Abstract
Background Early-life exposure to famine may influence the occurrence of chronic diseases and aging in midlife among those exposed. This study aims to explore the relationship between exposure to the Chinese Great Famine and aging in middle-aged individuals. Methods Participants born in 1963–1965 (unexposed), 1959–1961 (in utero exposure), and 1955–1957 (childhood exposure) from the Kailuan Study were included. Their biological age at 2010, 2014, and 2018 was investigated, and age acceleration (biological age minus actual age) was calculated to assess aging. Logistic regression analysis was employed to describe the relationship between famine exposure and the aging risk. Subgroup and sensitivity analysis were conducted to explore differences and stability in this relationship among different groups. Results A total of 17,543 participants were included in this study. Among them, 12,762 (72.7%) were male, and 4,781 (27.3%) were female, with 2,543 participants experiencing aging events. Compared to unexposed participants, those exposed during childhood and in utero exhibited a 1.69-fold (OR = 1.69, 95%CI: 1.53–1.87) and 1.22-fold (OR = 1.22, 95%CI: 1.08–1.37) increased risk of aging. Subgroup analysis revealed an interaction with income (P for interaction = 0.008), and additional interaction analysis suggested that increasing income could partially mitigate the detrimental effects of early-life famine exposure. Furthermore, experiencing famine in severely affected regions exacerbated the risk of aging (OR = 1.41, 95%CI: 1.21–1.63). Conclusion Exposure to famine in utero or during childhood may elevate the risk of midlife aging among exposed individuals, and these relationships are influenced by the severity of famine exposure. Increasing income may also help mitigate these effects.
Trial registration: Kailuan study, ChiCTRTNRC11001489. Registered July 19, 2015 Retrospectively registered, https//www.chictr.org.cn/showprojEN.html?proj=8050&u_atoken=af46a0dee8d73f320bb5459ab7bbcfa9&u_asig=1a0c381017255295896468605e00cf .
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77283-z.
Keywords: Famine, Aging, Income, In utero, Childhood
Introduction
Aging, a process that has garnered considerable research interest in recent years, is characterized by a decline in the body’s immune function and an increased risk of complications [1]. A comprehensive understanding of the mechanisms and triggers that contribute to aging is pivotal for addressing the challenges posed by an aging population. Research has underscored the influence of nutritional status, physical activity, and external environmental factors on the initiation of aging [2]. Recent studies suggest that nutritional deficiencies during the intrauterine and childhood phases may precipitate an earlier onset and heightened risk of cerebral hemorrhage in individuals during middle age [3]. Furthermore, early-life adversities, such as natural disasters or conflicts, have been correlated with an increased prevalence of metabolic diseases and elevated systemic inflammation. Consequently, this research posits that events of early-life adversity may also have significant implications for the biological aging process.
Between 1959 and 1961, China endured the most catastrophic famine since World War II, marking a period of extreme hardship and widespread malnutrition that led to millions of fatalities. This three-year calamity not only resulted in an unprecedented loss of life due to starvation but also perpetuated a legacy of malnutrition and heightened disease vulnerability among the survivors [4]. The famine, epitomized by acute food scarcity, had enduring implications for the health outcomes of the affected populations. Notably, Clair et al. were the first to identify a correlation between famine exposure and an elevated risk of schizophrenia, laying the groundwork for subsequent investigations into the famine’s far-reaching health consequences [5]. In the aftermath, comprehensive studies have been rigorously conducted to elucidate the extensive impacts of this famine, contributing to a broader understanding of the interplay between early-life adversities and long-term health.
Based on this, we hypothesize that exposure to famine during the intrauterine and childhood periods may exacerbate the risk of aging in individuals during middle age. Despite the reduction in large-scale famines due to natural causes with socio-economic development, uneven distribution of nutritional resources persists, influenced by factors such as wars and public health events (e.g., COVID-19), leading to persistent malnutrition in early life [6, 7]. Therefore, this study aims to explore the relationship between famine exposure and the risk of aging in middle age through an extensive and large-scale prospective cohort study from northern China.
Methods
Study population
All participants in this study were derived from the Kailuan Study (registered at https://www.chictr.org.cn/, registration number: ChiCTR2000029767). Initiated in 2006, the Kailuan Study was originally launched by the Kailuan Group in Tangshan, with the aim of detecting and mitigating cardiovascular and cancer risks among the local population. The comprehensive research design has been delineated in preceding studies [8, 9]. Starting in 2006, the population in the region has been subjected to biennial health examinations. These examinations encompass general assessments, laboratory tests, imaging studies, and questionnaire surveys.
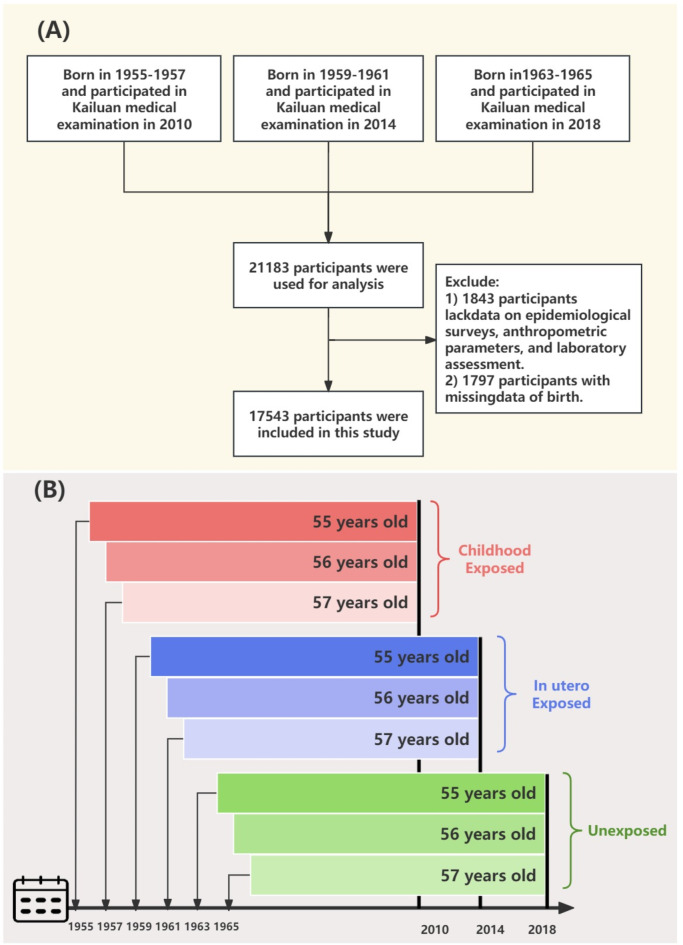
To ensure age homogeneity among the unexposed, in utero exposed, and childhood exposed groups (thereby mitigating the influence of age as a confounding factor), individuals born in the years 1963–1965, 1959–1961, and 1955–1957 were included, who participated in the health examinations conducted in 2010, 2014, and 2018. The initial cohort consisted of 21,183 participants. We excluded 1,843 individuals due to incomplete information regarding marital status, educational attainment, income level, smoking habits, and alcohol consumption. Furthermore, to accurately determine whether participants were born in regions severely affected by the famine, an additional 1,797 participants were excluded because of missing birthplace data (Fig. 1(A)). Additionally, imputation was performed for missing continuous variables, incorporating 823 participants with missing body mass index (BMI) data, 285 with missing waist circumference (WC) data, 47 with missing uric acid (UA) levels, and 96 with missing high-density lipoprotein cholesterol (HDL-C) values. All participants provided informed consent, and the study’s methodology was in strict compliance with the principles of the Helsinki Declaration.
Fig. 1.
(A) Flowchart depicting the study population in the Kailuan Study. (B) Detailed grouping methods for the study population in the current study.
Famine and severity
Participants were stratified based on their birth years relative to the Chinese famine’s timeframe (1959–1961), resulting in three distinct groups: the unexposed group (born in 1963–1965), the in utero exposed group (born in 1959–1961), and the childhood exposed group (born in 1955–1957) [10]. To mitigate the confounding effects of normal aging, follow-up was concluded at varying years for each cohort: 2018 for the unexposed group, 2014 for the in utero exposed group, and 2010 for the childhood exposed group. This strategy was employed to ensure that the age range of participants in each group was consistently controlled at 55–57 years. Figure 1 (B) provides detailed grouping methods for the study population in the current study.
While the initial study location of Tangshan did experience a significant famine, it is crucial to acknowledge that the extent of famine varied considerably across different regions in China. Drawing on methodologies outlined in prior research, we adopted a classification system for famine severity based on reported excess mortality rates across various provinces. Specifically, participants originating from provinces with an excess mortality rate below 50% were designated as experiencing non-severe famine exposure. In contrast, individuals born in provinces with a mortality rate exceeding 50% were identified as having been severely exposed. The provinces classified under the severely exposed category include Jilin, Guangdong, Jiangsu, Shandong, Fujian, Hubei, Liaoning, Guangxi, Hunan, Gansu, Henan, Sichuan, Qinghai, Guizhou, and Anhui [11]. However, it is important to note that even within these provinces, local variations in famine severity could exist.
Definition of outcome
The primary outcome of this study is aging, operationalized through the use of the Bioage package to construct a phenotypic age formula leveraging existing variables. Specifically, aging is defined by a phenoage advance greater than 0, where phenoage advance is calculated as the difference between Phenotypic age and chronological age: phenoage advance = Phenotypic age - chronological age. The calculation of phenoage and age acceleration involved several specific biomarkers and variables. The Phenotypic age itself is derived from a formula that integrates various biomarkers and chronological age, calculated as follows: First, a base value xb is determined by the equation: xb = − 12.579–0.0351 × albumin (Alb) – 0.0091 × lymph + 0.2931 × lnCRP + 0.0856 × glucose + 0.0104 × creatinine + 0.0743 × white blood cell count + 0.0827 × chronological age. The specific biomarkers included in the formula were Alb, lymph, CRP, glucose, creatinine, and white blood cell count. Subsequently, Phenotypic age is computed using 141.8 + ln [–0.00581 × (–1.37211 × exp(xb)) / 0.0071982] / 0.08742. This comprehensive approach allows for a nuanced analysis of aging by comparing biological markers of aging with chronological age, thereby quantifying the extent of biological aging beyond what is expected by chronological age alone.
Definition of the covariates
All participants in this study voluntarily agreed to have their laboratory data collected at Kailuan General Hospital. They visited the hospital after fasting for at least 8 h to undergo blood tests, including measurements of triglycerides (TG), HDL-C, fasting blood glucose (FBG), albumin (Alb), UA, hemoglobin (HB), and C-reactive protein (CRP). In conjunction with these tests, participants also completed routine questionnaire surveys, which captured information on age, sex, education level, type of employment, income, smoking status, alcohol consumption, and regular physical activity. Regular physical exercise is defined as engaging in physical activity at least 3 times per week, with each session lasting 30 min or more. Body measurements taken included waist circumference (WC), height, and weight. A normal WC is defined as less than 90 cm for men and less than 80 cm for women. The BMI was calculated by dividing the weight in kilograms (kg) by the square of the height in meters (m^2), with a BMI of 28 kg/m^2 or greater indicating obesity. Information on comorbidities, including hypertension, diabetes, and fatty liver, was also collected [12].
Statistical analysis
All statistical analyses were performed using SAS version 9.4 and R version 4.2.0. Two-sided P-values less than 0.05 were deemed statistically significant. Continuous variables were described as mean ± standard deviation or median (IQR) based on the distribution’s normality. Differences among groups were assessed using one-way Analysis of Variance (ANOVA) or non-parametric tests, contingent on the distribution normality of the data. Categorical variables were presented as numbers (percentages) and the differences between groups were evaluated using the chi-square test. The Bioage package facilitated the generation of the formula for calculating phenotypic age, thus determining the phenotypic age for each participant [13]. Logistic regression analysis was employed to elucidate the relationship between famine exposure and aging. Multivariate analysis adjusted for age, CRP, sex, type of employment, educational level, regular physical exercise, income, smoking, and alcohol consumption, hypertension, diabetes, WC, BMI, HDL-C, UA, Alb, and TG. Subgroup and interaction analyses were conducted to investigate the impact of famine exposure on aging across different population segments. Sensitivity analyses were carried out to ensure the robustness of the results, which included the exclusion of participants with significant nutritional variations, anemia, advanced age, and those diagnosed with cancer.
Results
Baseline characteristics of the study participants
A total of 17,543 participants were included from the Kailuan Study, with an average age of 54.60 ± 1.08 years. The cohort comprised 12,762 men (72.7%) and 4,781 women (27.3%). Among these, 2,543 participants exhibited aging events. As delineated in Table 1, those exposed to the famine during childhood or in utero demonstrated a higher phenotypic age and a larger proportion of males compared to the unexposed group. Additionally, these individuals were more likely to be engaged in physical labor and had lower levels of education and income. Behavioral patterns revealed that famine-exposed participants were more inclined to smoke and consume alcohol. Physically, they exhibited lower waist circumference and BMI values. From a laboratory perspective, these participants also showed reduced levels of TG.
Table 1.
Baseline characteristics.
| Groups | Unexposed | Childhood exposed | In utero exposed | P value |
|---|---|---|---|---|
| n | 5723 | 7766 | 4054 | |
| Age (mean (SD), years) | 54.37(1.15) | 54.53(0.94) | 54.93(0.98) | < 0.001 |
| Phenoage (mean (SD), years) | 48.38(6.78) | 49.59(6.35) | 49.61(6.25) | < 0.001 |
| Men (%) | 3873(67.7) | 6083(78.3) | 2806(69.2) | < 0.001 |
| Work type (Physical labor, %) | 1223(21.4) | 6966(89.7) | 3879(95.7) | < 0.001 |
| High school and above (%) | 2064(36.1) | 1239(16.0) | 606(14.9) | < 0.001 |
| Regular physical exercise (%) | 813(14.2) | 982(12.6) | 669(16.5) | < 0.001 |
| Income (> 3000 ¥, %) | 3648(63.7) | 4411(56.8) | 2669(65.8) | < 0.001 |
| Current smoker (%) | 1633(28.5) | 3304(42.5) | 1799(44.4) | < 0.001 |
| Current drinker (%) | 1005(17.6) | 1747(22.5) | 803(19.8) | < 0.001 |
| Fatty liver (%) | 2826(49.4) | 3535(45.5) | 1966(48.5) | < 0.001 |
| Hypertension (%) | 2814(49.2) | 3671(47.3) | 1723(42.5) | < 0.001 |
| Diabetes (%) | 882(15.4) | 865(11.1) | 668(16.5) | < 0.001 |
| WC (median [IQR], cm) | 89.0[83.0,94.0] | 88.0[82.0,95.0] | 86.0[82.0,92.0] | < 0.001 |
| BMI (median [IQR], kg/m2) | 25.08[23.37,27.44] | 24.90[22.86,27.06] | 24.80[22.86,27.03] | < 0.001 |
| HDL-C (median [IQR], mmol/L) | 1.44[1.23,1.67] | 1.48[1.22,1.83] | 1.31[1.09,1.51] | < 0.001 |
| TG (median [IQR], mmol/L) | 1.42[1.00,2.12] | 1.32[0.91,1.99] | 1.33[0.90,2.03] | < 0.001 |
| Uric acid (median [IQR], µmol/L) | 319.3[266.0,379.0] | 299.1[252.0,358.0] | 325.0[274.8,389.0] | < 0.001 |
| Hb (median [IQR], g/L) | 152.0[141.0,161.0] | 149.0[140.0,158.0] | 151.0[140.8,160.0] | < 0.001 |
Note: WC: waist circumference; BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride; Hb: hemoglobin.
Relationship between famine and aging
The logistic regression outcomes presented in Table 2 reveal that, compared to participants unexposed to famine, those exposed during childhood (OR = 1.69, 95%CI: 1.53–1.87) and in utero (OR = 1.22, 95%CI: 1.08–1.37) exhibited significantly increased risks of aging in the crude models. Even after adjusting for potential risk factors, the heightened risk persisted among participants exposed during childhood (OR = 1.67, 95%CI: 1.51–2.22) and in utero (OR = 1.46, 95%CI: 1.12–1.92).
Table 2.
The association between famine exposure and aging.
| Groups | Events/Total | Crude | Adjusted | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Unexposed | 641/5723 | Ref. | Ref. | |||
| Childhood exposed | 1363/7766 | 1.69(1.53,1.87) | < 0.001 | 1.67(1.25,2.22) | < 0.001 | |
| In utero exposed | 539/4054 | 1.22(1.08,1.37) | 0.002 | 1.46(1.12,1.92) | 0.006 | |
Note: Adjusted models included age, CRP, sex, work type, educational level, regular physical exercise, income, smoke, drink, hypertension, diabetes, WC, BMI, HDL, uric acid, Alb and TG.
CRP: C-reactive protein; WC: waist circumference; BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; Alb: albumin; TG: triglyceride.
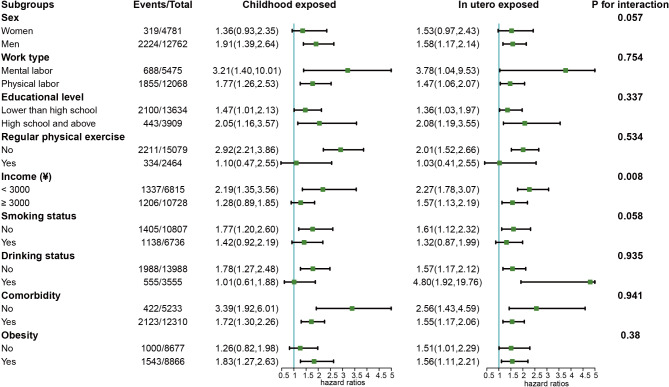
To delve deeper into the influence of famine exposure on aging across different demographics, subgroup analyses were conducted, considering variables such as gender, occupation, education level, regular physical exercise, income, smoking, drinking, comorbidities, waist circumference, and BMI (Fig. 2). These analyses revealed a consistent relationship across most subgroups, with income emerging as a significant modifier of the effect (p for interaction = 0.008). Specifically, among participants earning less than 3000 yuan, those exposed to famine during childhood (OR = 2.19, 95%CI: 1.35–3.56) and in utero (OR = 2.27, 95%CI: 1.78–3.07) faced an elevated risk of aging.
Fig. 2.
Subgroup analysis of the association between famine exposure and aging. Note: The model was adjusted for age, CRP, sex, work type, educational level, regular physical exercise, income, smoke, drink, hypertension, diabetes, WC, BMI, HDL, uric acid, Alb and TG, except for the stratified factors. CRP: C-reactive protein; WC: waist circumference; BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; Alb: albumin; TG: triglyceride.
Further additive joint analyses (Figure S1) underscored that, relative to participants with concurrent low income and famine exposure, the risk of aging decreased for those with low income but without famine exposure (OR = 0.55, 95%CI: 0.34–0.87), those with high income and famine exposure (OR = 0.51, 95%CI: 0.37–0.69), and those with high income without famine exposure (OR = 0.36, 95%CI: 0.21–0.61).
Severity of famine and sensitivity analysis
The differential impact of famine severity on aging risk was substantiated, as evidenced in Table S1. Participants from areas severely affected by famine exhibited a significantly increased risk of aging in comparison to those from areas with less severe famine exposure (OR = 1.41, 95%CI: 1.21–1.63). Moreover, the population was stratified into five distinct groups for further analysis. Relative to participants with no famine exposure, those exposed during childhood to areas of low severity did not experience a statistically significant increase in aging risk (OR = 1.36, 95%CI: 0.94–2.20). Conversely, individuals exposed during childhood to areas of high severity (OR = 1.58, 95%CI: 1.19–2.10), exposed in utero to low-severity (OR = 1.50, 95%CI: 1.15–1.97), and high-severity areas (OR = 2.92, 95%CI: 1.74–4.79) all demonstrated elevated risks of aging (Table 3).
Table 3.
Famine severity exposure and aging.
| Groups | Events/Total | OR (95% CI) | P value |
|---|---|---|---|
| Unexposed | 641/5723 | Ref. | |
| In childhood exposed and less severe region | 1308/7476 | 1.36 (0.94, 2.20) | 0.940 |
| In childhood exposed and severe region | 55/290 | 1.58 (1.19, 2.10) | 0.001 |
| In utero exposed and less severe region | 501/3871 | 1.50 (1.15, 1.97) | 0.003 |
| In utero exposed and severe region | 38/183 | 2.92 (1.74, 4.79) | < 0.001 |
Note: Models were adjusted for age, CRP, sex, work type, educational level, regular physical exercise, income, smoke, drink, hypertension, diabetes, WC, BMI, HDL, uric acid, Alb and TG.
CRP: C-reactive protein; WC: waist circumference; BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; Alb: albumin; TG: triglyceride.
Comprehensive sensitivity analyses were executed to ascertain the stability of these findings (Table S2). By excluding participants with significant nutritional variations, anemia, advanced age, and cancer diagnoses, the analyses affirmed the consistency of the primary outcomes. Both childhood and in utero exposures to famine conditions were linked with an augmented risk of aging in middle age, underscoring the lasting health implications of early-life famine exposure.
Discussion
This investigation leverages a prospective, repeated-measure cohort design, characterized by age homogeneity among participants but differing in terms of famine exposure. This study uniquely demonstrates that individuals exposed to famine during childhood or in utero exhibit a significantly elevated risk of aging in middle age, compared to those not exposed to famine. Notably, analyses stratified by income reveal that the adverse effects of famine exposure on aging are more pronounced in lower-income populations. This finding suggests that higher income levels may offer a protective buffer against the detrimental impacts of early-life famine exposure. Furthermore, the study elucidates that the severity of famine exposure is a critical factor, with more severe famine conditions correlating with an increased risk of aging. This research underscores the complex interplay between early-life environmental factors, socio-economic status, and long-term health outcomes, highlighting the importance of addressing social determinants of health in mitigating aging risks.
Recent research has illuminated the long-term health effects of famine exposure, and this study is one of the most comprehensive analyses on the relationship between exposure to the Chinese Great Famine and aging. Consistent with our prior findings, exposure during in utero (HR = 2.14, 95%CI: 1.78–2.56) and childhood (HR = 2.49, 95%CI: 2.16–2.88) is significantly associated with an increased risk of cancer and cancer-related mortality in adulthood [14]. We focused on middle-aged individuals (55–57 years old) to more accurately capture aging challenges [15, 16]. Comparing these outcomes globally, studies on the Leningrad Siege show persistent cardiovascular risks and aging effects [17]. Similarly, Cheng et al. found that Dutch Famine survivors experienced accelerated biological aging six decades later [18]. These findings underscore the universal and long-lasting impacts of early-life famine exposure on health, irrespective of geographical and cultural differences.
Beyond the scope of aging, studies have shown that early life famine exposure is linked to a higher prevalence of cardiovascular diseases in later life. Chen et al. highlighted the sex-specific effects of fetal exposure to the 1959–1961 Chinese famine, showing a significant association with an increased risk of adult hypertension, particularly in males [19]. Further supporting this, Meng et al. demonstrated that prenatal famine exposure is associated with specific adulthood obesity patterns and an increased risk of type 2 diabetes, further emphasizing the profound and lasting impact of early life nutritional deprivation on long-term health outcomes [20]. Research from the Netherlands indicates irreversible effects of prenatal famine exposure on cerebral blood perfusion, particularly in the prefrontal cortex of males, potentially mediated by metabolic parameters like mid-life blood glucose levels [21]. Furthermore, exposure during pregnancy is suggested to impact gland development significantly, affecting thyroid function and health by age 50 [22]. These findings underscore the heightened susceptibility to diseases in middle age among individuals exposed to famine during early life. A comprehensive review by Heike et al. reveals that such exposure induces DNA methylation changes in genes associated with neuronal, neuroendocrine, and immune functions, leading to genetic-level qualitative changes [23]. As individuals age, these methylation abnormalities grow exponentially, contributing to the deterioration in neurological and mental health, and ultimately accelerating aging across various bodily organs and systems [24].
The interaction analysis between famine exposure and income is noteworthy. Higher income levels may mitigate the adverse effects of early life famine exposure through several potential mechanisms. Improved nutrition and dietary diversity are likely significant factors, as higher income allows for better access to a variety of nutrient-rich foods, which is critical for long-term health and aging [25]. Access to quality healthcare also plays a crucial role; individuals with higher incomes can afford better healthcare services, enabling early detection and treatment of health issues, thereby reducing the risk of aging-related diseases [26]. Reduced stress is another important factor; financial stability associated with higher income reduces chronic stress, which is known to have a detrimental impact on health and accelerate aging [27]. Additionally, higher income may facilitate healthier living conditions and access to resources that promote overall well-being, further mitigating the impacts of early life adversity [28]. These findings align with previous studies that highlight the buffering effect of socio-economic status on health outcomes following early life adversities [29].
In essence, exposure to famine during childhood and in utero represents a critical phase of early-life malnutrition. Recent global survey research underscores that malnutrition is second only to congenital anomalies in contributing to the burden of non-fatal diseases among children, with a pronounced impact on infants and toddlers under the age of 5 [30]. The immediate repercussions of early malnutrition not only precipitate health issues in affected children but also levy an economic strain on their families. Preliminary research delineates a correlation between severe childhood malnutrition and liver dysfunction indicators, such as fatty degeneration and hypoalbuminemia. Early nutritional deficiencies are linked to significant impairments in hepatic peroxisomes and mitochondria, alongside compromised metabolic liver functions. Without timely intervention, these detriments exacerbate with age, fostering metabolic irregularities and precipitating premature aging [31].
These findings lend credence to the “catch-up growth” hypothesis posited by Barker et al., which intimates that early-life adversities imprint lasting vulnerabilities on infants. Fetal growth restrictions due to malnutrition may instigate catch-up growth in later stages, markedly elevating disease susceptibility in adulthood [32]. Intriguingly, interaction analysis reveals that the detrimental impacts of early famine exposure might be mitigated by subsequent increases in income, ostensibly linked to enhancements in life quality and dietary habits. This observation aligns with Meng et al.’s analysis of the Jia Dao Biobank data in China, which associates prenatal famine exposure with heightened cardiovascular disease risks, moderated by the participants’ subsequent urban or rural residencies (p for interaction < 0.001) [33].
This body of work underscores the pivotal role of nutritional catch-up and dietary quality improvement in diminishing disease risks among those who endured early famine exposure. Income fluctuations throughout life significantly influence malnutrition risk, with lower-income families experiencing more pronounced weight loss and nutritional deficiencies [34]. The China Health and Nutrition Survey further elucidates that higher income levels engender dietary diversity [35], highlighting the global challenge of nutritional intake inequality exacerbated by economic disparities. Consequently, even for individuals who experienced famine, enhancements in socioeconomic status may partially rectify the damage inflicted by early malnutrition [36].
This study offers valuable insights into the long-term effects of famine exposure, but several limitations must be acknowledged. First, the cohort is drawn from an industrial city in northern China, specifically Hebei, where famine conditions were relatively moderate. This regional specificity may limit the generalizability of our findings to areas with more extreme famine conditions. Second, the cohort predominantly comprises male participants, which may not fully reflect gender-specific responses to famine. Although no significant gender interaction was detected, this could be due to the study’s limited ability to capture gender differences, as prior research has suggested sex-based variations in metabolic responses to famine [37–40], particularly regarding biological aging [18]. To address this, we performed subgroup analyses by sex, finding that famine significantly accelerated biological aging in men but not in women. However, the interaction effect was not significant, possibly due to the smaller sample size of female participants. Additionally, the absence of detailed data on adult dietary habits limits our ability to explore how post-famine nutrition might influence long-term health outcomes. This gap complicates the interpretation of the nuanced relationship between early-life famine exposure and aging processes [41]. Lastly, while we utilized PhenoAge as our primary measure of biological aging [42], the study did not incorporate newer third-generation biomarkers like DunedinPace due to dataset limitations [43]. Future research should include these advanced biomarkers to enhance the reliability and comprehensiveness of findings across different biological aging measures.
Conclusion
In summary, this study highlights that early-life famine exposure, whether during in utero or childhood phases, is associated with an elevated risk of aging in midlife. These effects are influenced by factors such as income levels and famine severity. From a public health perspective, it is crucial for individuals with such exposures to maintain healthy dietary habits and lifestyle choices. Proactive management of these aspects can help mitigate age-related diseases. This underscores the need for targeted health interventions and policies to improve long-term health outcomes for populations with historical famine exposure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the staff and participants of the Kailuan Study for their important contributions.
Author contributions
Tao Ma: Methodology, Software, Writing- original draft; Xiao-Meng Hao: Writing- original draft; Xiao-Wei Zhang: Methodology, Software; Xin-Yu Liu: Supervision, Validation; Yi-Ming Wang: Validation, Resources, Visualization; Qing-Song Zhang: Validation, Supervision; Jin Zhang: Conceptualization, Supervision, Validation, Resources, Project administration.
Funding
This work was financially supported by grants from Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009 A) to Prof. Jin Zhang.
Data availability
Data will be made available upon reasonable request (Jin Zhang; E-mail: evergreen176700@163.com: ).
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Kailuan General Hospital and Tianjin Medical University Cancer Institute and Hospital and adhered to the principles of the Declaration of Helsinki. Participants or their legal representatives provided written informed consent. The Kailuan Study was retrospectively registered in the Chinese Clinical Trial Register (ChiCTR) on 24 August, 2011 (ChiCTR-TNRC-11001489; http://www.chictr.org.cn/showprojen.aspx?proj=8050).
Competing interests
The authors declare no competing interests.
Transparency statement
The manuscript’s guarantor affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Role of the funding source
The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Ma, Xiao-Meng Hao and Xiaowei Zhang contributed equally to this work.
References
- 1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G: Hallmarks of aging: An expanding universe. Cell 2023, 186(2):243–278. [DOI] [PubMed]
- 2. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E: From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571(7764):183–192. [DOI] [PMC free article] [PubMed]
- 3. Li Y, Li Y, Gurol ME, Liu Y, Yang P, Shi J, Zhuang S, Forman MR, Wu S, Gao X: In utero exposure to the Great Chinese Famine and risk of intracerebral hemorrhage in midlife. Neurology 2020, 94(19):e1996-e2004. [DOI] [PMC free article] [PubMed]
- 4. Jiang H, Yu Y, Li L, Xu W: Exposure to the Great Famine in Early Life and the Risk of Obesity in Adulthood: A Report Based on the China Health and Nutrition Survey. Nutrients 2021, 13(4). [DOI] [PMC free article] [PubMed]
- 5. St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P et al: Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. Jama 2005, 294(5):557–562. [DOI] [PubMed]
- 6. Bendavid E, Boerma T, Akseer N, Langer A, Malembaka EB, Okiro EA, Wise PH, Heft-Neal S, Black RE, Bhutta ZA: The effects of armed conflict on the health of women and children. Lancet (London, England) 2021, 397(10273):522–532. [DOI] [PMC free article] [PubMed]
- 7. Burgos R, García-Almeida JM, Matía-Martín P, Palma S, Sanz-Paris A, Zugasti A, Alfaro JJ, Fullana AA, Continente AC, Chicetru MJ et al: Malnutrition management of hospitalized patients with diabetes/hyperglycemia and COVID-19 infection. Reviews in endocrine & metabolic disorders 2022, 23(2):205–213. [DOI] [PMC free article] [PubMed]
- 8. Liu C, Liu T, Zhang Q, Jia P, Song M, Zhang Q, Ruan G, Ge Y, Lin S, Wang Z et al: New-Onset Age of Nonalcoholic Fatty Liver Disease and Cancer Risk. JAMA network open 2023, 6(9):e2335511. [DOI] [PMC free article] [PubMed]
- 9. Liu T, Liu CA, Zhang QS, Zhang Q, Wang YM, Song MM, Lin SQ, Deng L, Wu SL, Shi HP: Early-onset and later-onset cancer: trends, risk factors, and prevention in Northern China. Science China Life sciences 2024. [DOI] [PubMed]
- 10. Xu Y, Yi Q, Shan S, Zhou J, Li S, Hou L, Ye X, Ying J, Song P, An L: Chinese famine exposure in early life and metabolic obesity phenotype in middle age: Results from the China health and retirement longitudinal study. Frontiers in endocrinology 2022, 13:975824. [DOI] [PMC free article] [PubMed]
- 11. Wu S, Zhao X, Li L, Han X, Liu Q, Chen S, Geng H, VanEvery H, Zhang X: In Utero and Early-Childhood Exposure to the Great Chinese Famine and Adult Arterial Stiffness: A Cross-Sectional Study. Arteriosclerosis, thrombosis, and vascular biology 2024, 44(3):755–757. [DOI] [PubMed]
- 12. Liu T, Wang Y, Wang X, Liu C, Zhang Q, Song M, Song C, Zhang Q, Shi H: Habitually Skipping Breakfast Is Associated with the Risk of Gastrointestinal Cancers: Evidence from the Kailuan Cohort Study. Journal of general internal medicine 2023, 38(11):2527–2536. [DOI] [PMC free article] [PubMed]
- 13. Kwon D, Belsky DW: A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. GeroScience 2021, 43(6):2795–2808. [DOI] [PMC free article] [PubMed]
- 14. Zhang X, Wang G, Forman MR, Fu Q, Rogers CJ, Wu S, Gao X: In utero and childhood exposure to the Great Chinese Famine and risk of cancer in adulthood: the Kailuan Study. The American journal of clinical nutrition 2021, 114(6):2017–2024. [DOI] [PubMed]
- 15. Spiteri K, Broom D, Bekhet AH, de Caro JX, Laventure B, Grafton K: Barriers and Motivators of Physical Activity Participation in Middle-aged and Older-adults - A Systematic Review. Journal of aging and physical activity 2019, 27(4):929–944. [DOI] [PubMed]
- 16. Dmitrieva NI, Gagarin A, Liu D, Wu CO, Boehm M: Middle-age high normal serum sodium as a risk factor for accelerated biological aging, chronic diseases, and premature mortality. EBioMedicine 2023, 87:104404. [DOI] [PMC free article] [PubMed]
- 17. Rotar O, Moguchaia E, Boyarinova M, Kolesova E, Khromova N, Freylikhman O, Smolina N, Solntsev V, Kostareva A, Konradi A et al: Seventy years after the siege of Leningrad: does early life famine still affect cardiovascular risk and aging?Journal of hypertension 2015, 33(9):1772–1779; discussion 1779. [DOI] [PubMed]
- 18. Cheng M, Conley D, Kuipers T, Li C, Ryan C, Taubert J, Wang S, Wang T, Zhou J, Schmitz LL et al: Accelerated biological aging six decades after prenatal famine exposure. medRxiv : the preprint server for health sciences 2023. [DOI] [PMC free article] [PubMed]
- 19. Chen H, Nembhard WN, Stockwell HG: Sex-specific effects of fetal exposure to the 1959–1961 Chinese famine on risk of adult hypertension. Maternal and child health journal 2014, 18(3):527–533. [DOI] [PubMed]
- 20. Meng R, Lv J, Yu C, Guo Y, Bian Z, Yang L, Chen Y, Zhang H, Chen X, Chen J et al: Prenatal famine exposure, adulthood obesity patterns and risk of type 2 diabetes. International journal of epidemiology 2018, 47(2):399–408. [DOI] [PMC free article] [PubMed]
- 21. de Rooij SR, Mutsaerts H, Petr J, Asllani I, Caan MWA, Groot P, Nederveen AJ, Schwab M, Roseboom TJ: Late-life brain perfusion after prenatal famine exposure. Neurobiology of aging 2019, 82:1–9. [DOI] [PubMed]
- 22. Keestra SM, Motoc I, Ravelli ACJ, Roseboom TJ, Finken MJJ: Thyroid Function at Age Fifty After Prenatal Famine Exposure in the Dutch Famine Birth Cohort. Frontiers in endocrinology 2022, 13:836245. [DOI] [PMC free article] [PubMed]
- 23. Eichenauer H, Ehlert U: The association between prenatal famine, DNA methylation and mental disorders: a systematic review and meta-analysis. Clinical epigenetics 2023, 15(1):152. [DOI] [PMC free article] [PubMed]
- 24. Wang K, Liu H, Hu Q, Wang L, Liu J, Zheng Z, Zhang W, Ren J, Zhu F, Liu GH: Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal transduction and targeted therapy 2022, 7(1):374. [DOI] [PMC free article] [PubMed]
- 25. Zhang J, Zhao A: Dietary Diversity and Healthy Aging: A Prospective Study. Nutrients 2021, 13(6). [DOI] [PMC free article] [PubMed]
- 26. De Vogli R, Gimeno D, Kivimaki M: Socioeconomic inequalities in health in 22 European countries. The New England journal of medicine 2008, 359(12):1290; author reply 1290–1291. [DOI] [PubMed]
- 27. Schneiderman N, Ironson G, Siegel SD: Stress and health: psychological, behavioral, and biological determinants. Annual review of clinical psychology 2005, 1:607–628. [DOI] [PMC free article] [PubMed]
- 28. Braveman P, Egerter S, Williams DR: The social determinants of health: coming of age. Annual review of public health 2011, 32:381–398. [DOI] [PubMed]
- 29. Ben-Shlomo Y, Kuh D: A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International journal of epidemiology 2002, 31(2):285–293. [PubMed]
- 30. Guthold R, White Johansson E, Mathers CD, Ross DA: Global and regional levels and trends of child and adolescent morbidity from 2000 to 2016: an analysis of years lost due to disability (YLDs). BMJ global health 2021, 6(3). [DOI] [PMC free article] [PubMed]
- 31. van Zutphen T, Ciapaite J, Bloks VW, Ackereley C, Gerding A, Jurdzinski A, de Moraes RA, Zhang L, Wolters JC, Bischoff R et al: Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. Journal of hepatology 2016, 65(6):1198–1208. [DOI] [PubMed]
- 32. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ: Weight in infancy and death from ischaemic heart disease. Lancet (London, England) 1989, 2(8663):577–580. [DOI] [PubMed]
- 33. Meng R, Yu C, Guo Y, Bian Z, Si J, Nie J, Yang L, Chen Y, Du H, Zhou L et al: Early famine exposure and adult disease risk based on a 10-year prospective study of Chinese adults. Heart (British Cardiac Society) 2020, 106(3):213–220. [DOI] [PMC free article] [PubMed]
- 34. Kosec K, Song J: The effects of income fluctuations on undernutrition and overnutrition across the lifecycle. Health economics 2021, 30(10):2487–2509. [DOI] [PubMed]
- 35. Ren Y, Li H, Wang X: Family income and nutrition-related health: Evidence from food consumption in China. Social science & medicine (1982) 2019, 232:58–76. [DOI] [PubMed]
- 36. Turner C, Kalamatianou S, Drewnowski A, Kulkarni B, Kinra S, Kadiyala S: Food Environment Research in Low- and Middle-Income Countries: A Systematic Scoping Review. Advances in nutrition (Bethesda, Md) 2020, 11(2):387–397. [DOI] [PMC free article] [PubMed]
- 37. Cheng M, Sommet N, Kerac M, Jopp DS, Spini D: Exposure to the 1959–1961 Chinese famine and risk of non-communicable diseases in later life: A life course perspective. PLOS global public health 2023, 3(8):e0002161. [DOI] [PMC free article] [PubMed]
- 38. Yu C, Wang J, Wang F, Han X, Hu H, Yuan J, Miao X, Yao P, Wei S, Wang Y et al: Victims of Chinese famine in early life have increased risk of metabolic syndrome in adulthood. Nutrition (Burbank, Los Angeles County, Calif) 2018, 53:20–25. [DOI] [PubMed]
- 39. Yao WY, Li L, Jiang HR, Yu YF, Xu WH: Transgenerational associations of parental famine exposure in early life with offspring risk of adult obesity in China. Obesity (Silver Spring, Md) 2023, 31(1):279–289. [DOI] [PubMed]
- 40. Zhang Y, Pu J, Ding Y, Wu L, Yin Y, Sun M, Gu Y, Zhang D, Zhang Z, Zheng Q et al: Sex Differences at Early Old Stage in Glycolipid Metabolism and Fatty Liver in Offspring Prenatally Exposed to Chinese Great Famine. Frontiers in nutrition 2022, 9:913966. [DOI] [PMC free article] [PubMed]
- 41. Mohajeri MH: Nutrition and Aging. International journal of molecular sciences 2023, 24(11). [DOI] [PMC free article] [PubMed]
- 42. Gao X, Geng T, Jiang M, Huang N, Zheng Y, Belsky DW, Huang T: Accelerated biological aging and risk of depression and anxiety: evidence from 424,299 UK Biobank participants. Nature communications 2023, 14(1):2277. [DOI] [PMC free article] [PubMed]
- 43. Belsky DW, Caspi A, Corcoran DL, Sugden K, Poulton R, Arseneault L, Baccarelli A, Chamarti K, Gao X, Hannon E et al: DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife 2022, 11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request (Jin Zhang; E-mail: evergreen176700@163.com: ).