Abstract
MYCN-amplified RB1 wild-type (MYCNampRB1+/+) retinoblastoma is a rare and aggressive subtype, often resistant to standard therapies. Identifying unique MRI features is crucial for diagnosing this subtype, as biopsy is not recommended. This study aimed to differentiate MYCNampRB1+/+ from the most prevalent RB1-/- retinoblastoma using pretreatment MRI and radiomics. Ninety-eight unilateral retinoblastoma patients (19 MYCN cases and 79 matched controls) were included. Tumors on T2-weighted MR images were manually delineated and validated by experienced radiologists. Radiomics analysis extracted 120 features per tumor. Several combinations of feature selection methods, oversampling techniques and machine learning (ML) classifiers were evaluated in a repeated fivefold cross-validation machine learning pipeline to yield the best-performing prediction model for MYCN. The best model used univariate feature selection, data oversampling (duplicating MYCN cases), and logistic regression classifier, achieving a mean AUC of 0.78 (SD 0.12). SHAP analysis highlighted lower sphericity, higher flatness, and greater gray-level heterogeneity as predictive for MYCNampRB1+/+ status, yielding an AUC of 0.81 (SD 0.11). This study shows the potential of MRI-based radiomics to distinguish MYCNampRB1+/+ and RB1-/- retinoblastoma subtypes.
Keywords: Retinoblastoma, MRI; radiomics , MYCN-amplification
Subject terms: Paediatric cancer, Eye cancer, Head and neck cancer, Cancer imaging, Cancer genetics
Introduction
Retinoblastoma is the most prevalent malignant ocular neoplasm in pediatric patients with an incidence of 1:17,000 live births and is typically diagnosed before the age of five1. The initiation of tumorigenesis primarily involves biallelic inactivation of the RB1 tumor suppressor gene, resulting in RB1-/- retinoblastoma. It has been established that distinct molecular subtypes of retinoblastoma exist, offering potential avenues for individualized therapeutic interventions2–8.
In 2013 a subtype of retinoblastoma was identified, characterized by a significant amplification of MYCN in the tumor without detectable pathogenic variants in the RB1 alleles(MYCNampRB1+/+). In this subtype, MYCN amplification was identified as the key driver in initiating retinal tumors, observed in approximately 1–2% of retinoblastoma cases9–11. Although MYCN amplifications can also be found in RB1-/- tumors, RB1 wild-type (RB1+/+) tumors with MYCN amplification (MYCNampRB1+/+) show a clear inclination and exhibiting unique clinical characteristics12,13. A previous study revealed that this MYCNampRB1+/+ retinoblastoma manifests as unilateral large tumors at an early age, displaying distinctive histopathological features characterized by poorly differentiated neuroblastic cells and limited rosette formations9. Moreover, this subtype demonstrated relative resistance to conventional therapeutic interventions14. The diagnosis of retinoblastoma in children under 12 months of age frequently indicates an inherited condition with increased risk of bilateral disease and subsequent significant loss of vision, which enhances efforts to pursue eye-saving therapeutic approaches. However, caution must be exercised when considering eye salvage in this aggressive subtype of retinoblastoma. Hence, early identification of patients with MYCNampRB1+/+ retinoblastoma holds significant clinical importance, as it may facilitate the implementation of a tailored treatment strategy.
Clinical detection of genetic characteristics in retinoblastoma is challenging due to limited availability of histopathologic material for molecular testing. Tumor biopsy is contraindicated due to the risks of local tumor seeding and metastasis. Additionally, there is a decreasing availability of enucleated specimens as eye-saving treatment options are more commonly employed, even in advanced-stage cases15,16. Therefore, the development of methods to stratify tumor subtypes in-vivo and implement targeted therapies is crucial. A promising technique involves obtaining cell-free tumor DNA from the aqueous humor17, however this approach requires an invasive procedure and is not yet in widespread use. Another emerging, and non-invasive technique, is magnetic resonance imaging (MRI), which is widely utilized in retinoblastoma for diagnostic support, disease staging, and screening for intracranial trilateral disease18–20. Qualitative analysis of MR images showed MYCNampRB1+/+ retinoblastoma can be differentiated from the subtype RB-/- retinoblastoma in a case–control study with very young unilaterally affected retinoblastoma patients21. The MYCN-amplified RB1 wild-type more frequently exhibits specific features such as a peripheral location, peritumoral hemorrhage, subretinal hemorrhage with fluid–fluid level and retinal folding with vitreous enclosure21. In addition to qualitative analysis of MR images, these same images can be utilized for quantitative data extraction through radiomics—a potential tool in oncology research to enhance personalized medicine22. Some exploratory radiomics studies have been conducted in the field of retinoblastoma23,24. Notably, Li et al.'s 2022 study demonstrated that their radiomics model for predicting postlaminar optic nerve tumor invasion, a significant risk factor for metastasis, surpassed radiologists’ assessments in terms of sensitivity. Nevertheless, existing literature concerning the differentiation of retinoblastoma subtypes remains limited.
Consequently, the objective of this study was to distinguish MYCNampRB1+/+ retinoblastoma from RB1-/- retinoblastoma utilizing quantitative radiomics on pretreatment MR imaging.
Methods
This retrospective multicenter case–control study was conducted in compliance with the STARD (Standards for Reporting Diagnostic accuracy studies) checklist and received approval from the institutional review board of the Amsterdam UMC. The necessity to obtain informed consent from participants was waived (IRB number IRB00002991).
Patients
This study utilized a patient cohort from a multicenter retrospective case–control study by Jansen et al.21. This patients were included from 10 tertiary retinoblastoma referral centers in 5 countries. In that study patients were included when 1) retinoblastoma was histopathologically confirmed; 2) unilateral disease; 3) availability of pretreatment MR images obtained after 1995 and at least included T1-weighted and T1-weighted contrast-enhanced MR images, and 4) availability genetic of analysis of tumor material. Cases of MYCNampRB1+/+ retinoblastoma subtype were matched with controls (RB1-/- retinoblastoma with proven pathogenic variants in both alleles of the RB1 gene) based on age, MRI scan date, and referral site at a ratio of 1:4. If controls could not be obtained in the same institution, controls from different institutions were used. The patient cohort consist of 110 retinoblastoma patients, including 22 MYCNampRB1+/+ retinoblastoma cases and 88 RB1-/- retinoblastoma matched controls. In the current study, patients from the cohort were excluded if no T2-weighted MR images were available or if the images could not be processed or analyzed through the radiomics analysis pipeline (e.g. if tumor sizes were too small). If an MYCNampRB1+/+ retinoblastoma case was excluded its controls were subsequently also deleted.
Quantitative MR imaging feature extraction and statistical methods for predicting MYCN-status
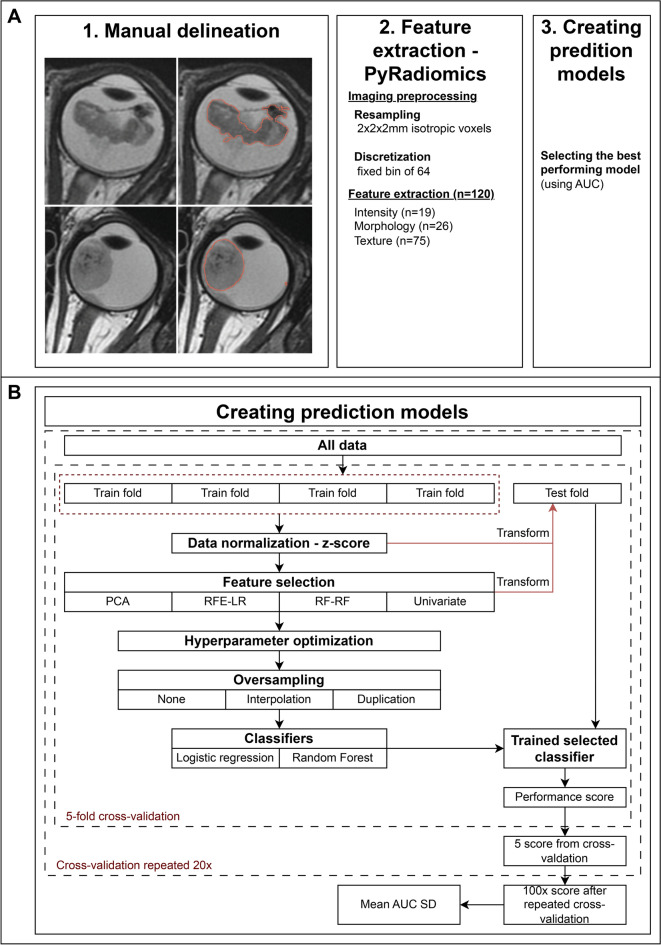
To enable quantitative feature extraction from MR images, the whole tumors were manually delineated on T2-weighted MR images using 3D Slicer (Version 4.10.1, MIT, USA). The delineation excluded non-enhancing areas of pure retinal detachment and subretinal fluid or hemorrhage. Expert radiologists (P.d.G. and M.d.J.) subsequently validated the manual delineations. All image processing and feature calculations were performed using the PyRadiomics package (version 3.1.0)25. MR images and delineations were resampled to 2x2x2 mm isotropic voxels and discretized using a fixed bin width of 6425. A total of 120 radiomics features were extracted from the delineated tumors, which could be divided into three main categories: intensity (n = 19), morphology (n = 26), and texture (n = 75). A previously used framework was employed to train a prediction models for MYCN-status and evaluate their performance using a cross-validation procedure26.
This pipeline consists of a repeated cross-validation scheme that evaluates different combinations of feature selection methods, oversampling techniques, and classifiers to obtain an average classification performance for each configuration. Features were scaled using a z-score normalization. To reduce redundancy, features with a linear correlation above 0.8 with another feature were removed (removing the feature with the highest average correlation with the feature set). Four dimension reduction strategies were used: principal component analysis (PCA), recursive feature elimination in nested cross-validation using logistic regression (RFE-LR) and using random forests (RFE-RF), and a univariate selection method based the f-statistic from ANOVA (selecting the top 10 percentile). To address the inherently imbalanced dataset, oversampling of MYCNampRB1+/+ retinoblastoma cases was applied using duplication of cases and interpolation (SMOTE). Two classifiers were evaluated: a logistic regression classifier (with L1 regularization) and a random forest classifier27. Model hyperparameters were optimized in nested cross-validation. For each combination of the aforementioned methods, a stratified repeated fivefold cross-validation approach was used to assess model generalizability. In every cross-validation fold, the model was trained on 80% of the samples and validated on the holdout subset of 20% of the samples. The fivefold cross-validation was repeated twenty times to further limit chance findings. Model performance was evaluated using the receiver-operator characteristic curve area-under-the-curve (AUC) was generated. The average AUC (with standard deviation [SD]) was calculated across the cross-validation iterations. The Brier score loss was calculated to additionally assess model calibration (0.0 being optimal28). For the best-performing combination of methods, the contribution of individual features to predictions were evaluated by calculating the SHAP values from a model trained on the whole dataset29. To assess whether the best-performing combination of methods exhibited significant performance beyond chance, random permutations were conducted. the repeated cross-validation procedure was repeated 1000 time using randomly shuffled labels, generating 1000 mean (cross-validated) AUCs representative of “random guessing”. The resulting p value was defined as the fraction of repeated cross-validation iterations where the permutation mean AUC equaled or exceeded the observed mean AUC30. A schematic flowchart is shown in Fig. 1.
Fig. 1.
Work flowchart of the study. (1) Manual delineation of the whole-tumor and validated by expert radiologists; (2) Processing data through PyRadiomics (version 3.1.0). (2.1) Resampling of MR imaging to 2×2×2 mm isotropic voxels and discretization by a 64 fixed bin. (2.2) Extracting 120 radiomics features divided into three categories: intensity (n = 19), morphology (n = 26), texture (n = 76); 3) Schematic framework of the creation of prediction models for MYCN-status utilizing different combinations of feature selection methods, oversampling techniques, and classifiers; (4) Selection and evaluation of the best-performing model. (4.1) Selection of the best-performing model by the highest mean AUC after the fivefold cross-validation which was 20 times repeated. (4.2) Evaluating the highest selected model by “random guessing” in which the original mean AUC was compared to permuted mean AUC. The permuted mean AUC was calculated by randomly shuffling the MYCN status and using it as input in the fivefold cross-validation which was repeated 20 times. This shuffling and 20 times repeated fivefold cross-validation was repeated 1000 times to obtain the permuted mean AUC.
Results
Patients
Out of 110 patients in the cohort, 98 (89%) were included. Baseline characteristics of the patients are shown in Table 1 and examples of a case (MYCNampRB1+/+ retinoblastoma) and a control (RB1-/- retinoblastoma) are shown in Fig. 2. A summary of MR imaging parameters can be found in Table S1. Twelve patients could not be evaluated: 1) one MYCNampRB1+/+ patient had extensive extra-ocular disease outside of the analysis frame; 2) one MYCNampRB1+/+ patient had no T2-weighted imaging; 3) eight matched control patients were subsequently excluded along with the abovementioned two MYCNampRB1+/+ patients, and 4) two other control scans showed tumors with diameters below 5.38 mm and volume less than 63 mm3 which were too small for PyRadiomics evaluation after resampling.
Table 1.
Patient demographics.
| Total patients | Cases (MYCNampRB1+/+ retinoblastoma) |
Controls (RB1-/- retinoblastoma) |
p value | |
|---|---|---|---|---|
| Patients n (%) | 98 | 20 (20%) | 78 (80%) | |
| Female sex n (%) | 44 (45%) | 7 (35%) | 37 (47%) | .440† |
| Laterality (left eye) n (%) | 49 (50%) | 9 (45%) | 40 (51%) | .803† |
| Age in months at scan date; median, [IRQ], (range) | 8 [5-12], (0-67) | 7 [4-8], (3-42) | 9 [5-13], (0-67) | .059§ |
| MRI examination year, median [IRQ], (range) | 2011 [2007–2015], (2001–2021) | 2014 [2009–2017], (2002–2021) | 2011 [2006–2015], (2001–2021) | .546§ |
p values were derived from † Fisher’s exact test or § Mann Whitney U –test.
Fig. 2.
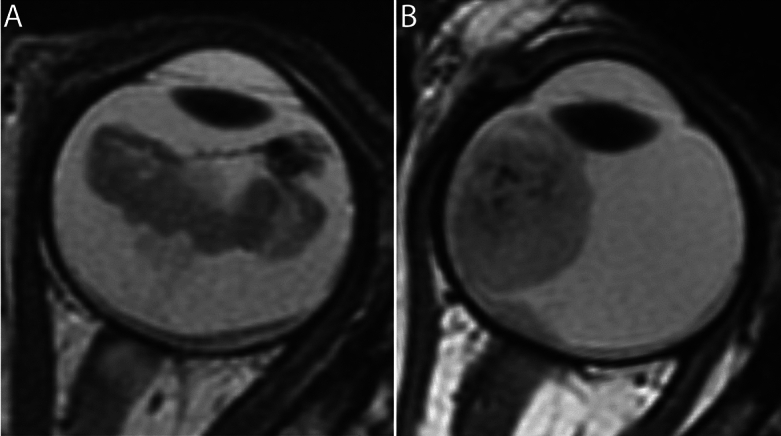
MRI phenotype MYCN-amplified RB1 wild-type (MYCNampRB1+/+) retinoblastoma versus RB1 pathogenic variation (RB1−/−) retinoblastoma. (A) Axial 2D T2-weighted MR image of a 42-month-old patient with MYCNampRB1+/+ retinoblastoma. The tumor has a diffuse growth pattern and is plaque shaped. Also, the intensity in the tumor varies greatly. (B) Axial 2D T2-weighted MR image of a 35-month-old patient with RB1−/− retinoblastoma. The tumor has an endophytic growth pattern and is dome shaped.
Classification performance
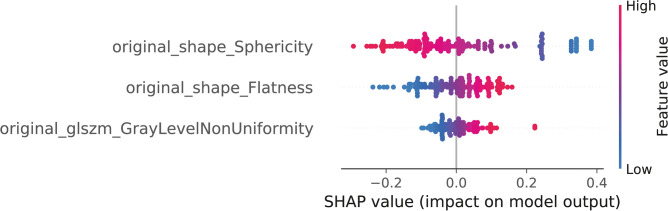
The best-performing combinations of methods was constructed by univariate feature selection, duplication of the MYCN features as means of oversampling, and logistic regression as classifier resulting in a mean AUC of 0.78 (SD 0.12; p = 0.001) with a mean Brier score of 0.20 (SD 0.05). The other combinations of methods had AUCs ranging 0.60 (SD 0.15) to 0.78 (SD 0.12). Performance of all radiomics models is shown in Table 2. For the best-performing combinations of methods, the SHAP values of the individual features are shown in Fig. 3. Of these three features, none were within the intensity category, two (66%) within the morphology category, and one (33%) within the texture category. The morphology features were sphericity (morphologic resemblance to a sphere) and flatness, indicating that MYCNampRB1+/+ tumors were less spherical and were flatter compared to RB1-/- retinoblastoma. The selected texture feature was gray level non uniformity indicating MYCNampRB1+/+ tumors were more heterogeneous in terms of gray level distribution. Using these three features with logistic regression yielded a mean cross-validation AUC of 0.81 (SD 0.11).
Table 2.
Radiomics models predicting MYCN-status.
| Feature selection | Oversampling | Classifier | Mean AUC | AUC SD | Mean brier score | Brier score SD |
|---|---|---|---|---|---|---|
| Univariate | Duplicate | Logistic Regression | 0.78 | 0.12 | 0.20 | 0.05 |
| Univariate | Interpolate | Logistic Regression | 0.78 | 0.12 | 0.20 | 0.05 |
| Univariate | None | Logistic Regression | 0.77 | 0.12 | 0.15 | 0.03 |
| RFE-RF | None | Logistic Regression | 0.76 | 0.12 | 0.15 | 0.03 |
| RFE-RF | Duplicate | Logistic Regression | 0.76 | 0.13 | 0.21 | 0.05 |
| RFE-RF | Interpolate | Logistic Regression | 0.75 | 0.13 | 0.21 | 0.05 |
| RFE-LR | Duplicate | Logistic Regression | 0.75 | 0.15 | 0.22 | 0.06 |
| RFE-LR | None | Logistic Regression | 0.74 | 0.15 | 0.16 | 0.05 |
| RFE-LR | Interpolate | Logistic Regression | 0.74 | 0.15 | 0.22 | 0.06 |
| RFE-LR | None | Random Forest | 0.66 | 0.13 | 0.16 | 0.03 |
| RFE-LR | Duplicate | Random Forest | 0.65 | 0.13 | 0.17 | 0.03 |
| PCA | Duplicate | Logistic Regression | 0.64 | 0.15 | 0.23 | 0.04 |
| RFE-RF | None | Random Forest | 0.64 | 0.13 | 0.16 | 0.03 |
| PCA | Interpolate | Logistic Regression | 0.64 | 0.15 | 0.23 | 0.05 |
| PCA | Interpolate | Random Forest | 0.64 | 0.15 | 0.18 | 0.04 |
| PCA | Duplicate | Random Forest | 0.64 | 0.15 | 0.17 | 0.03 |
| RFE-RF | Duplicate | Random Forest | 0.64 | 0.13 | 0.17 | 0.03 |
| Univariate | Interpolate | Random Forest | 0.64 | 0.14 | 0.21 | 0.05 |
| RFE-LR | Interpolate | Random Forest | 0.63 | 0.14 | 0.18 | 0.03 |
| RFE-RF | Interpolate | Random Forest | 0.63 | 0.13 | 0.18 | 0.03 |
| PCA | None | Logistic Regression | 0.63 | 0.15 | 0.19 | 0.04 |
| PCA | None | Random Forest | 0.63 | 0.16 | 0.16 | 0.03 |
| Univariate | None | Random Forest | 0.62 | 0.15 | 0.17 | 0.04 |
| Univariate | Duplicate | Random Forest | 0.60 | 0.15 | 0.19 | 0.05 |
Results from all cross-validation analyses (PCA: principal component analysis; RFE-RF: a recursive feature elimination approach using a random forest in nested cross-validation; Univariate: a univariate selection method based on ANOVA testing that retained the top 10 percentile features; AUC: area under the curve; SD: standard deviation; Brier score assesses the model calibration and refinement [0.0 being optimal]).
Fig. 3.
All features in the best-performing model with univariate feature selecting, duplicate scaling, and a logistic regression as classifier. Features in three categories were assessed: Intensity features, texture features and morphology features. Intensity features comprised of first order statistics [19 features]. Morphology features comprised of shaped-based 2D features [10 features] and 3D features [16 features]. Texture features comprised on features based on gray-level co-occurrence matrices (GLCM) [24 features], gray-level run length matrices (GLRLM) [16 features], gray-level size zone matrices (GLSZM) [16 features], neighborhood gray-tone difference matrices (NGTDM) [5 features], and gray-level dependence matrices (GLDM) [14 features]. Original_shape_Sphericity = original shape sphericity; original_shape_Flatness = original shape flatness; original_glszm_GrayLevelNonUniformity = Original glszm gray level non uniformity.
Discussion
Due to their aggressive nature and relative resistance to typical chemotherapy approaches, identification of patients with MYCN-amplified RB1 wild-type (MYCNampRB1+/+) retinoblastoma might be clinically valuable in the future, although current treatment guidelines are not yet stratified according to genetic somatic alterations. As tissue biopsy is not considered safe in retinoblastoma, we sought to determine whether quantitative analysis of MR imaging could be used to identify this rare retinoblastoma subtype. However, there are promising results regarding aqueous humor liquid biopsy as a surrogate for tumor biopsy31. The various radiomics models based on the pretreatment T2-weighted MR images showed that MYCNampRB1+/+ and RB1-/- retinoblastoma patients could be differentiated with an AUC of at least 0.60 up until 0.78. Eventually, the models could supplement the known clinical, ophthalmologic and qualitative MR imaging findings for pretreatment recognition of MYCNampRB1+/+ retinoblastoma.
The use of radiomics analyses in research on retinoblastoma is scarce and only a limited number of published studies successfully used quantitative image analysis of retinoblastoma. A recent study investigating the ability of MR imaging-based radiomics features of the tumor to differentiate retinoblastoma with and without postlaminar optic nerve invasion resulted in a model with an AUC of 0.8424. In that study, a total of 2058 features were extracted and after feature selection only nine strongly correlated MRI features remained and were implemented in their prognostic model. Though individually appraising radiomics features is challenging, all of the features in our model could be linked to the tumor characteristics of MYCNampRB1+/+ on MRI as reported by Jansen et al21. The two morphological features, sphericity and flatness, were respectively lower and higher in MYCNampRB1+/+ tumors compared to RB1-/- controls, indicating that the MYCNampRB1+/+ tumors were less spherical and more flat. This is consistent with the qualitative observations by Jansen et al. showing a more diffuse growth pattern in MYCNampRB1+/+ tumors versus the bulkier mass lesions in RB1-/- tumors21. The texture feature in our model, gray level non uniformity was found to be higher in MYCNampRB1+/+ retinoblastoma compared to RB1-/- controls. This suggests an increased variability in grey levels within MYCNampRB1+/+ tumors as a sign of intratumoral heterogeneity, which could be related to the more aggressive nature of this subtype of retinoblastoma. Notably, this is in line with two recently identified features of peritumoral hemorrhage and subretinal hemorrhage associated with the fast-growing MYCNampRB1+/+ tumors21. This correlation between qualitative features and quantitative features strengthens the suggestion that radiomics models may well become powerful supportive tools for subtype recognition in retinoblastoma, especially when predictive models combine clinical characteristics and ophthalmological and qualitative radiological features.
There were several limitations of this study. The subtype MYCNampRB1+/+ is rare and cases with MR imaging available world-wide are limited and MR imaging acquisition protocols and quality varied within and between centers. Another consequence of the rarity of this tumor subtype was the unavailability of a separate validation cohort for the current study. To address this, a fivefold cross-validation was used to assess model generalization. Also, the case–control design of the study might have inflated the accuracy results, as there was an overrepresentation of MYCNcases in the study setting compared to clinical practice32. Last, some of the strong clinical predictors of MYCNampRB1+/+ such as a young-age at presentation and unilaterality could not be incorporated in our analyses due to the case–control study design9. Combination of these strong clinical predictors with the quantitative features obtained through radiomics might result in an even an better prediction of higher AUC for predicting MYCN status.
In conclusion, this study demonstrated the feasibility of distinguishing between MYCNampRB1+/+ and RB1-/- tumors using quantitative imaging analysis. Facilitating early detection of this more aggressive retinoblastoma subtype, MYCNampRB1+/+, and may aid in selecting personalized treatments, ultimately improving future outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- RB1-/-
Pathogenic variants in the RB1 gene
- MYCNampRB1+/+
MYCN-amplified RB1 wild-type
- MRI
Magnetic resonance imaging
- STARD
Standards for Reporting Diagnostic accuracy studies
- PCA
Principal component analysis
- RFE-LR
Recursive feature elimination approach using a logistic regression in nested cross-validation
- RFE-RF
Recursive feature elimination approach using random forest in nested cross-validation
- Univariate
Univariate selection method based on ANOVA testing that retained the top 10 percentile features
- AUC
Area under the curve
- SD
Standard deviation
Author contributions
Guarantors of integrity of entire study, C.M.d.B., R.W.J., P.M., P.d.G. ; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, C.M.d.B., R.W.J., A.H.S., P.M., S. Sirin., J.D. ; clinical studies, C.M.d.B., J.L.J., A.H.S., A.K.M., P.M., O.E.U., H.G., R.C.B., S. Sen, M.K., S. Sirin, H.J.B., P.G., C.J.D., M.C., A.C.M., P.d.G. ; experimental studies, C.M.d.B., R.W.J., S.v.E., S. Sen, M.C., R.B., J.D. ; statistical analysis, C.M.d.B., R.W.J., M.C., M.C.d.J. ; and manuscript editing, C.M.d.B., R.W.J., L.C., S.G., S.v.E., J.L.J., A.R., A.H.S., A.K.M., P.M., O.E.U., G.B.H., H.G., H.C.B., K.E.N., R.C.B., S. Sen, M.K. S. Sirin, H.J.B., P.G., C.J.D., M.C., R.B., J.D., A.C.M., M.C.d.J., P.d.G.
Funding
Initiation and coordination of this study was funded by Stichting Kinderen Kankervrij (KIKA) (grant no. 342) and the Hanarth Foundation (grant for project titled MRI-based Deep Learning Segmentation and Quantitative Radiomics in Retinoblastoma: A Next Step Toward Personalized Interventions). Departmental funding was received by Casey Eye Institute, Oregon Health & Science University (A.H.S., A.K.M., and O.E.U.) from the National Institutes of Health (grant P30 EY010572) in addition to unrestricted departmental funding from Research to Prevent Blindness. Departmental funding was received by Emory Eye Center, Ocular Oncology Service (O.E.U., G.B.H., and H.G.) via National Eye Institute core grant P30 EY006360.
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request, only if permission of the providing center is obtained.
Declarations
Conflict of interests
C.M.d.B. No relevant relationships. R.W.J. No relevant relationships. L.C. No relevant relationships. S.G. No relevant relationships. S.v.E. No relevant relationships. J.L.J. No relevant relationships. A.R. No relevant relationships. A.H.S. No relevant relationships. A.K.M. No relevant relationships. P.M. No relevant relationships. O.E.U. No relevant relationships. G.B.H. No relevant relationships. H.G. No relevant relationships. H.C.B. No relevant relationships. K.E.N. No relevant relationships. R.C.B. Consultant for Aileron Therapeutics. S. Sen No relevant relationships. M.K. No relevant relationships. S. Sirin No relevant relationships. H.J.B. No relevant relationships. P.G. No relevant relationships. C.J.D. No relevant relationships. M.C. No relevant relationships. R.B. No relevant relationships. J.D. No relevant relationships. A.C.M. No relevant relationships. M.C.d.J. No relevant relationships. P.d.G. No relevant relationships.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76933-6.
References
- 1.Moll, A. C. et al. Incidence and survival of retinoblastoma in The Netherlands: a register based study 1862–1995. Br J Ophthalmol 81(7), 559–562 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pritchard, E. M., Dyer, M. A. & Guy, R. K. Progress in Small Molecule Therapeutics for the Treatment of Retinoblastoma. Mini Rev Med Chem 16(6), 430–454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubry, A., Yu, T. & Bremner, R. Preclinical studies reveal MLN4924 is a promising new retinoblastoma therapy. Cell Death Discov 6, 2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kooi, I. E. et al. Loss of photoreceptorness and gain of genomic alterations in retinoblastoma reveal tumor progression. Ebiomedicine 2(7), 660–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshar, A. R. et al. Next-Generation Sequencing of Retinoblastoma Identifies Pathogenic Alterations beyond RB1 Inactivation That Correlate with Aggressive Histopathologic Features. Ophthalmology 127(6), 804–813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaiquevich, P. et al. Treatment of Retinoblastoma: What Is the Latest and What Is the Future. Front. Oncol. 12, 822330 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapatai, G. et al. Gene expression profiling identifies different sub-types of retinoblastoma. Br J Cancer 109(2), 512–525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, J. et al. A high-risk retinoblastoma subtype with stemness features, dedifferentiated cone states and neuronal/ganglion cell gene expression. Nat Commun 12(1), 5578 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rushlow, D. E. et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol 14(4), 327–334 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Ewens, K. G. et al. Phosphorylation of pRb: mechanism for RB pathway inactivation in MYCN-amplified retinoblastoma. Cancer Med 6(3), 619–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh, H. P. et al. An immature, dedifferentiated, and lineage-deconstrained cone precursor origin of N-Myc-initiated retinoblastoma. Proc Natl Acad Sci U S A 119(28), e2200721119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price, E. A. et al. MYCN amplification levels in primary retinoblastoma tumors analyzed by Multiple Ligation-dependent Probe Amplification. Ophthalmic Genet 42(5), 604–611 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Lillington, D. M. et al. High level amplification of N-MYC is not associated with adverse histology or outcome in primary retinoblastoma tumours. Br J Cancer 87(7), 779–782 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zugbi, S., et al., Clinical, Genomic, and Pharmacological Study of MYCN-Amplified RB1 Wild-Type Metastatic Retinoblastoma. Cancers, 2020. 12(9). [DOI] [PMC free article] [PubMed]
- 15.Abramson, D. H., Gobin, Y. P. & Francis, J. H. Orbital Retinoblastoma Treated with Intra-arterial Chemotherapy. Ophthalmology 128(10), 1437 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Zhou, C., et al., Eye-Preserving Therapies for Advanced Retinoblastoma: A Multicenter Cohort of 1678 Patients in China. Ophthalmology, 2021. [DOI] [PubMed]
- 17.Berry, J. L. et al. Genomic cfDNA Analysis of Aqueous Humor in Retinoblastoma Predicts Eye Salvage: The Surrogate Tumor Biopsy for Retinoblastoma. Molecular cancer research : MCR 16(11), 1701–1712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Jong, M. C. et al. Diagnostic Accuracy of Intraocular Tumor Size Measured with MR Imaging in the Prediction of Postlaminar Optic Nerve Invasion and Massive Choroidal Invasion of Retinoblastoma. Radiology 279(3), 817–826 (2016). [DOI] [PubMed] [Google Scholar]
- 19.de Graaf, P. et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol 42(1), 2–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong, M. C. et al. Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 15(10), 1157–1167 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Jansen, R. W. et al. MRI Features for Identifying MYCN-amplified RB1 Wild-type Retinoblastoma. Radiology 307(5), e222264 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambin, P. et al. Radiomics: the bridge between medical imaging and personalized medicine. Nature reviews Clinical oncology 14(12), 749–762 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Strijbis, V. I. J. et al. Multi-view convolutional neural networks for automated ocular structure and tumor segmentation in retinoblastoma. Sci Rep 11(1), 14590 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Z., et al., MRI-based radiomics model can improve the predictive performance of postlaminar optic nerve invasion in retinoblastoma. The British Journal of Radiology, 2022. 95(1130). [DOI] [PMC free article] [PubMed]
- 25.van Griethuysen, J. J. M. et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 77(21), e104–e107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cysouw, M. C. F. et al. Machine learning-based analysis of [(18)F]DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur J Nucl Med Mol Imaging 48(2), 340–349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breiman, L. Random Forests. Machine Learning 45, 5–32 (2001). [Google Scholar]
- 28.Murphy, A.H., A New Vector Partition of the Probability Score. Journal of Applied Meteorology and Climatology, 1973. 12(4).
- 29.Lundberg, S.M. and S.-I. Lee, A unified approach to interpreting model predictions. Advances in neural information processing systems, 2017. 30.
- 30.Ojala, M. and G.C. Garriga, Permutation tests for studying classifier performance. Journal of machine learning research, 2010. 11(6).
- 31.Xu, L., et al., Establishing the Clinical Utility of ctDNA Analysis for Diagnosis, Prognosis, and Treatment Monitoring of Retinoblastoma: The Aqueous Humor Liquid Biopsy. Cancers (Basel), 2021. 13(6). [DOI] [PMC free article] [PubMed]
- 32.Yuan, W. et al. Temporal bias in case-control design: preventing reliable predictions of the future. Nat Commun 12(1), 1107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request, only if permission of the providing center is obtained.