Abstract
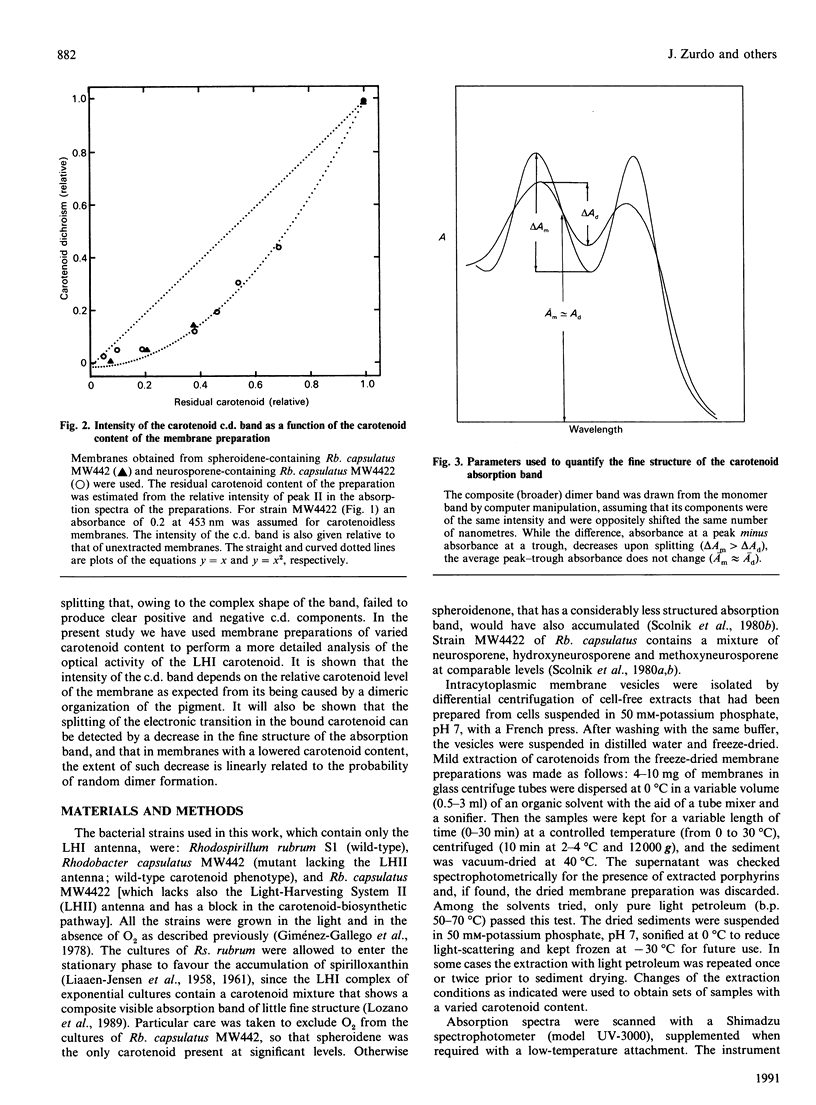
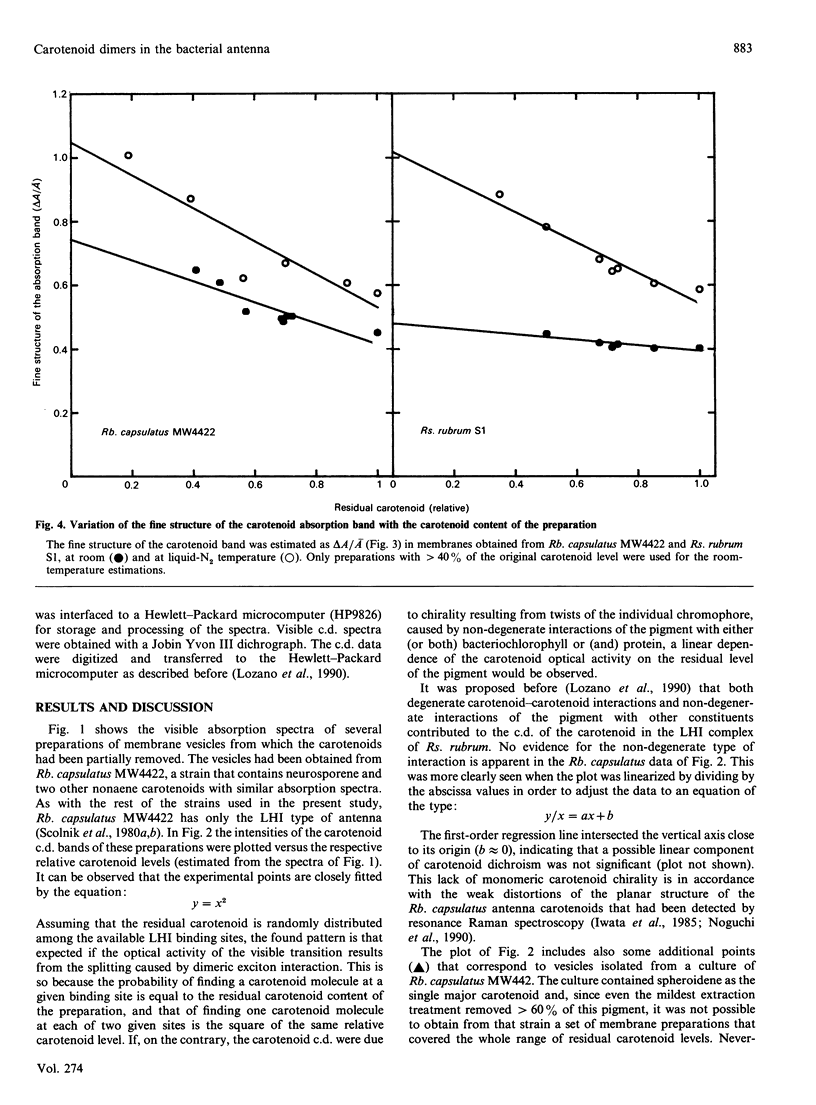
The carotenoid content of intracytoplasmic membrane vesicles isolated from purple phototrophic bacteria was reduced to a variable extent by mild extraction with light petroleum. Using preparations obtained from Rhodobacter capsulatus strains that contained the Light Harvesting System I (LHI) complex as the only major photosynthetic holochrome, it was shown that the visible circular dichroism of the carotenoids increased with the square of the membrane carotenoid content, as expected from being caused by dimeric exciton interaction. No chirality resulting from twists of the individual planar chromophore was detected. Therefore the contribution to carotenoid optical activity of non-degenerate interactions with bacteriochlorophyll or the apoprotein does not appear to be significant. The broadening of the absorption band of the bound pigment, caused by the splitting of the monomer transition, was demonstrated in membrane vesicles of both Rb, capsulatus and Rhodospirillum rubrum as a decrease of the fine structure of the band. Furthermore, the dimeric organization of the carotenoid pigments in the bacterial LHI complex accounted for the observed quantitative relationship between the fine structure of the band and the carotenoid content of the membrane.
Full text
PDF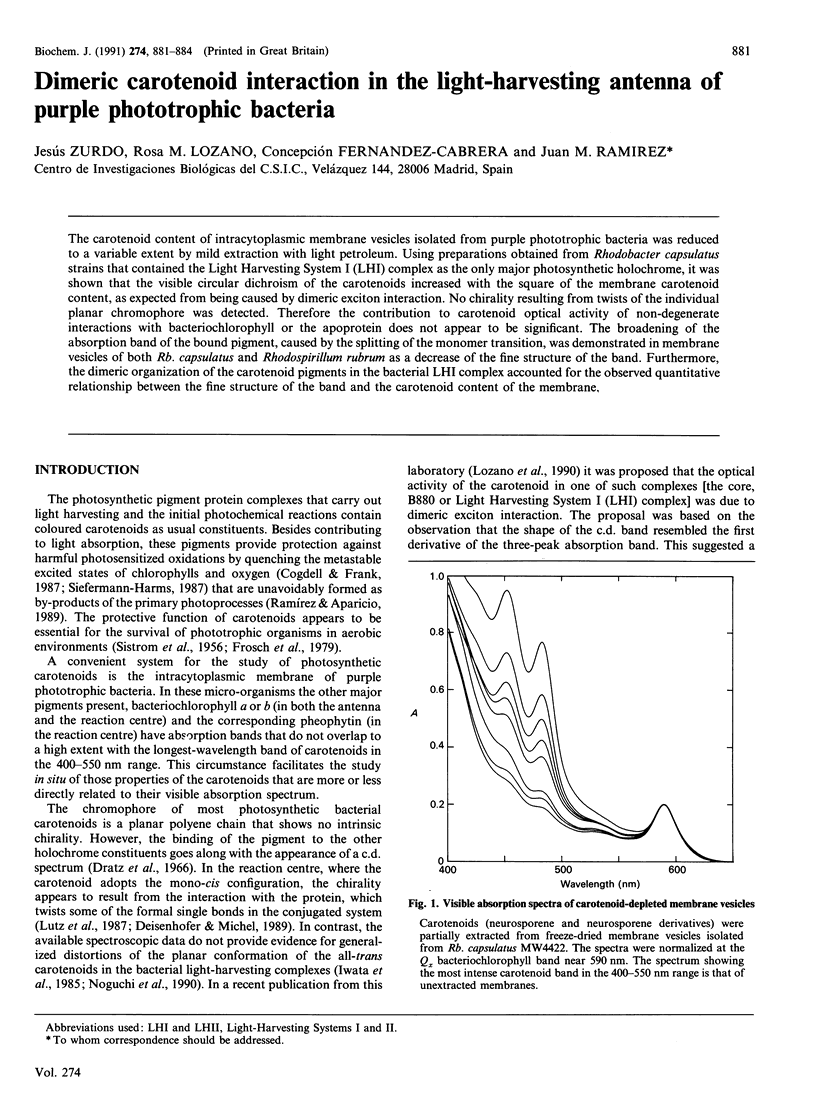

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cogdell R. J., Frank H. A. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895(2):63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratz E. A., Schultz A. J., Sauer K. Chlorophyll-chlorophyll interactions. Brookhaven Symp Biol. 1966;19:303–318. [PubMed] [Google Scholar]
- JENSEN S. L., COHEN-BAZIRE G., NAKAYAMA T. O., STANIER R. Y. The path of carotenoid synthesis in a photosynthetic bacterium. Biochim Biophys Acta. 1958 Sep;29(3):477–498. doi: 10.1016/0006-3002(58)90003-9. [DOI] [PubMed] [Google Scholar]
- JENSEN S. L., COHEN-BAZIRE G., STANIER R. Y. Biosynthesis of carotenoids in purple bacteria: a reevaluation based on considerations of chemical structure. Nature. 1961 Dec 23;192:1168–1172. doi: 10.1038/1921168a0. [DOI] [PubMed] [Google Scholar]
- Lozano R. M., Fernández-Cabrera C., Ramírez J. M. The contribution of the carotenoid to the visible circular dichroism of the light-harvesting antenna of Rhodospirillum rubrum. Biochem J. 1990 Sep 1;270(2):469–472. doi: 10.1042/bj2700469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., GRIFFITHS M., STANIER R. Y. The biology of photosynthetic bacterium which lacks colored carotenoids. J Cell Physiol. 1956 Dec;48(3):473–515. doi: 10.1002/jcp.1030480309. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Walker M. A., Marrs B. L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J Biol Chem. 1980 Mar 25;255(6):2427–2432. [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]


