we know. We believe that this is an example of convergent evolution, although it does appear that there has been accelerated change in the line connecting the rattlesnake to the ancestral vertebrate line. Detailed evidence for the amino acid sequence of the protein has been deposited as Supplementary Publication SUP 50162 (16 pages) at the British Library Document Supply Centre, Boston Spa. Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1991) 273, 5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Hermoso J., Meyer T. E., Bartsch R. G., Kamen M. D. Cytochrome c2 sequence variation among the recognised species of purple nonsulphur photosynthetic bacteria. Nature. 1979 Apr 12;278(5705):659–660. doi: 10.1038/278659a0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Melis K., Stout C. D. The amino acid sequence of the dihaem cytochrome c4 from the bacterium Azotobacter vinelandii. Biochem J. 1984 Aug 15;222(1):217–227. doi: 10.1042/bj2220217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Meyer T. E., Bartsch R. G., Kamen M. D. The amino acid sequence of cytochrome c' from the purple sulphur bacterium Chromatium vinosum. Biochem J. 1979 Mar 1;177(3):819–823. doi: 10.1042/bj1770819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Meyer T. E., Kamen M. D., Schichman S. A., Sawyer L. A reassessment of the structure of Paracoccus cytochrome c-550. J Mol Biol. 1981 Apr 5;147(2):351–356. doi: 10.1016/0022-2836(81)90445-9. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M. L., Darga L. L., Goodman M., Czelusniak J. Evolution of cytochrome C investigated by the maximum parsimony method. J Mol Evol. 1981;17(4):197–213. doi: 10.1007/BF01732758. [DOI] [PubMed] [Google Scholar]
- Bahl O. P., Smith E. L. Amino acid sequence of rattlesnake heart cytochrome c. J Biol Chem. 1965 Sep;240(9):3585–3593. [PubMed] [Google Scholar]
- CRICK F. H. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- Carlson S. S., Mross G. A., Wilson A. C., Mead R. T., Wolin L. D., Bowers S. F., Foley N. T., Muijsers A. O., Margoliash E. Primary structure of mouse, rat, and guinea pig cytochrome c. Biochemistry. 1977 Apr 5;16(7):1437–1442. doi: 10.1021/bi00626a031. [DOI] [PubMed] [Google Scholar]
- Chan S. K., Tulloss I., Margoliash E. Primary structure of the cytochrome c from the snapping turtle, Chelydra serpentina. Biochemistry. 1966 Aug;5(8):2586–2597. doi: 10.1021/bi00872a016. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R. Substrate specificity of a proteolytic enzyme isolated from a mutant of Pseudomonas fragi. J Biol Chem. 1980 Feb 10;255(3):839–840. [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Swen H. M., Wolthers B. G. Structure of papain. Nature. 1968 Jun 8;218(5145):929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. The human somatic cytochrome c gene: two classes of processed pseudogenes demarcate a period of rapid molecular evolution. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9625–9629. doi: 10.1073/pnas.85.24.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J. Sequence of the lacI gene. Nature. 1978 Aug 24;274(5673):765–769. doi: 10.1038/274765a0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Aspects of molecular evolution. Annu Rev Genet. 1973;7:343–380. doi: 10.1146/annurev.ge.07.120173.002015. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Gibbs A. J., McIntyre G. A. The diagram, a method for comparing sequences. Its use with amino acid and nucleotide sequences. Eur J Biochem. 1970 Sep;16(1):1–11. doi: 10.1111/j.1432-1033.1970.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Heller J., Smith E. L. Neurospora crassa cytochrome c. II. Chymotryptic peptides, tryptic peptides, cyanogen bromide peptides, and the complete amino acid sequence. J Biol Chem. 1966 Jul 10;241(13):3165–3180. [PubMed] [Google Scholar]
- Hennig B. Change of cytochrome c structure during development of the mouse. Eur J Biochem. 1975 Jun 16;55(1):167–183. doi: 10.1111/j.1432-1033.1975.tb02149.x. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. A reinvestigation of residues 64-68 and 175 in papain. Evidence that residues 64 and 175 are asparagine. Biochem J. 1970 Feb;116(4):689–692. doi: 10.1042/bj1160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H., Holmquist R. Evolutionary clock: nonconstancy of rate in different species. Science. 1972 Aug 11;177(4048):530–532. doi: 10.1126/science.177.4048.530. [DOI] [PubMed] [Google Scholar]
- LIGHT A., FRATER R., KIMMEL J. R., SMITH E. L. CURRENT STATUS OF THE STRUCTURE OF PAPAIN: THE LINEAR SEQUENCE, ACTIVE SULFHYDRYL GROUP, AND THE DISULFIDE BRIDGES. Proc Natl Acad Sci U S A. 1964 Nov;52:1276–1283. doi: 10.1073/pnas.52.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer F., Simon A. M. Neurospora crassa and Humicola lanuginosa cytochromes c: more homology in the heme region. Biochem Biophys Res Commun. 1974 Jan 23;56(2):317–323. doi: 10.1016/0006-291x(74)90844-4. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E. PRIMARY STRUCTURE AND EVOLUTION OF CYTOCHROME C. Proc Natl Acad Sci U S A. 1963 Oct;50:672–679. doi: 10.1073/pnas.50.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- MATSUBARA H., SMITH E. L. HUMAN HEART CYTOCHROME C. CHYMOTRYPTIC PEPTIDES, TRYPTIC PEPTIDES, AND THE COMPLETE AMINO ACID SEQUENCE. J Biol Chem. 1963 Aug;238:2732–2753. [PubMed] [Google Scholar]
- Mitchel R. E., Chaiken I. M., Smith E. L. The complete amino acid sequence of papain. Additions and corrections. J Biol Chem. 1970 Jul 25;245(14):3485–3492. [PubMed] [Google Scholar]
- Narita K., Chitani K. The complete amino acid sequence in baker's yeast cytochrome c. J Biochem. 1969 Feb;65(2):259–267. [PubMed] [Google Scholar]
- Scarpulla R. C., Agne K. M., Wu R. Cytochrome c gene-related sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):739–743. doi: 10.1073/pnas.79.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. B., Schilling J. W., Wilson A. C. Adaptive evolution in the stomach lysozymes of foregut fermenters. 1987 Nov 26-Dec 2Nature. 330(6146):401–404. doi: 10.1038/330401a0. [DOI] [PubMed] [Google Scholar]
- Timkovich R., Dickerson R. E., Margoliash E. Amino acid sequence of Paracoccus denitrificans cytochrome c550. J Biol Chem. 1976 Apr 25;251(8):2197–2206. [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]


