Abstract
Introduction: Heat stress is one of the environmental causes of damage to the testis, whose effects are less known before puberty. The aim of the present study was to investigate the impact of photobiomodulation (PBM) on the testis of prepubertal mice subjected to hyperthermia.
Methods: Twenty-four three-week-old prepubertal male mice were allocated to the following groups: I) control, II) scrotal hyperthermia (Hyp), and III) Hyp+PBM (n=8/each group). In order to induce hyperthermia, the scrotum was placed in water at 43 °C for 20 minutes every other day for a total duration of 10 days. In the Hyp+PBM group, after hyperthermia induction, the testis of the mice was subjected to laser irradiation at a wavelength of 890 nm (0.03 J/cm2 for 30 seconds) for 35 days. After the mice were sacrificed, the testis and epididymis were removed for testing.
Results: Compared with those of the Hyp group, the sperm parameters of the laser irradiation group improved notably. In addition, histological examinations revealed that the final number of testis cells and the volume of tissue in the Hyp+PBM group were dramatically greater than those in the Hyp group. The analysis of molecular data revealed an increase in the expression of mitotic genes and testosterone levels and a decrease in the formation of reactive oxygen species (ROS) and the expression of the apoptotic gene in the testis subjected to PBM.
Conclusion: Based on the present findings, laser therapy can reduce complications caused by scrotal hyperthermia during prepuberty and ameliorate spermatogenesis during puberty.
Keywords: Male, Testis, Low-level laser therapy, Spermatogenesis, Hyperthermia
Introduction
The term “prepuberty” in mice refers to the time from birth to approximately three weeks after birth. The transition of gonocytes into spermatogonial stem cells occurs during this period, and male gonad activity is low.1 Additionally, in prepuberty, the nurse cells of testicular tissue, known as Sertoli cells, mature to form an appropriate niche for the proliferation and differentiation of germ cells.2 Additionally, two other unique features include hypothalamic-pituitary-gonadal axis quiescence and very low serum testosterone levels.3 During prepuberty, the testis is susceptible to damage due to environmental stressors such as heat shock. Heat stress (HS) can impact the growth and development of the testis and lead to failure of spermatogenesis and infertility in adulthood.4,5
The testis that has been exposed to high temperatures prior to puberty, in sauna, hot water, and prolonged cycling, exhibits significant molecular and morphological changes. Oxidative stress plays a vital role in the induction of cell death signaling following HS, which causes oligospermia and azoospermia in the future by impairing the testicular cells.6,7 In addition, the overproduction of reactive oxygen species (ROS) can cause destruction of the epithelium of the seminiferous tubules, programmed cell death of Leydig cells in the interstitial tissue, and disruption of steroidogenesis.8
Photobiomodulation (PBM) is a modern physiotherapy in which the beneficial effects on cell proliferation and tissue repair have been investigated.9 Several studies have reported the positive impacts of PBM on sperm production and the structure of testicular tissue. Some evidence confirms that the use of laser irradiation at different wavelengths improves testicular tissue and sperm parameters, including motility and viability.10,11 It has been proven that lasers reduce cell vulnerability by stabilizing cell DNA against oxidative stress.12 Considering the modulation of mitochondrial respiratory chain function after laser irradiation, the use of PBM is an appropriate method for regulating the activity and metabolism of cells.13 Also, PBM can play a pivotal role in maintaining the integrity of the spermatogenic epithelium by regulating the function of Sertoli cells and maintaining the mitotic and miotic cycles of germ cells.14
Despite the effects of PBM on the testis and the evidence that exposure to HS is related to damage to testis function and fertility, the impacts of this stress during prepuberty have been less investigated. The purpose of designing this experimental project was to determine whether laser therapy can improve the histological parameters of testis and spermatogenesis in prepubertal male mice induced by scrotal hyperthermia.
Materials and Methods
Animals
In the present experiment, 24 prepubertal male mice (3 wks., 15-20 g) were purchased from the animal lab of Institute Pasture, Tehran, Iran. In accordance with NIH guidelines, all the mice were kept under controlled laboratory conditions. Here, three groups were considered (n = 8/each group): I) control (Cont), II) scrotal hyperthermia (Hyp), and III) scrotal hyperthermia + PBM 0.03 j/cm2 (Hyp + PBM 0.03 J/cm2). In order to identify animals, their tails were marked by color.
Induction Of Scrotal Hyperthermia
On the 22nd day after birth, the animals were injected with ketamine (50 mg/kg) to induce anesthesia. In the Hyp and Hyp + PBM groups, the scrotum was submerged in water heated to 43°C for a duration of 20 min. every other day over a period of 10 days. In the Cont group, the aforementioned process took place at 35 °C.15,16
Photobiomodulation
In the current study, a low-level pulsed laser with the presented characteristics was used. The wavelength was 890 nm and the spot size was 1cm2. The pulse frequency was 80 Hz and each point of exposure was 30 seconds (for each testis). Twenty-four hours after the last induction of hyperthermia, the testis of the mice was subjected to laser therapy at an energy density of 0.03 J/cm2 every other day for 5 weeks. Finally, all the animals were euthanized by cervical dislocation, their epididymis were removed for sperm analysis, and the testis were removed for histological and molecular tests.10
Sperm Parameters
To conduct sperm analysis, the epididymal tail was meticulously isolated from nearby tissues and subsequently placed in a dish with Ham’s F10 solution. Afterwards, it was placed in the incubator and kept at 35 °C for 15-20 minutes. Then, 10 μL from each sample were applied onto the slide, and both progressive and non-progressive sperms were observed. The sperms were counted with a Neubauer slide. Additionally, eosin-nigrosin dye was used to assess sperm viability.
Tissue Preparation
For histological assessments, the mice were anesthetized via ketamine injection (50 mg/kg) and were scarified by cervical dislocation, and their right testis were transferred to Bouin’s solution for 48 hours. Finally, the testis samples were embedded in paraffin blocks. To determine the number of testicular cells and tissue volume, 5 µm slices were prepared from the samples and were stained with hematoxylin and eosin (H&E).
The Volume of the Testis
The Cavalieri technique and the following formula were used to measure the volume of testicular tissue (17):
The given equation represents the sum of counted points, ΣP, and the ratio of the area of the probe points to the magnification, a/p. The t shows the measure of the distance between tissue slices.
The Number of Testicular Cells
The optical dissector method was utilized to determine the quantity of cells in the testis. The following formula was also used for calculation17:
In the given equation, ΣQ represents the total count of germ and somatic cells. The value of h is the height of the dissector and the value of t is the real thickness of the tissue section. Σp refers to the overall count of fields that were included. a/f denotes the probe area divided by the magnification factor, whereas BA indicates the thickness of the microtome section.
Serum Testosterone Levels
To check the serum level of testosterone, first, blood was collected from the hearts of the animals under deep anesthesia. After clotting, the blood samples were centrifuged at 5500 rpm (5 minutes). Finally, the extracted serum was kept at -80 °C temperature until testing with a special ELISA kit.
Measurement of Total ROS Formation
The assessment of ROS in the testis was conducted via spectrofluorimetry with dichlorofluorescein diacetate (DCFDA: CAS Number 2044-85-1). In this study, 50 mg of tissue was mixed with 100 μL of a 20 μM solution of DCFDA. The samples were then incubated in a dark environment at 37 °C for 45 minutes. Afterward, the testis were broken down into smaller fragments using a sonicator. The lysed samples were centrifuged at 1500 revolutions per minute for 5 minutes at 4 °C. Finally, the samples were evaluated via a spectrofluorometer at a wavelength of 488 nm.18
Evaluation of C-kit, Pcna, and Caspase-3 Expression at the mRNA Level
To determine the expression of the C-kit, Pcna, and Caspase-3 genes, the left testis of each animal was immediately transferred to -80 °C for RNA stabilization and stored until use. Next, DNase I was added to remove genomic contamination from the extracted RNA samples. cDNA was subsequently synthesized via a Fermentas’ commercial kit. The relative gene expression was evaluated via the QuantiTect SYBR Green Real-Time Polymerase Chain Reaction kit (Takara Bio Inc., Japan) and quantified via the TaqMan method. Primer 3 Plus software was used to design all pairs of primers (forward and reverse), which were checked with the primer-blast tool before use. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene in this study (Table 1).
Table 1. Primer Design .
| Genes | Primers Sequences | Product Size (bp) |
| Caspase-3 | F: AGTGGGATTGATGAGGAGATGG | 240 |
| R: AGTGGAGTGTAGGGAGAAGGA | ||
| C-kit | F: GCATCACCATCAAAAACGTG | 332 |
| R: GATAGTCAGCGTCTCCTGGC | ||
| Pcna | F: GATGTGGAGCAACTTGGAAT | 160 |
| R: AGCTCTCCACTTGCAGAAA | ||
| GAPDH | F: CAGAACATCATCCCAGCCTCC | 293 |
| R: TTGGCAGGTTTCTCAAGACGG |
Statistical Analysis
The normality of the data was assessed via the Shapiro-Wilk test, and SPSS software version 21 (SPSS, Chicago, IL, USA) was used for data analysis. One-way ANOVA and Tukey’s post hoc test were used, with all the data reported as the mean ± standard deviation. A significance level of P < 0.05 was considered.
Results
Sperm Parameters
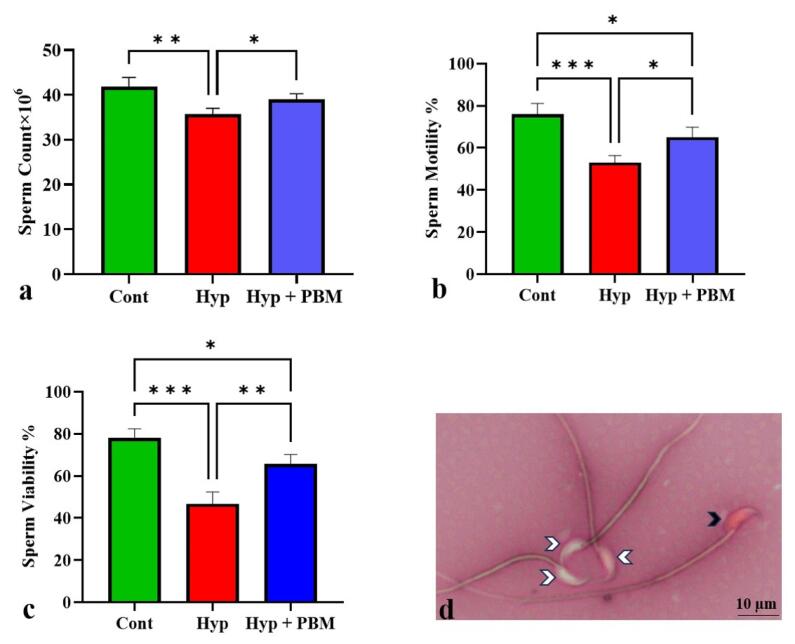
Our analysis revealed dramatic decreases in sperm count, motility, and viability in the Hyp group compared with the Cont group (P= 0.001, P =0.0001, and P =0.0006, respectively). Compared with those in the Hyp group, these parameters were significantly greater in the Hyp + PBM group (P = 0.04, P = 0.01, and P = 0.007, respectively) (Figure 1, Table 2).
Figure 1.
Photobiomodulation Therapy Improved Sperm Parameters. *P < 0.05, **P< 0.01, ***P < 0.001. The data were displayed as Mean ± SD (n = 4). a) sperm count, b) sperm motility, c) sperm viability, and d) photomicrograph of sperm stained with eosin-nigrosin *100. The white arrowheads indicate viable sperm and the black arrowhead indicates non-viable sperm
Table 2. Data Presented as Mean ± SD in Different Groups .
| Variable | Groups (Mean±SD) | ||
| Cont | Hyp | Hyp+PBM | |
| Sperm count | 41.86 ± 2.05 | 35.76 ± 1.27 | 38.99 ± 1.28 |
| Sperm motility | 76 ± 5.16 | 53 ± 3.36 | 65 ± 4.76 |
| Sperm viability | 78 ± 4.35 | 46.67 ± 5.68 | 65.67 ± 4.5 |
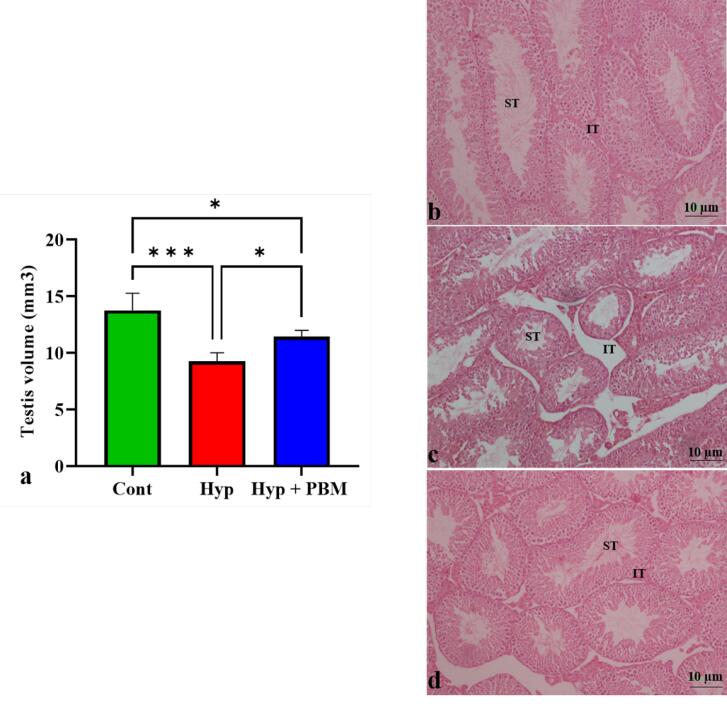
| Testis volume | 13.75 ± 1.53 | 9.25 ± 0.75 | 11.43 ± 0.57 |
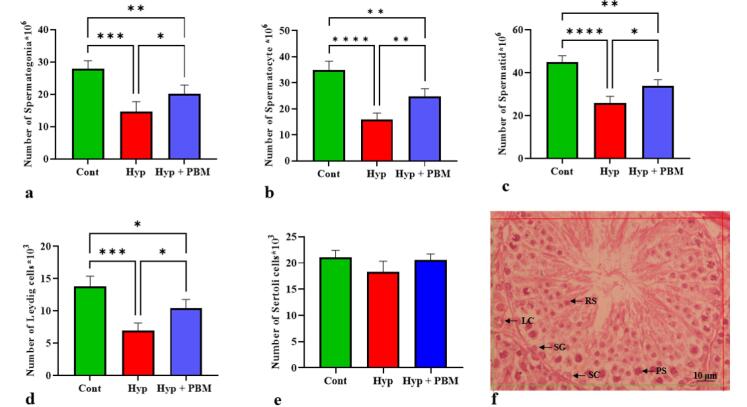
| Number of spermatogonia | 27.92 ± 2.49 | 14.7 ± 3.04 | 20.19 ± 2.66 |
| Number of spermatocyte | 34.94 ± 3.33 | 16 ± 2.37 | 24.82 ± 2.85 |
| Number of spermatid | 44.84 ± 3.09 | 26 ± 2.95 | 33.85 ± 2.9 |
| Number of Leydig | 13.85 ± 1.5 | 6.91 ± 1.19 | 10.41 ± 1.36 |
| Number of Sertoli | 21.08 ± 1.33 | 18.34 ± 2.01 | 20.58 ± 1.13 |
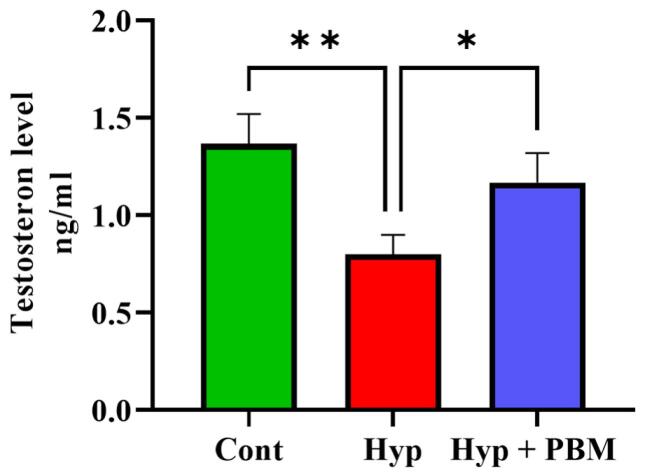
| Serum level of testosterone | 1.36 ± 0.15 | 0.8 ± 0.1 | 1.1 ± 0.15 |
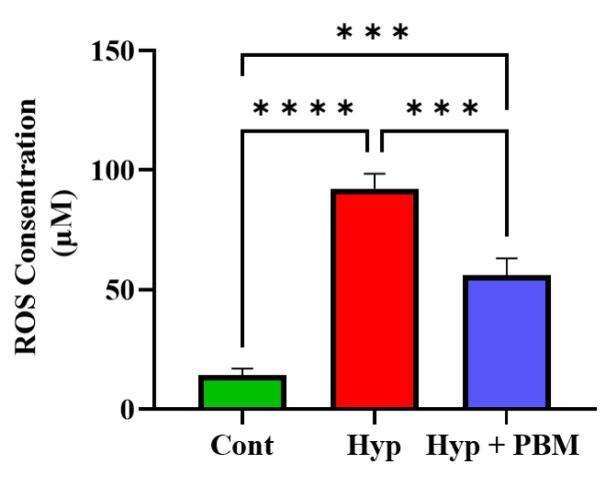
| ROS | 14.14 ± 2.8 | 92.17 ± 6.12 | 56.01 ± 7.04 |
Testis Volume
Stereological analysis revealed that the volume of the testis was lower in the induced scrotal hyperthermia than in the Cont group (P =0.0005). Compared with that in the Hyp group, the volume of testis tissue in the Hyp + PBM group was significantly greater (P =0.03) (Figure 2, Table 2).
Figure 2.
Laser irradiation increased the volume of testis. a) The volume of testicular tissue increased in mice subjected to laser irradiation. *P < 0.05 and ***P < 0.001. The values were presented as Mean ± SD (n = 5). Photomicrograph of hematoxylin and eosin staining of testis tissue *10. b) Cont group, c) Hyp group, and d) Hyp + PBM group. ST stands for seminiferous tubules and IT stands for interstitial tissue
Number of Testicular Cells
The number of spermatogonia, spermatocyte, spermatid, and Leydig cells was significantly lower in the Hyp group than in the Cont group (P =0.0002, P <0.0001, P <0.0001, and P =0.0001, respectively). The number of these cells in the Hyp + PBM group was significantly greater than that in the Hyp group (P =0.04, P =0.004, P =0.01, P =0.01, respectively) (Figure 3, Table 2).
Figure 3.
The Total Quantity of Testicular Cells Increased After Photobiomodulation. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. The data were provided as Mean ± SD (n = 5). a) spermatogonia, b) spermatocyte, c) spermatid, d) Leydig, and e) Sertoli. f) Photomicrograph of a testis tissue section stained with H&E *40. SG: Spermatogonia, PS: Primary spermatocyte, RS: Round spermatid, SC: Sertoli cell, LC: Leydig cell
Testosterone Level
ELISA analysis revealed that the Hyp group presented significantly lower serum testosterone levels than the Cont group (P =0.005). Compared with those in the group that did not receive laser irradiation, the levels of serum testosterone significantly increased in the Hyp + PBM group (P =0.03) (Figure 4, Table 2).
Figure 4.
The serum testosterone level experienced an increase following the use of photobiomodulation. *P < 0.05 and **P < 0.01. The values were reportedas Mean ± SD (n = 4). There was no notable difference observed between the Cont and Hyp + PBM groups
ROS Formation
Our analysis showed that ROS formation in the Hyp group was dramatically greater than that in the Cont group (P =0.005). Compared with those in mice with induced scrotal hyperthermia, the ROS levels in mice exposed to PBM were significantly lower (P =0.03) (Figure 5, Table 2).
Figure 5.
ROS formation increased in the testis of mice exposed to hyperthermia. Photobiomodulation decreased total ROS in the testis of the mice. ***P < 0.001 and ****P < 0.0001. The data were presented as Mean ± SD (n = 4)
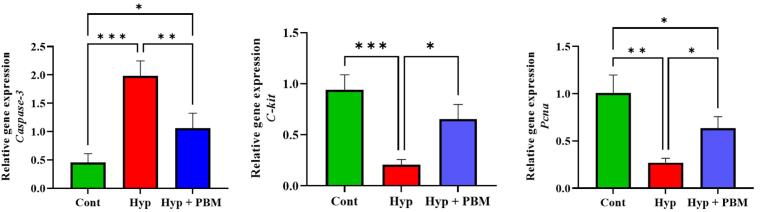
Pcna, C-kit, and Caspase-3 Genes Expression
Real-time PCR data analysis revealed that the expression levels of Pcna and C-kit were significantly lower in the Hyp group than those in the Cont group (P =0.001 and P =0.0008, respectively). However, the expression of caspase-3 was greater in the Hyp group than in the Cont group (P =0.0005). Compared with those in the Hyp group, the expression levels of mitotic genes and apoptotic genes in the Hyp + PMB group increased and decreased, respectively (P =0.03, P =0.01, and P =0.007, respectively) (Figure 6, Table 3).
Figure 6.
Mitotic Genes Expression Upregulated and Apoptotic Gene Expression Downregulated in the Testis Tissue of the Mice Exposed to Laser Irradiation. *P < 0.05, **P < 0.01 and ***P < 0.001. The values were reported as Mean ± SD (n = 4). a) Caspase-3, b) C-kit, and c) Pcna
Table 3. Gene expression data presented as Mean ± SD in different groups .
| Variable | Groups (Mean±SD) | ||
| Cont | Hyp | Hyp+PBM | |
| Pcna | 1 ± 0.18 | 0.26 ± 0.04 | 0.6 ± 0.12 |
| C-kit | 0.94 ± 0.14 | 0.2 ± 0.05 | 0.65 ± 0.14 |
| Caspase-3 | 045 ± 015 | 1.97 ± 0.26 | 1.06 ± 0.26 |
Discussion
Hyperthermia is considered an environmental stress whose effects on the male reproductive system before puberty have been less studied. On the basis of the results obtained from this experimental research, hyperthermia before puberty can cause histological and molecular changes in the testis. HS increases testicular cell activity and the need for oxygen.19 Following the increase in oxygen demand and lack of supply, the mitochondrial function of Sertoli, Leydig and spermatogonial stem cells is disrupted, leading to an increase in ROS levels in the testis.20 Our findings showed that the induction of oxidative stress before puberty by HS could be the key to initiating apoptotic pathways by increasing the expression of Caspase-3. Therefore, the number of germ and somatic cells in mice subjected to scrotal hyperthermia decreases dramatically, which is associated with testicular atrophy and affects the final number of sperm during puberty. Our observations also revealed that oxidative stress before puberty is associated with the cessation of mitosis and a decrease in the mRNA levels of the C-kit and Pcna genes in germ cells. A reduction in mitosis is associated with a decrease in the number of spermatocytes and spermatids, which ultimately leads to a decrease in sperm parameters and testicular cells.21
In this research, the number of Sertoli cells did not decrease because of their resistance to heat; however, heat stress disrupted the balance of oxidants and antioxidants and the function of the Sertoli cell.16 Dysfunction of this cell leads to disintegrity the spermatogenic epithelium and reduces the quantity and quality of sperm during puberty.22 The results demonstrated a reduction in Leydig cells in mice induced by scrotal hyperthermia. HS plays a crucial role in inducing cell death by damaging intracellular organelles such as the endoplasmic reticulum 23. The death of Leydig cells is linked to a disturbance in the generation and secretion of testosterone. Low levels of testosterone at the onset of puberty lead to the cessation of spermatogenesis and reduced sperm production.24
Previous evidence has demonstrated the potential of PBM in tissue repair.25 In line with past research, in the present study, the histological and molecular parameters were significantly improved in mice exposed to laser irradiation. Laser therapy has beneficial effects on various cells and tissues. In cells, the mitochondria is the first site where laser photons have an effect.13,26 PBM balances the levels of oxidants and antioxidants by increasing the mitochondrial membrane potential and improving the electron transfer cycle. Previous studies have shown that near-infrared wavelengths can regulate cell metabolism by increasing the mitochondrial membrane potential, producing ATP, and decreasing ROS levels.27 Additionally, PBM stimulates mitochondrial biogenesis by increasing the levels of PGC1α, transcription factor A, and uncoupling protein 2.28,29 In line with these findings, our observations in this study revealed that a wavelength of 890 nm reduced oxidative stress and improved the activity of testicular tissue and sperm production. In testicular tissue, Sertoli cells support other cells. Many mitochondria are present in the cytoplasm of these cells.30 Therefore, damage to the mitochondria of Sertoli cells following HS before maturation acts as a source of ROS production.31 Laser light reduces the level of oxidants and increases the level of antioxidants, such as glutathione, with effects on the mitochondrial membrane in these cells. Moreover, laser therapy improves the Sertoli cell metabolism.32 The proper functioning of these cells ensures the division and differentiation of other cells in the spermatogenic epithelium and the process of sperm production during puberty.
In addition, the results of the present study revealed that the number of Leydig cells and testosterone levels in PBM-treated mice increased notably. Like in Sertoli cells, many mitochondria are present in the cytoplasm of these cells. Therefore, by affecting the mitochondria of Leydig cells and improving their activity, the laser increases androgen and testosterone production at the beginning of puberty, which stimulates spermatogenesis.33 This finding aligns with previous research. Hasani et al10 investigated the effects of laser therapy in a mice model of transient scrotal hyperthermia. Their results demonstrated an increase in both Leydig cell number and testosterone production. In male gonads, spermatogonial cells are considered stem cells. Maintaining the anatomical and functional niches of these cells is necessary for their survival and mitotic division.34 Before puberty, HS causes changes in the spermatogonial cell niche by inducing oxidative stress, which ultimately affects their behavior.35 Laser irradiation preserves the niche by reducing the level of oxidants and preventing cell death. Additionally, PBM stimulates mitosis and self-renewal in these cells by inducing the expression of C-kit and Pcna, which ultimately maintains the population of spermatogonia and increases the number of spermatocytes and spermatids.36,37 Germ cell proliferation is the reason for the increase in the final number of sperms during puberty. These observations are consistent with a study conducted by Tabatabaee et al,38 who demonstrated that a laser with a wavelength of 890 nm (0.03 J/cm2) increased the number of spermatogonial cells and improved spermatogenesis in mice undergoing scrotal hyperthermia. The results obtained from this study emphasize that laser therapy can be used to reduce testicular damage caused by HS during prepuberty.
Conclusion
The data collected from this study show that HS disrupts spermatogenesis during puberty through the induction of oxidative stress in the prepubertal testis. PBM which has a positive effect on the mitochondria of testicular cells decreases the level of ROS and the expression of caspase-3. In addition, PBM stimulates the division of spermatogonial cells by increasing the expression of mitotic genes, leading to an increase in the germ cell population and an improvement in sperm parameters during puberty.
Acknowledgments
We would like to express our appreciation to the Laser application in the medical sciences research center at Shahid Beheshti University of Medical Sciences in Tehran, Iran, for their financial support of this research project (grant number: 30219).
Authors’ Contribution
Conceptualization: Mohsen Norouzian and Fatemeh Fadaei Fathabadi.
Data curation: Mohsen Norouzian, Fatemeh Fadaei Fathabadi, and Fakhroddin Aghajanpour.
Formal analysis: Hojjat-Allah Abbaszadeh, Hamid Nazarian, and Reza Soltani.
Funding acquisition: Mohsen Norouzian.
Investigation: Fakhroddin Aghajanpour, Azar Afshar, and Reza Soltani.
Methodology: Hojjat-Allah Abbaszadeh, Hamid Nazarian, Azar Afshar, and Homayoon Bana Derakhshan.
Project administration: Mohsen Norouzian and Fatemeh Fadaei Fathabadi.
Resources: Mohsen Norouzian.
Software: Homayoon Bana Derakhshan, Azar Afshar, Reza Soltani, and Hamid Nazarian.
Supervision: Mohsen Norouzian and Fatemeh Fadaei Fathabadi.
Validation: Hojjat-Allah Abbaszadeh, Hamid Nazarian, and Homayoon Bana Derakhshan.
Visualization: Homayoon Bana Derakhshan, Fakhroddin Aghajanpour, and Reza Soltani.
Writing–original draft: Fakhroddin Aghajanpour, Azar Afshar, and Hojjat-Allah Abbaszadeh.
Writing–review editing: Fakhroddin Aghajanpour, Azar Afshar, and Mohsen Norouzian.
Competing Interests
All the writers confirm that they have no personal concerns or biases that could affect the findings of this study.
Ethical Approval
The protocols of this research were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1400.730).
Funding
None.
Please cite this article as follows: Aghajanpour F, Abbaszadeh HA, Nazarian H, Afshar A, Soltani R, Bana Derakhshan H, et al. Photobiomodulation improves histological parameters of testis and spermatogenesis in adult mice exposed to scrotal hyperthermia in the prepubertal phase. J Lasers Med Sci. 2024;15:e49. doi:10.34172/jlms.2024.49.
References
- 1.Couto-Santos F, Souza AC, Bastos DS, Ervilha LO, Dias FC, de Sales Araújo L, et al. Prepubertal exposure to arsenic alters male reproductive parameters in pubertal and adult rats. Toxicol Appl Pharmacol. 2020;409:115304. doi: 10.1016/j.taap.2020.115304. [DOI] [PubMed] [Google Scholar]
- 2.Rebourcet D, O’Shaughnessy PJ, Pitetti JL, Monteiro A, O’Hara L, Milne L, et al. Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development. 2014;141(10):2139–49. doi: 10.1242/dev.107029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism. 2018;86:3–17. doi: 10.1016/j.metabol.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Singh SK. Prepubertal exposure to perfluorononanoic acid interferes with spermatogenesis and steroidogenesis in male mice. Ecotoxicol Environ Saf. 2019;170:590–9. doi: 10.1016/j.ecoenv.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Allen CM, Lopes F, Mitchell RT, Spears N. How does chemotherapy treatment damage the prepubertal testis? Reproduction. 2018;156(6):R209–33. doi: 10.1530/rep-18-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrashekar KN, Muralidhara Muralidhara. Evidence of oxidative stress and mitochondrial dysfunctions in the testis of prepubertal diabetic rats. Int J Impot Res. 2009;21(3):198–206. doi: 10.1038/ijir.2009.9. [DOI] [PubMed] [Google Scholar]
- 7.Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage A review. Int J Hyperthermia. 2014;30(7):513–23. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 8.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80(5):913–9. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, et al. Photobiomodulation—underlying mechanism and clinical applications. J Clin Med. 2020;9(6):1724. doi: 10.3390/jcm9061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasani A, Khosravi A, Rahimi K, Afshar A, Fadaei-Fathabadi F, Raoofi A, et al. Photobiomodulation restores spermatogenesis in the transient scrotal hyperthermia-induced mice. Life Sci. 2020;254:117767. doi: 10.1016/j.lfs.2020.117767. [DOI] [PubMed] [Google Scholar]
- 11.Safian F, Ghaffari Novin M, Karimi M, Kazemi M, Zare F, Ghoreishi SK, et al. Photobiomodulation with 810 nm wavelengths improves human sperms’ motility and viability in vitro. Photobiomodul Photomed Laser Surg. 2020;38(4):222–31. doi: 10.1089/photob.2019.4773. [DOI] [PubMed] [Google Scholar]
- 12.Espey BT, Kielwein K, van der Ven H, Steger K, Allam JP, Paradowska-Dogan A, et al. Effects of pulsed-wave photobiomodulation therapy on human spermatozoa. Lasers Surg Med. 2022;54(4):540–53. doi: 10.1002/lsm.23399. [DOI] [PubMed] [Google Scholar]
- 13.Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 2018;94(2):199–212. doi: 10.1111/php.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dadras S, Abdollahifar MA, Nazarian H, Ghoreishi SK, Fallahnezhad S, Naserzadeh P, et al. Photobiomodulation improved stereological parameters and sperm analysis factors in streptozotocin-induced type 1 diabetes mellitus. J Photochem Photobiol B. 2018;186:81–7. doi: 10.1016/j.jphotobiol.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Aghajanpour F, Soltani R, Afshar A, Abbaszadeh HA, Fadaei Fathabadi F, Moeinian N, et al. Sertoli cell-conditioned medium can improve blood-testis-barrier function and spermatogenesis in azoospermia mice induced by scrotal hyperthermia: an experimental study. Int J Reprod Biomed. 2024;22(1):17–30. doi: 10.18502/ijrm.v22i1.15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshar A, Aliaghaei A, Nazarian H, Abbaszadeh HA, Naserzadeh P, Fadaei Fathabadi F, et al. Curcumin-loaded iron particle improvement of spermatogenesis in azoospermic mouse induced by long-term scrotal hyperthermia. Reprod Sci. 2021;28(2):371–80. doi: 10.1007/s43032-020-00288-2. [DOI] [PubMed] [Google Scholar]
- 17.Ghaffari Novin M, Sabbagh Alvani M, Mafi Balani M, Aliaghaei A, Afshar A, Aghajanpour F, et al. Therapeutic effects of edaravone on azoospermia: free radical scavenging and autophagy modulation in testicular tissue of mice. J Reprod Infertil. 2022;23(2):73–83. doi: 10.18502/jri.v23i2.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayoubi M, Naserzadeh P, Hashemi MT, Rostami MR, Tamjid E, Tavakoli MM, et al. Biochemical mechanisms of dose-dependent cytotoxicity and ROS-mediated apoptosis induced by lead sulfide/graphene oxide quantum dots for potential bioimaging applications. Sci Rep. 2017;7(1):12896. doi: 10.1038/s41598-017-13396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kastelic JP, Wilde RE, Bielli A, Genovese P, Rizzoto G, Thundathil J. Hyperthermia is more important than hypoxia as a cause of disrupted spermatogenesis and abnormal sperm. Theriogenology. 2019;131:177–81. doi: 10.1016/j.theriogenology.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Reyes JG, Farias JG, Henríquez-Olavarrieta S, Madrid E, Parraga M, Zepeda AB, et al. The hypoxic testicle: physiology and pathophysiology. Oxid Med Cell Longev. 2012;2012:929285. doi: 10.1155/2012/929285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giampietri C, Petrungaro S, Coluccia P, D’Alessio A, Starace D, Riccioli A, et al. Germ cell apoptosis control during spermatogenesis. Contraception. 2005;72(4):298–302. doi: 10.1016/j.contraception.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6):769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Y, Li J, He S. Zinc protects against heat stress-induced apoptosis via the inhibition of endoplasmic reticulum stress in TM3 Leydig cells. Biol Trace Elem Res. 2022;200(2):728–39. doi: 10.1007/s12011-021-02673-7. [DOI] [PubMed] [Google Scholar]
- 24.Handelsman DJ. Testosterone, spermatogenesis, and unravelling the mysteries of puberty. Endocrinology. 2020;161(9):bqaa120. doi: 10.1210/endocr/bqaa120. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aryan A, Aghajanpour F, Dashtdar M, Hejazi F, Salimi M, Afshar A, et al. Exploring intercellular dynamics: ultra-weak biophoton emission as a novel indicator of altered cell functions and disease in oligospermia mice. J Lasers Med Sci. 2023;14:e65. doi: 10.34172/jlms.2023.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics. 2013;6(10):829–38. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan RA, Mermier CM, Bisoffi M, Trujillo KA, Conn CA. Dietary stimulators of the PGC-1 superfamily and mitochondrial biosynthesis in skeletal muscle A mini-review. J Physiol Biochem. 2014;70(1):271–84. doi: 10.1007/s13105-013-0301-4. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Dong T, Li P, Wu MX. Noninvasive low-level laser therapy for thrombocytopenia. Sci Transl Med. 2016;8(349):349ra101. doi: 10.1126/scitranslmed.aaf4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porubska B, Vasek D, Somova V, Hajkova M, Hlaviznova M, Tlapakova T, et al. Sertoli cells possess immunomodulatory properties and the ability of mitochondrial transfer similar to mesenchymal stromal cells. Stem Cell Rev Rep. 2021;17(5):1905–16. doi: 10.1007/s12015-021-10197-9. [DOI] [PubMed] [Google Scholar]
- 31.Sun W, Ni Z, Li R, Chang X, Li W, Yang M, et al. Flurochloridone induces Sertoli cell apoptosis through ROS-dependent mitochondrial pathway. Ecotoxicol Environ Saf. 2021;216:112183. doi: 10.1016/j.ecoenv.2021.112183. [DOI] [PubMed] [Google Scholar]
- 32.Rezaei F, Bayat M, Nazarian H, Aliaghaei A, Abaszadeh HA, Naserzadeh P, et al. Photobiomodulation therapy improves spermatogenesis in busulfan-induced infertile mouse. Reprod Sci. 2021;28(10):2789–98. doi: 10.1007/s43032-021-00557-8. [DOI] [PubMed] [Google Scholar]
- 33.Adikara YA, Samik A, Yudaniayanti IS, Adikara TS, Hestianah EP, Utama S. Effect of laser acupuncture shoot on ova point of male Mojosari duck (Anas plathyrhynchos) on the number of Sertoli and Leydig cells. KnE Life Sci. 2017;3(6):650–7. doi: 10.18502/kls.v3i6.1194. [DOI] [Google Scholar]
- 34.Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82(5):381–8. doi: 10.1532/ijh97.05088. [DOI] [PubMed] [Google Scholar]
- 35.Moreno Acosta OD, Boan AF, Hattori RS, Fernandino JI. Notch pathway is required for protection against heat stress in spermatogonial stem cells in medaka. Fish Physiol Biochem. 2023;49(3):487–500. doi: 10.1007/s10695-023-01200-w. [DOI] [PubMed] [Google Scholar]
- 36.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):348–64. doi: 10.1109/jstqe.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prabhu V, Rao BSS, Rao ACK, Prasad K, Mahato KK. Photobiomodulation invigorating collagen deposition, proliferating cell nuclear antigen and Ki67 expression during dermal wound repair in mice. Lasers Med Sci. 2022;37(1):171–80. doi: 10.1007/s10103-020-03202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabatabaee F, Darabi S, Soltani R, Aghajanpour F, Afshar A, Abbaszadeh HA, et al. Therapeutic effects of exosome therapy and photobiomodulation therapy on the spermatogenesis arrest in male mice after scrotum hyperthermia. J Lasers Med Sci. 2024;15:e3. doi: 10.34172/jlms.2024.03. [DOI] [PMC free article] [PubMed] [Google Scholar]