Abstract
Primary cultures of intrahepatic bile duct epithelial (IBDE) cells isolated from duckling livers were successfully grown for studies of duck hepatitis B virus (DHBV). The primary IBDE cells were characterized by immunohistochemistry using CAM 5.2, a cytokeratin marker which was shown to react specifically to IBDE cells in duck liver tissue sections and in primary cultures of total duck liver cells. Immunofluorescence assay using anti-duck albumin, a marker for hepatocytes, revealed that these IBDE cultures did not appear to contain hepatocytes. A striking feature of these cultures was the duct-like structures present within each cell colony of multilayered IBDE cells. Normal duck serum in the growth medium was found to be essential for the development of these cells into duct-like structures. When the primary cultures of duck IBDE cells were acutely infected with DHBV, dual-labeled confocal microscopy using a combination of anti-DHBV core proteins and CAM 5.2 or a combination of anti-pre-S1 proteins and CAM 5.2 revealed that the IBDE cell colonies contained DHBV proteins. Immunoblot analysis of these cells showed that the DHBV pre-S1 and core proteins were similar to their counterparts in infected primary duck hepatocyte cultures. Southern blot analysis of infected IBDE preparations using a digoxigenin-labeled positive-sense DHBV riboprobe revealed the presence of hepadnavirus covalently closed circular (CCC) DNA, minus-sense single-stranded (SS) DNA , double-stranded linear DNA, and relaxed circular DNA. The presence of minus-sense SS DNA in the acutely infected IBDE cultures is indicative of DHBV reverse transcriptase activity, while the establishment of a pool of viral CCC DNA reveals the ability of these cells to maintain persistent infection. Taken collectively, the results from this study demonstrated that primary duck IBDE cells supported hepadnavirus replication as shown by the de novo synthesis of DHBV proteins and DNA replicative intermediates.
Hepatitis B virus (HBV) infection poses a major public health threat in many countries where the infection is endemic. Recent estimates revealed that there are approximately 350 million HBV chronic carriers worldwide, with over 1 million deaths occurring annually from HBV-related diseases (2, 25). Alpha interferon and lamivudine (a nucleoside analogue) are the only approved treatments for chronic HBV infection. However, both treatment strategies are effective in suppressing viral replication in only 30 to 40% of patients (10, 16, 21). There is clearly a need to seek alternative antiviral treatment strategies for this important disease.
Significant progress has been made in identifying potential antiviral therapies for HBV disease. In this regard, duck HBV (DHBV), a HBV-related avian hepadnavirus, has been used extensively for the evaluation of new anti-HBV agents (38). DHBV has proved a valuable replication and pathogenesis model for HBV infection because it readily establishes a persistent noncytopathic infection in ducklings in a manner similar to that of perinatal HBV infection (39). Within the liver, the relaxed circular (RC), the double-stranded linear (DSL), the single-stranded (SS), and the covalently closed circular (CCC) DNA replicative intermediates produced during productive DHBV infection are similar to those of species found in HBV-infected individuals (48). These hepadnavirus DNA replicative intermediates serve as important markers during antiviral therapy, as their level of expression is indicative of treatment success (48).
To date, all antiviral agents tested against HBV have proved virustatic rather than virucidal, with cessation of therapy resulting in the return of all hepadnavirus replicative intermediates to at least pretreatment levels (7, 38). This relapse appears to be due to the persistence of the hepadnavirus CCC DNA. The CCC DNA, representing the transcriptionally active template, exists in the nuclei of infected cells and is found as a viral minichromosome (6, 31). This form of viral DNA does not undergo semiconservative replication and therefore is not a direct target for present antiviral agents. Thus, during antiviral treatment the CCC DNA level in infected cells generally remains stable (38).
Another contributing factor to the relapse phenomenon may be the presence of hepadnavirus replication within the liver or in extrahepatic sites where antiviral agents may be less effective in cells other than hepatocytes (27, 28, 33, 34). Immunohistochemical (IHC) and in situ hybridization (ISH) studies of tissues derived from congenitally DHBV-infected ducks treated with antiviral agents have shown the retention of virus in intrahepatic bile duct epithelial (IBDE) cells despite virus clearance from hepatocytes (24, 27, 28, 33–35). It has been postulated that the inability of the antiviral agents to clear the virus from IBDE cells has important implications for therapy, since these cells may constitute an ongoing reservoir of replicating virus that allows persistent infection in the liver and reinfection of hepadnavirus-free hepatocytes after cessation of antiviral therapy (23, 24, 27, 28, 33–35). IBDE cells are not the only nonhepatocyte cells to harbor hepadnaviruses. Spleen cells, pancreatic islet and acinar cells, and cells of the lymphoid organs from infected humans (5, 9), Pekin ducks (14, 15, 27, 45), and woodchucks (13, 20) have all been shown to contain and express hepadnavirus proteins and DNA. It is now well established that replication of hepadnaviruses occurs predominantly in hepatocytes, but it is not known whether active viral replication occurs in other cells containing hepadnavirus markers. Studies in this area have been hampered by the lack of suitable cell culture systems that can support hepadnavirus replication. Recent technical advances in isolating primary cultures of mammalian IBDE cells have allowed for such studies to now be considered. While these cultures have been developed mainly as a model to study the pathophysiology of human bile duct diseases (1, 3, 18), there is also a role for their potential application in the studies of hepadnavirus replication. This study aimed to culture primary duck IBDE cells in order to investigate whether they can support hepadnavirus replication.
MATERIALS AND METHODS
Animals.
Pekin-Aylesbury ducklings negative for DHBV were obtained from a commercial supplier (Tegal, Sydney, New South Wales, Australia) with assistance from Robert Dixon (University of New South Wales, Sydney, Australia). The serum from each duckling was collected and tested for DHBV DNA by dot blot hybridization as described previously (11). All protocols involving the use of ducklings were approved by the Animal Experimentation Ethics Committee, Royal Melbourne Hospital Research Foundation, Melbourne, Australia.
Enzymes and antibodies.
Collagenase and hyaluronidase were purchased from Worthington Biochemical Corporation, Lakewood, N.J., while pronase was obtained from Roche Molecular Biochemicals, Mannheim, Germany. Monoclonal antibodies to DHBV pre-S1 protein and to duck Kupffer cells were kind gifts from J. Pugh (MicroBioTest Inc., Sterling, Va.), while rabbit polyclonal antibodies to DHBV core protein were kindly provided by A. Jilbert (Institute of Medical and Veterinary Science, Adelaide, Australia). CAM 5.2 and fluorescein isothiocyanate (FITC)-conjugated CAM 5.2 were purchased from Becton Dickinson, Paramus, N.J. FITC-conjugated, tetramethyl rhodamine isocyanate (TRITC)-conjugated, horseradish peroxidase (HRP)-conjugated, and alkaline phosphatase (AP)-conjugated secondary antibodies were from Dako (Carpinteria, Calif.). Texas red-conjugated anti-mouse immunoglobulins were purchase from Pharmacia-Amersham, Uppsala, Sweden.
Isolation of duck IBDE cells.
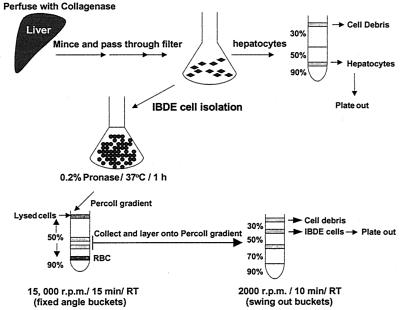
The procedure employed for the isolation of IBDE cells is a modified method of Sirica and Gainey (40) (Fig. 1). Briefly, liver from a 7-day-old duckling was surgically removed and perfused via the hepatic vein as described previously (46). The liver was perfused with 0.05% (wt/vol) collagenase in Dulbecco's modified Eagle's medium (DMEM) F/12 (Gibco-BRL) supplemented with 100 U of penicillin/ml and 100 μg of streptomycin/ml after flushing with Hanks balanced salt solution, pH 7.4 (Gibco-BRL), containing 50 mM EGTA. The collagenase-digested liver was then minced into small fragments and allowed to incubate in DMEM F/12 containing 0.05% (wt/vol) collagenase and 0.05% (wt/vol) hyaluronidase for 30 min at 37°C. The minced tissue was washed several times in DMEM F/12 and passed through a crude sieve to obtain a total liver cell suspension. An aliquot of the cell suspension was processed for primary hepatocyte culture (see below) while the rest of the hepatocytes in the total liver cells were lysed by incubation in 100 ml of 0.2% (wt/vol) pronase in DMEM F/12 for 45 min at 37°C. DNase I was then added to a final concentration of 100 μg/ml to digest released cellular DNA, which may cause cell clumping. After incubation for a further 15 min, the enzymes were removed from the cell suspension by several washes in DMEM F/12. The cells were then resuspended in a minimal volume of medium and layered onto a Percoll (Pharmacia) gradient comprised of 20 ml of 50% (vol/vol) and 5 ml of 90% (vol/vol) isotonic Percoll. The gradients were centrifuged at 15,000 rpm for 15 min at room temperature (RT) in a JA20 Beckman centrifuge to separate lysed hepatocytes and other cell debris from the nonparenchymal cells. Cells banding lower down the 50% Percoll gradient were collected, washed, and overlaid onto a second gradient comprised of 2.5 ml of 30% (vol/vol), 2.5 ml of 50% (vol/vol), 2.5 ml of 70% (vol/vol), and 1 ml of 90% (vol/vol) isotonic Percoll; Percoll density marker beads were also layered on top of a parallel gradient. The gradient was centrifuged at 2,000 × g for 15 min at RT, and cells banding at each Percoll interface were collected, washed, and resuspended in growth medium, which was DMEM F/12 containing 5% (vol/vol) fetal calf serum, 100 μg of soybean trypsin inhibitor/ml, 0.02% (wt/vol) glucose, 450 ng of hydrocortisone 21-hemisuccinate/ml, 1× insulin-transferrin-selenium (ITS)-A supplement (Gibco-BRL), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 1.5% (vol/vol) dimethyl sulfoxide (DMSO), and 1.5% (vol/vol) duckling sera (virus-free). Cells found banding at each Percoll interface were designated from the top to the bottom of the gradient as fractions F1 (1.04/1.06 g/cm3), F2 (1.06/1.08 g/cm3), and F3 (1.08/1.11 g/cm3). The cells from each interface were seeded onto 12-well plates (Nunc) and coverslips (12-mm diameter) which had been thinly coated with rat tail collagen according to the manufacturer's instructions (Collaborative Research Products, Bedford, Mass.). The cells were maintained by medium changing every second day.
FIG. 1.
Schematic diagram of the method used for the isolation of IBDE cells from duckling liver.
Primary duck hepatocyte cultures.
For primary cultures of duck hepatocytes, an aliquot of total liver cells from the above-mentioned IBDE cell isolation procedure was overlaid onto a Percoll gradient comprised of 30% (vol/vol), 50% (vol/vol), and 90% (vol/vol) isotonic Percoll. The gradient was centrifuged at 2,000 × g for 10 min at RT. A yellow layer of cells banding at the 50% and 90% Percoll interface was collected, washed several times in growth medium, and seeded onto 12-well plates and coverslips (12-mm diameter). The culture medium was changed every second day.
Acute DHBV infection.
The primary duck hepatocytes or primary IBDE cells at day 1 of culture were inoculated with 100 viral genome equivalents (vge)/ml of DHBV (Australia strain) derived from pooled duckling sera (4, 12). The cells were incubated for 2 h with occasional rocking prior to the addition of growth medium. At appropriate times postinfection (p.i.), cells were harvested for the analysis of viral proteins and DNA replicative intermediates.
Preparation of labeled probes.
A full-length clone of the Australian strain of DHBV in the plasmid pT3T7 was used as template for the generation of a digoxigenin (DIG)-labeled riboprobe (37). The riboprobe containing DIG-labeled UTP was synthesized using the DIG RNA labeling kit (Roche Diagnostics, Sydney, Australia) according to the manufacturer's protocol. The riboprobe generated with T7 polymerase in the reaction mix detects the minus strand of DHBV DNA.
Detection of DHBV replicative intermediates.
The procedures for extraction of DHBV total DNA and CCC DNA have been described previously by Luscombe et al. (27). For hepadnavirus CCC DNA analysis, 0.5 ml of lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 150 mM NaCl, 1% [wt/vol] sodium dodecyl sulfate [SDS]) was added to the cell culture to lyse the cells, followed by the addition of 2.5 M KCl to a final concentration of 0.25 M. The resultant insoluble protein complex was removed by centrifugation. The viral DNA was then extracted using phenol:chloroform (1:1) and precipitated with ethanol. For total DHBV DNA analysis, cells were disrupted in Tris lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 10 mM EDTA, and 0.5% [wt/vol] SDS), digested with 100 mg of proteinase K/ml for 2 h at 50°C, and processed for viral DNA extraction as described above.
Assay of DHBV virions from culture medium.
DHBV DNA in virions secreted into the culture medium was assayed by the pronase-DNase I method (22). Infected culture medium was collected at various days p.i. and centrifuged for 5 min at 10,000 × g to remove cellular debris. The clarified medium was then incubated in 0.5 mg of pronase/ml for 1 h at 37°C to degrade free nucleocapsids. Viral DNA released from the degraded nucleocapsids was removed by the addition of magnesium acetate to a final concentration of 6 mM, followed by digestion in DNase I (at a final concentration of 50 μg/ml) for 30 min at 37°C. Secreted virions were lysed by the addition of EDTA and SDS to a final concentration of 10 mM and 0.5%, respectively. DNA was then extracted and prepared for Southern blot analysis.
Immunoblot assay.
The method for protein analysis was essentially that described by Lin et al. (24). Briefly, protein samples in Tris lysis buffer separated on a SDS–12% (wt/vol) polyacrylamide gel electrophoresis (PAGE) gel were transferred onto a nylon membrane (Amersham, Little Chalfont, United Kingdom). The blot was air dried prior to being blocked with 3% (wt/vol) skim milk in PBST buffer (phosphate-buffered saline [PBS] in 0.3% [vol/vol] Tween 20) for 1 h at RT. The blot was then incubated with primary antibodies diluted in PBST followed by reactivity with the appropriate HRP-conjugated secondary antibody. An enhanced chemiluminescence (ECL) system (Amersham) was employed as the protein detection system and was used according to the manufacturer's protocol.
Southern hybridization.
DNA was subjected to gel electrophoresis and processed for Southern hybridization as described previously by Luscombe et al. (28). The DNA was transferred onto nylon membranes (Roche Molecular Biochemicals) and baked for 30 min at 120°C. The hybridization and detection methods specified by Roche Molecular Biochemicals were employed. Briefly, the membrane was prehybridized for 2 h in a solution containing 50% (vol/vol) deionized formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.5% (vol/vol) Sarkosyl, 1% (wt/vol) SDS, and 2% (wt/vol) blocking reagent (Roche Molecular Biochemicals). The DIG-labeled riboprobe was then added, with hybridization proceeding overnight at 42°C. After hybridization, the membrane was washed twice with 0.1% (wt/vol) SDS in 2× SSC for 15 min and then twice with 0.1% (wt/vol) SDS in 0.5× SSC for 30 min at 50°C. For ECL detection of the DIG-labeled DNA, the membrane was incubated in blocking solution (1% [wt/vol] blocking reagent [Roche Molecular Biochemicals], 0.1 M maleic acid [pH 7.5], and 150 mM NaCl) for 1 h at RT followed by the addition of anti-DIG-AP (Roche Molecular Biochemicals) diluted 1:20,000 in blocking solution. After 30 min of incubation at RT, the membrane was subjected to two 15-min washes in washing buffer (0.1 M maleic acid [pH 7.5], 150 mM NaCl, and 0.3% [vol/vol] Tween 20). After equilibrating the membrane in detection buffer (0.1 M Tris [pH 9.6] and 100 mM NaCl) for 5 min, CDP-Star (Roche Molecular Biochemicals) diluted 1/100 in detection buffer was added to the membrane. For detection of the chemiluminescent signal, the membrane was exposed to Fuji medical X-ray film. DIG-labeled DNA Molecular Weight Marker VII from Roche Molecular Biochemicals was used as a marker for hepadnavirus DNA replicative intermediates.
IFA.
Coverslip cultures were fixed with absolute methanol for 5 min at RT or with cold ethanol:acetic acid (3:1) for 5 min. Alternatively, the cells were fixed in 2% (wt/vol) paraformaldehyde in PBS, pH 7.3, for 20 min and then permeabilized with 0.1% (vol/vol) Triton X-100 for 30 min. Fixed cells were processed for immunofluorescence assay (IFA) as described previously (29). Briefly, the cells were incubated in blocking buffer (1% [wt/vol] bovine serum albumin in PBS) for 30 min at RT and then incubated with primary antibody for 1 h at RT. After several washes in PBS, the cells were reacted for 1 h at RT with the appropriate secondary-conjugated antibody diluted in blocking buffer containing Evans Blue (Dako). For double-labeling studies, the procedure was repeated with the appropriate antibodies. Coverslip preparations were mounted in fluorescent mounting medium (Dako) and viewed with a Zeiss Axioskop or a Bio-Rad MRC 1024 laser confocal system attached to a Zeiss microscope. Image collection parameters were adjusted to minimize cross-channel leak-over and tested using appropriate single- and double-labeled preparations. Photographs were taken using Kodak Ektachrome film.
IHC.
Duck liver tissue was processed for IHC as described previously (27). Alternatively, coverslip cultures were fixed in 100% (vol/vol) methanol and processed for IHC. To remove endogenous peroxidase, cells were incubated in 1% (vol/vol) H2O2 in PBS for 10 min. The antibody reaction conditions were as described for IFA. Following HRP or AP reaction with diaminobenzidine (DAB) (Dako) or Fast Red (Sigma), the cells were rinsed with PBS, counterstained with Mayer hematoxylin, mounted in Clearmount (Zymed, South San Francisco, Calif.), and viewed with an Olympus BHS microscope. Photographs were taken using Kodak Ektachrome film.
RESULTS
Characterization of the primary culture of duck IBDE cells.
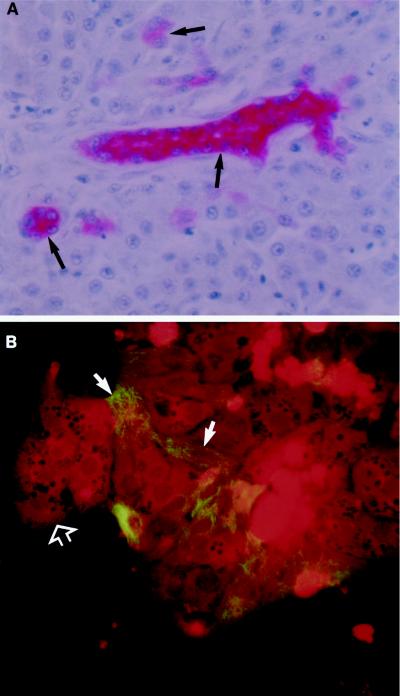
CAM 5.2, comprising antibodies to cytokeratin, is a reliable marker for duck IBDE cells (27, 28, 34). In this study, the specificity of CAM 5.2 was investigated using duck liver, kidney, and pancreas tissue sections and primary coverslip cultures of total duck liver cells. In duck liver tissue sections processed for IHC, only the cytoplasm of duck IBDE cells in the liver tissue section were positively stained with CAM 5.2; no hepatocyte staining was evident (Fig. 2A). Furthermore, cells in duck pancreas and kidney tissue sections were not stained with CAM 5.2 (results not shown). When primary coverslip cultures of total duck liver cells were processed for IFA, CAM 5.2 staining was observed as fluorescent filaments in the cytoplasm of epithelium-like cells; these fluorescent cells composed a small proportion (<10%) of the total liver cell population (Fig. 2B). Hepatocytes, which were easily recognized by their characteristic morphology, showed no detectable fluorescence (Fig. 2B). Presumably, the fluorescent staining cells detected in the primary liver cell culture corresponded to the CAM 5.2-positive cells detected by IHC in liver tissue sections (Fig. 2A) and therefore represented IBDE cells. It must be noted that there are a wide variety of commercially available cytokeratin markers with known reactivity to mammalian IBDE cells (19, 40). However, most were found not to react with duck IBDE cells in liver tissue sections or in primary duck liver cultures (results not shown).
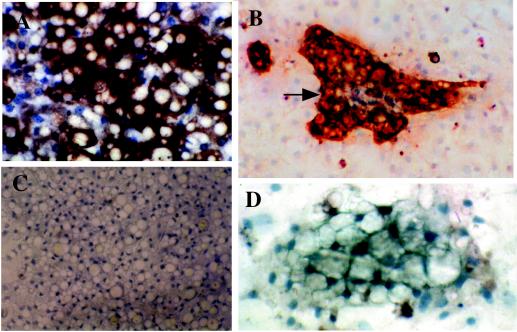
FIG. 2.
Reactivity of CAM 5.2 to duck IBDE cells. (A) Duck liver tissue sections were processed for immunoalkaline phosphatase staining as described in Materials and Methods. After reactivity with the substrate, Fast Red, the tissues were counterstained with Mayer's hematoxylin. Pink precipitates were detected in the cytoplasm of bile duct cells (arrows); no precipitates were detected in hepatocytes. (B) Primary cultures of total liver cells at day 2 of culture were fixed in 100% methanol for 5 min and processed for IFA using CAM 5.2. Fluorescent filaments were detected in approximately 10% of the total liver cells (solid arrows); these fluorescent cells were epithelium-like in morphology. Fluorescent staining was not observed in hepatocytes (open arrow) which were identified by the characteristic polygonal morphology; hepatocytes appeared dull red because of the Evans Blue stain.
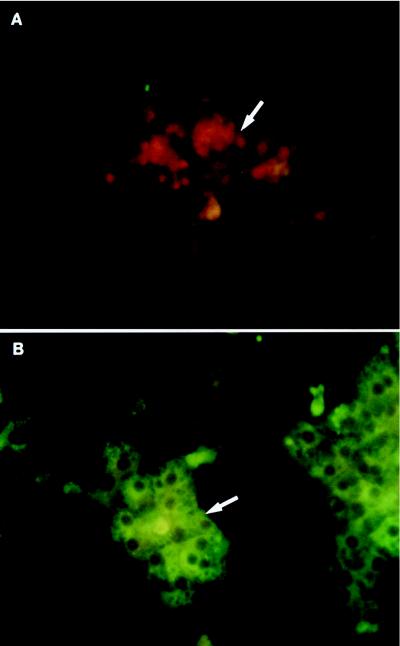
The primary culture of duck IBDE cells was established following isolation of nonparenchymal cells from the total liver cell population. Hepatocytes were lysed by pronase digestion following collagenase and hyaluronidase perfusion of the duck liver. Lysed hepatocytes were removed from the pronase-treated cells by employing a two-step Percoll gradient procedure as described in Materials and Methods (Fig. 1). The majority of the cells which stained positive for CAM 5.2, as determined by IFA, were recovered at the 1.04/1.06 g/cm3 density interface; these cells were cultured and referred to as primary duck IBDE cells. Although some CAM 5.2-positive cells were detected in other fractions, they generally contained a high proportion of cells that stained positive for desmin (a marker for Stellate cells) when cultured. In addition, these cultures generally became overgrown with fibroblasts and were not used in this study. To determine whether hepatocytes have been removed by the pronase-Percoll gradient method, coverslip cultures of primary duck IBDE cells were processed at day 2 of culture for IFA using antibodies to duck albumin, a marker for hepatocytes. Cytoplasmic fluorescent staining was not detected in primary IBDE cultures using the anti-duck albumin (Fig. 3A), although cytoplasmic fluorescent staining was observed in parallel primary duck hepatocyte (PDH) cultures (Fig. 3B). Based on this analysis, it appears that the primary duck IBDE culture preparations did not contain contaminating hepatocytes.
FIG. 3.
Characterization of primary cultures of duck IBDE cells and PDHs by IFA using polyclonal antibodies to duck albumin. Coverslip cultures of primary duck IBDE (A) or PDH cultures (B) were fixed with cold ethanol:acetic acid (3:1) for 5 min at day 2 of culture and processed for IFA using a 1/200 dilution of goat anti-albumin. After reaction with FITC-conjugated anti-goat immunoglobulin G containing Evans Blue, the cells were mounted and viewed. Cytoplasmic fluorescent staining was not detected in the primary IBDE cultures (A, arrow) but was detected in parallel cultures of PDHs (B, arrow). IBDE cells in panel A appeared red because of the Evans Blue counterstain.
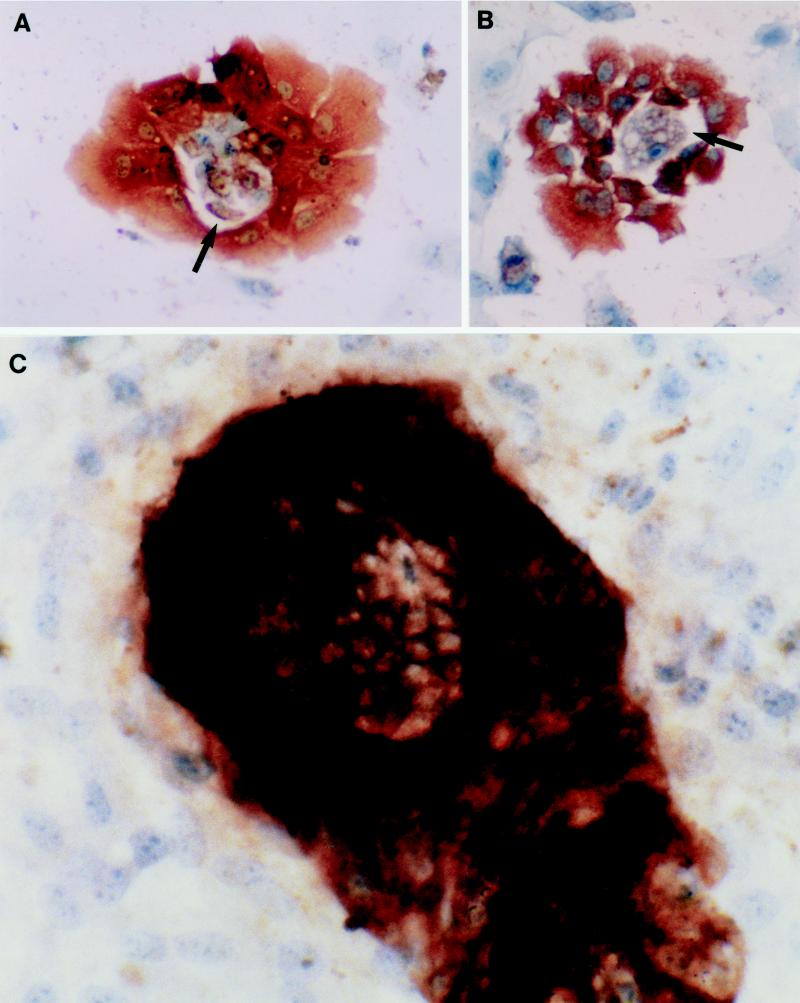
The growth characteristics of the primary duck IBDE cultures were investigated by IHC using CAM 5.2. Although the cells were plated to ensure 80% confluency, only 30% of the cells were adherent to the plate despite cell viability of 90% as determined by trypan blue exclusion assay. At 2 to 3 days of culture, positively stained CAM 5.2 cells were readily observed as cell clusters with the nuclei generally located at the basal region (Fig. 4A); a lumen was readily observed in these cell clusters. By day 5 of culture, these cells had formed colonies comprising multilayered cells, often with duct-like structures (Fig. 4B); approximately 30% of the culture comprised these distinct IBDE cell colonies. The primary IBDE cultures were maintained for 12 days, after which time the majority of the colonies were no longer positive for CAM 5.2. A common observation during the culture of IBDE cells was the detection of an epithelium-like cell monolayer among the IBDE colonies, usually observed after day 5 of culture. When examined by IHC using CAM 5.2, anti-desmin, anti-Kupffer, or anti-duck albumin, the monolayer was generally found to be negative for these markers (results not shown).
FIG. 4.
Phenotypic characterization of the primary duck IBDE cultures. At various days of culture, coverslips of primary IBDE cultures were fixed in 100% methanol for 5 min and processed for immunoperoxidase staining using CAM 5.2. After reactivity with the substrate, DAB, the cells were counterstained with Mayer's hematoxylin. Cytokeratin staining was detected as dark brown precipitates in the cytoplasm of cells at 2 (A and B) and 5 (C) days of culture. (A and B) Early in culture, IBDE cells grew as cell clusters which often contained a lumen (arrow). (C) By day 5 of culture, duct-like structures (arrow) were observed in IBDE colonies.
An important finding was the requirement of normal duck sera (virus-free) for the development of IBDE cells into multilayered colonies. Early attempts to culture these cells without the use of duck sera in the growth medium resulted in a monolayer culture. Although IBDE cells were detected at day 2 as determined by IHC using CAM 5.2, these cells did not develop into IBDE colonies with duct-like structures. The monolayer IBDE culture that formed lost CAM 5.2 reactivity (results not shown).
DHBV proteins in infected primary IBDE cultures.
To characterize the replication of DHBV in primary IBDE cells, the cells were acutely infected with positive duckling sera containing approximately 100 vge/ml. These studies were performed in parallel with acutely infected PDH cultures isolated from the same duck liver. IHC, IFA, and immunoblot assays were employed to characterize the DHBV proteins in the respective primary cultures. In IHC preparations using antibodies to pre-S1 and DAB as substrate, brown cytoplasmic staining was detected in DHBV-infected PDHs (Fig. 5A) and DHBV-infected IBDE cell colonies (Fig. 5B) at day 11 p.i. Brown cytoplasmic staining was not detected in mock-infected PDHs (Fig. 5C) or mock-infected IBDE cells (Fig. 5D). Interestingly, the epithelium-like cell monolayers of unknown identity which were observed later in the DHBV-infected IBDE culture did not appear to contain pre-S1 proteins (Fig. 5B).
FIG. 5.
Detection of DHBV pre-S1 in PDHs or primary cultures of IBDE cells acutely infected with DHBV. Coverslip PDH or IBDE cultures were infected with DHBV-positive duckling sera (A and B) or mock-infected as described in the Materials and Methods (C and D). At 11 days p.i. the cells were fixed in 100% methanol and processed for immunoperoxidase staining using anti-pre-S1. Cells were counterstained with Mayer's hematoxylin. Pre-S1 proteins were found localized in the cytoplasm of DHBV-infected PDHs (A) or in the cytoplasm of DHBV-infected IBDE cell colonies (B, arrow) as seen by the brown cytoplasmic staining. No pre-S1 staining was observed in mock-infected PDHs (C) or mock-infected IBDE cells (D).
To determine whether the cells that expressed hepadnavirus proteins were indeed IBDE cells, studies using dual-labeled IFA were performed. DHBV-infected primary IBDE coverslip cultures were processed for dual-labeled IFA using a combination of anti-DHBV core/CAM 5.2 or anti-pre-S1/CAM 5.2. Confocal microscopy analysis of preparations dual labeled with anti-DHBV core proteins and CAM 5.2 demonstrated cytoplasmic TRITC (Fig. 6A) and FITC staining (Fig. 6B), respectively, within the same cells. Two distinct fluorescent staining patterns were observed within the same cells; DHBV core proteins appeared as diffuse cytoplasmic TRITC staining (Fig. 6A), while CAM 5.2 exhibited striated fluorescent staining indicative of cytokeratin staining (Fig. 6B). Parallel studies using mock-infected cultures showed that fluorescent staining was not detected in cells reacted with anti-DHBV core but was observed with CAM 5.2 (results not shown). To ensure that the lack of anti-DHBV core staining in the mock preparation was not due to poor detection produced by the weaker TRITC fluoroprobe, parallel dual-labeled studies were performed where the fluoroprobes were switched such that the stronger-emitting FITC fluoroprobe reacted with anti-DHBV core while the TRITC fluoroprobe reacted with CAM 5.2. In these studies, FITC staining was not detected (Fig. 6C), while TRITC staining was observed as fluorescent filaments (Fig. 6D) in mock-infected IBDE cells. Thus, these findings confirmed the specificity of the anti-DHBV core.
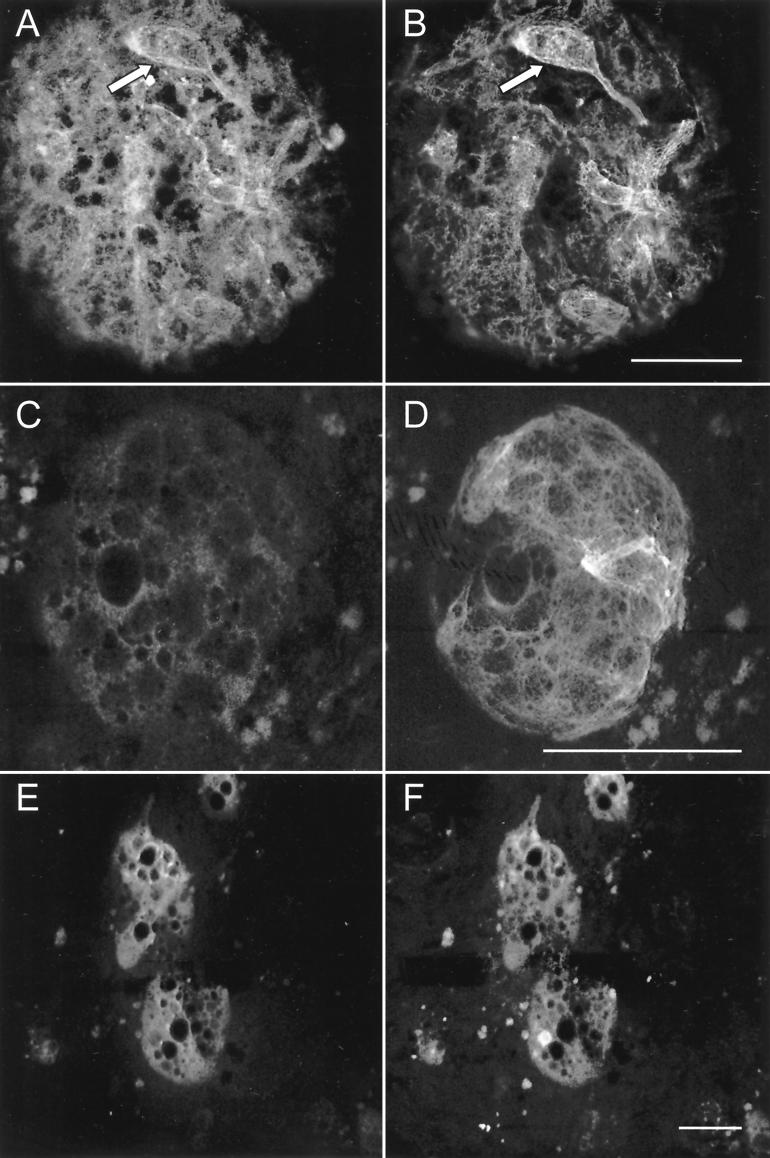
FIG. 6.
Colocalization of DHBV proteins and CAM 5.2-specific proteins in acutely infected or mock-infected primary cultures of IBDE cells. DHBV-infected (A and B) or mock-infected primary cultures of IBDE cells (C and D) were dual labeled with anti-DHBV core proteins (A and C) and CAM 5.2 (B and D) followed by staining with the appropriate TRITC-conjugated (A and D) and FITC-conjugated (B and C) secondary antibodies. DHBV-infected IBDE cell cultures were also dual labeled with anti-pre-S1/Texas red-conjugated secondary antibody (E) and FITC-conjugated CAM 5.2 (F). (A and B) Clusters of cells (arrows) emitting both TRITC and FITC signals can been seen in the IBDE colonies. Bars, 50 μm.
In preparations processed for dual labeling using anti-pre-S1/Texas red-conjugated secondary antibody (Fig. 6E) and FITC-conjugated CAM 5.2 (Fig. 6F), DHBV-infected IBDE cells containing both fluoroprobes were detected. Thus, these studies revealed that infected primary duck IBDE cells comprised CAM 5.2-positive cells that expressed DHBV pre-S1 and core proteins.
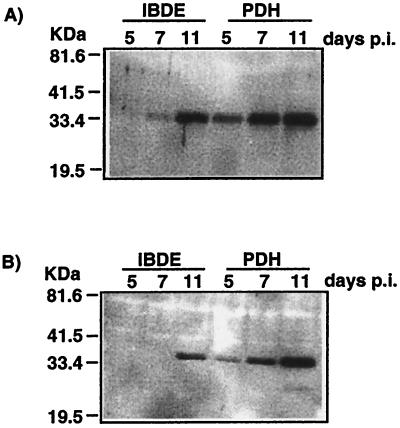
The DHBV proteins in the infected primary IBDE cultures were characterized further by immunoblot assay with parallel studies performed using DHBV-infected PDH preparations. In immunoblot assays, the DHBV core and pre-S1 proteins in DHBV-infected IBDE lysates migrated as a doublet of 34- to 36-kDa (Fig. 7A) and 36- to 38-kDa (Fig. 7B) species, respectively. These pre-S1 and core protein migration patterns were similar to those observed for DHBV-infected PDH preparations (Fig. 7A and B). Although the pre-S1 in the IBDE preparation was only detected by day 11 p.i., core proteins were detectable by day 7 p.i., with an increase in protein through to day 11 p.i. There were considerable difficulties in lysing infected IBDE cells for separation by SDS-PAGE gel analysis. Initial attempts to detect viral proteins in primary IBDE cultures using conventional methods (27) were unsuccessful. IBDE cells harvested in Tris lysis buffer containing 0.5% SDS required mechanical dissociation by being passed several times through a 25G needle followed by a cycle of freeze-thaw before SDS-PAGE analysis; this procedure was not necessary for DHBV-infected PDH preparations. The difficulties in lysing IBDE cells may be a reflection of the differences in membrane composition between biliary cells and hepatocytes (32), whereby the biliary cells may be more resistant to detergent treatment.
FIG. 7.
Immunoblot analysis of primary cultures of IBDE cells infected with DHBV. Parallel studies were performed with DHBV-infected PDH cultures. Duck IBDE cells or PDH cultures were infected with DHBV-positive duckling sera, and at various times p.i. the cells were harvested and processed for immunoblot analysis using anti-core proteins (A) and anti-pre-S1 (B).
DHBV replicative intermediates in acutely infected primary duck IBDE cultures.
During active DHBV replication, the viral RC DNA, DSL DNA, minus-polarity SS DNA, and CCC DNA are detected in infected primary duck hepatocyte cultures. To determine whether these viral DNA replicative intermediates were present within DHBV-infected primary duck IBDE cells, infected cultures were harvested at various times p.i. and processed for Southern blot analysis using a DIG-labeled DHBV riboprobe that hybridizes to the antisense (minus strand) viral DNA strand. In preparations processed for total DHBV DNA analysis, three distinct virus-specific DNA bands corresponding to the DHBV RC DNA, DSL DNA, and minus-polarity SS DNA were detected in infected primary duck IBDE preparations. The three viral replicative forms were detectable by day 4 p.i. and increased in quantity as infection progressed (Fig. 8A). This was similarly observed in parallel studies using infected PDH cultures; no differences in DNA banding patterns were observed between the IBDE and PDH preparations.
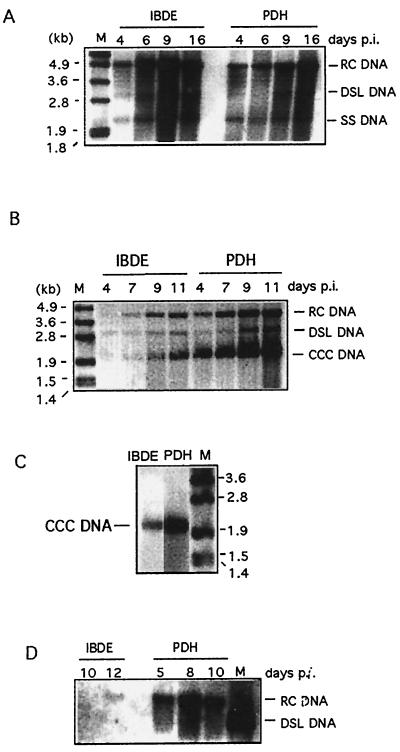
FIG. 8.
Detection of DHBV DNA replicative intermediates in infected duck IBDE cells or culture medium. Parallel studies were performed in DHBV-infected PDH cultures. At various days p.i., cells or culture media were harvested and processed for total DNA or CCC DNA analysis. Ten micrograms of DNA was loaded on each lane. A minus-strand DIG-labeled DHBV riboprobe was used. (A) Detection of RC, DSL, and SS DNA in all preparations of DHBV-infected IBDE or PDH cells processed for total DNA analysis. (B) Detection of RC, DSL, and CCC DNA in preparations of DHBV-infected IBDE or PDH cells processed for CCC DNA analysis. (C) DHBV CCC DNA in infected IBDE or PDH preparations remained as a supercoiled species after the respective DNA preparations were boiled for 1 min and quenched on ice prior to gel analysis. (D) Detection of RC DNA extracted from culture medium of DHBV-infected IBDE or PDH cultures. For panels A through C, M represents DIG-labeled DNA molecular size markers; for panel D, M represents a full-length cDNA of the Australian strain of DHBV removed from the plasmid pT3T7 by digestion with EcoRI.
The viral CCC DNA was also detected in both the infected IBDE and PDH cultures (Fig. 8B). The intensity of the CCC DNA band also increased as infection progressed. The DHBV CCC DNA from both the IBDE and PDH preparations remained a supercoiled species even after the DNA preparations were boiled and quenched prior to loading onto the gel, confirming their identity (Fig. 8C).
To determine whether virus particles were secreted in infected IBDE cultures, the culture medium was harvested at various days p.i. and assayed for enveloped virus. Parallel studies were performed for infected PDH cultures. The viral RC DNA extracted from IBDE culture medium was detected at day 12 p.i. (Fig. 8D), while the corresponding DNA extracted from the PDH preparations was detected as early as day 5 p.i. (Fig. 8D).
DISCUSSION
In this study, primary duck IBDE cells were successfully grown and maintained in culture following isolation from duckling liver. The IBDE cells were identified using CAM 5.2, a monoclonal antibody to cytokeratin that has previously been shown to be a reliable marker for duck bile ducts (27, 34). An important aspect to the culture of IBDE cells for the studies of hepadnavirus replication was to ensure the removal of hepatocytes that may interfere with the interpretation of the data. The use of pronase treatment in the lysis of hepatocytes followed by their removal by isotonic Percoll gradient centrifugation appeared sufficient for this purpose. IFA studies using anti-duck albumin, a marker for hepatocytes (1), demonstrated the absence of hepatocytes in the IBDE cultures.
A striking feature of the primary duck IBDE cultures was the duct-like structures present within each colony of multilayered cells (Fig. 4). These were observed as open ducts or enclosed ducts (Fig. 4 and 6). Similar duct-like appearance was reported by Sirica and Gainey (40) in the culture of primary IBDE cells isolated from rat livers. Methods for the primary cultures of mammalian IBDE cells isolated from rat, mouse, or human livers are well documented, and a number of strategies have been developed that allow cells to be maintained in culture (for a review see reference 1). Some have highlighted the need for inclusion of growth factors, such as insulin, epidermal growth factor, forskolin, bovine pituitary gland extract, bovine fetal serum, and triido-l-thryronine, for the promotion of cell proliferation and the maintenance of biliary epithelial phenotype (8, 17, 44). Other investigators have drawn attention to the importance of hepatocyte growth factor because of its potent mitogenicity for epithelial cells (41). Most have emphasized the requirement for collagen gel support for the maintenance of cellular phenotype.
Despite such measures, many studies have only established growth of primary mammalian IBDE cells as monolayer cultures rather than as three-dimensional duct-like structures. For the duck counterpart described in this study, the addition of normal duck and fetal bovine sera, DMSO, ITS solution, and hydrocortisone in the culture medium appeared sufficient to stimulate proliferation of biliary cells into duct-like structures without the addition of other growth factors. Duck sera were found to be essential for the development of duct-like structures in primary IBDE cultures. Presumably there are as-yet- unidentified growth factors in the duck sera that stimulated the differentiation of duck IBDE cells. The inclusion of DMSO into the culture medium was found to be necessary in inhibiting the growth of fibroblast, while the addition of ITS and hydrocortisone appeared to promote cell proliferation. Although mammalian IBDE cultures required a collagen support gel for phenotypic maintenance, this was not necessary for the primary duck IBDE cultures; culture plates were coated only with a thin layer of rat tail collagen to aid cell adherence rather than to provide cellular support.
The growth characteristic of primary duck IBDE cells shown in this study appeared similar to that described for the mammalian counterparts (17, 30, 40). The organization of duck IBDE cells into clusters containing a lumen early in culture (Fig. 4A and B) appears similar to that reported by Mano and colleagues in the culture of mouse cholangiocytes (30). It is likely that these IBDE cells developed into the multilayered cell colonies containing duct-like structures observed later in culture (Fig. 4C). In addition, these duck biliary cells were of a density similar to that of the mammalian bile duct cells (36). The primary duck IBDE could be maintained for 12 days, after which time there was significant loss of biliary phenotype as determined by the decrease in intensity or loss of CAM 5.2 staining. Also evident during the later stages of culture was the proliferation of epithelium-like cells that grew as a monolayer. The monolayer did not appear to represent Stellate or Kupffer cells, as shown by the lack of reactivity to the respective antibody markers. It is likely that these cells represent dedifferentiated IBDE cells that have lost the characteristic phenotypic marker but retained the capacity to proliferate (43).
This study is the first to report on the characterization of primary culture of duck intrahepatic biliary cells and the de novo synthesis of DHBV proteins and replicative intermediates. Only CAM 5.2 staining cell colonies were found to contain de novo synthesized viral proteins as demonstrated by dual-label confocal microscopy studies. Importantly, DHBV-infected primary IBDE cells cultured in growth medium without duck sera formed monolayer cultures that did not contain hepadnavirus proteins and DNA. In contrast, parallel infected PDH cultures grown in the absence of duck sera demonstrated the presence of hepadnaviral markers (results not shown). It must be noted that duck serum is not required for the growth of duck PDH cultures for DHBV studies (39). Thus, primary duck IBDE cultures grown as a monolayer did not support hepadnavirus replication. Interestingly, these observations further confirmed the apparent absence of hepatocytes in the DHBV-infected IBDE cultures.
This study demonstrated that the hepadnavirus proteins and DNA replicative intermediates detected in infected duck biliary cells were similar to their counterparts in infected PDHs. In terms of the DNA replicative intermediates, the presence of negative-sense SS DNA in infected IBDE cells is indicative of DHBV reverse transcriptase activity, while the presence of CCC DNA indicates that infection can be established in these cells. For PDHs, persistent DHBV infection is dependent on the maintenance and regulation of the transcriptionally active CCC DNA pool via a proposed intracellular conversion pathway (42, 47). There may be a role for such a pathway in infected IBDE cells, as there is clearly a pool of CCC DNA in these cells.
The findings from this study show that the intrahepatic bile duct can serve as an important site for hepadnavirus replication in the liver. These findings would suggest that hepadnavirus proteins and DNA detected in IBDE cells of liver tissues derived from in vivo studies (24, 27, 34) represent reservoirs of active viral replication. The susceptibility of bile ducts to virus infection is not unique to hepadnaviruses. Recent studies on hepatitis C virus (HCV) have demonstrated HCV replication in primary cultures of human extrahepatic bile duct, i.e., gallbladder epithelial cells (26). For both HCV and the hepadnavirus, the extent to which infected bile ducts are involved in intrahepatic spread of the virus and maintenance of viral persistence remains to be elucidated.
ACKNOWLEDGMENTS
Jia-Yee Lee was supported by the National Health and Medical Research Council of Australia (NH&MRC Project No. 970351).
We thank Scott Bowden, Victorian Infectious Diseases Reference Laboratory, Melbourne, Australia, for critical review of the manuscript.
REFERENCES
- 1.Alpini G, Phillips J O, Vroman B, LaRusso N F. Recent advances in the isolation of liver cells. Hepatology. 1994;20:494–514. [PubMed] [Google Scholar]
- 2.Beasley R P, Hwang L Y, Lin C C, Chien C S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan. Lancet. 1981;ii:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum A, Suchy F J. The intrahepatic cholangiopathies. Semin Liver Dis. 1998;18:263–269. doi: 10.1055/s-2007-1007162. [DOI] [PubMed] [Google Scholar]
- 4.Bishop N, Civitico G, Wang Y Y, Guo K J, Birch C, Gust I, Locarnini S. Antiviral strategies in chronic hepatitis B virus infection: I. Establishment of an in vitro system using the duck hepatitis B virus model. J Med Virol. 1990;31:82–89. doi: 10.1002/jmv.1890310204. [DOI] [PubMed] [Google Scholar]
- 5.Blum H E, Stowring L, Figus A, Montgomery C K, Haase A T, Vyas G N. Detection of hepatitis B virus DNA in hepatocytes, bile duct epithelium, and vascular elements by in situ hybridization. Proc Natl Acad Sci USA. 1983;80:6685–6688. doi: 10.1073/pnas.80.21.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock C T, Schranz P, Schroder C H, Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8:215–229. doi: 10.1007/BF01703079. [DOI] [PubMed] [Google Scholar]
- 7.Colacino J M, Staschke K A. The identification and development of antiviral agents for the treatment of chronic hepatitis B virus infection. Prog Drug Res. 1998;50:259–322. doi: 10.1007/978-3-0348-8833-2_6. [DOI] [PubMed] [Google Scholar]
- 8.de Groen P C, Vroman B, Laakso K, LaRusso N F. Characterization and growth regulation of a rat intrahepatic bile duct epithelial cell line under hormonally defined, serum-free conditions. In Vitro Cell Dev Biol Anim. 1998;34:704–710. doi: 10.1007/s11626-998-0066-1. [DOI] [PubMed] [Google Scholar]
- 9.Delladetsima J K, Vafiadis I, Tassopoulos N C, Kyriakou V, Apostolaki A, Smyrnoff T. HBcAg and HBsAg expression in ductular cells in chronic hepatitis B. Liver. 1994;14:71–75. doi: 10.1111/j.1600-0676.1994.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag J L, Schiff E R, Wright T L, Perrillo R P, Hann H W, Goodman Z, Crowther L, Condreay L D, Woessner M, Rubin M, Brown N A. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 11.Dixon R J, Jones N F, Freiman J S. Reduced duck hepatitis B virus viraemia in ducklings coinfected with the immunodepressive reticuloendotheliosis virus. J Med Virol. 1990;30:169–173. doi: 10.1002/jmv.1890300304. [DOI] [PubMed] [Google Scholar]
- 12.Freiman J S, Jilbert A R, Dixon R J, Holmes M, Gowans E J, Burrell C J, Wills E J, Cossart Y E. Experimental duck hepatitis B virus infection: pathology and evolution of hepatic and extrahepatic infection. Hepatology. 1988;8:507–513. doi: 10.1002/hep.1840080313. [DOI] [PubMed] [Google Scholar]
- 13.Frommel D, Crevat D, Vitvitsky L, Pichoud C, Hantz O, Chevalier M, Grimaud J A, Lindberg J, Trepo C G. Immunopathologic aspects of woodchuck hepatitis. Am J Pathol. 1984;115:125–134. [PMC free article] [PubMed] [Google Scholar]
- 14.Halpern M S, England J M, Deery D T, Petcu D J, Mason W S, Molnar-Kimber K L. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci USA. 1983;80:4865–4869. doi: 10.1073/pnas.80.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpern M S, McMahon S B, Mason W S, O'Connell A P. Viral antigen expression in the pancreas of DHBV-infected embryos and young ducks. Virology. 1986;150:276–282. doi: 10.1016/0042-6822(86)90288-6. [DOI] [PubMed] [Google Scholar]
- 16.Hoofnagle J H. Therapy of viral hepatitis. Digestion. 1998;59:563–578. doi: 10.1159/000007532. [DOI] [PubMed] [Google Scholar]
- 17.Joplin R. Isolation and culture of biliary epithelial cells. Gut. 1994;35:875–878. doi: 10.1136/gut.35.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- 19.Katayanagi K, Kono N, Nakanuma Y. Isolation, culture and characterization of biliary epithelial cells from different anatomical levels of the intrahepatic and extrahepatic biliary tree from a mouse. Liver. 1998;18:90–98. doi: 10.1111/j.1600-0676.1998.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 20.Korba B E, Cote P J, Wells F V, Baldwin B, Popper H, Purcell R H, Tennant B C, Gerin J L. Natural history of woodchuck hepatitis virus infections during the course of experimental viral infection: molecular virologic features of the liver and lymphoid tissues. J Virol. 1989;63:1360–1370. doi: 10.1128/jvi.63.3.1360-1370.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai C L, Chien R N, Leung N W, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray D F. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 22.Lenhoff R J, Summers J. Construction of avian hepadnavirus variants with enhanced replication and cytopathicity in primary hepatocytes. J Virol. 1994;68:5706–5713. doi: 10.1128/jvi.68.9.5706-5713.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin E, Luscombe C, Colledge D, Wang Y Y, Locarnini S. Long-term therapy with the guanine nucleoside analog penciclovir controls chronic duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 1998;42:2132–2137. doi: 10.1128/aac.42.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin E, Luscombe C, Wang Y Y, Shaw T, Locarnini S. The guanine nucleoside analog penciclovir is active against chronic duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 1996;40:413–418. doi: 10.1128/aac.40.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lok A S. Hepatitis B infection: pathogenesis and management. J Hepatol. 2000;32:89–97. doi: 10.1016/s0168-8278(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 26.Loriot M A, Bronowicki J P, Lagorce D, Lakehal F, Persico T, Barba G, Mergey M, Vons C, Franco D, Belghiti J, Giacca M, Housset C, Brechot C. Permissiveness of human biliary epithelial cells to infection by hepatitis C virus. Hepatology. 1999;29:1587–1595. doi: 10.1002/hep.510290527. [DOI] [PubMed] [Google Scholar]
- 27.Luscombe C, Pedersen J, Bowden S, Locarnini S. Alterations in intrahepatic expression of duck hepatitis B viral markers with ganciclovir chemotherapy. Liver. 1994;14:182–192. doi: 10.1111/j.1600-0676.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 28.Luscombe C, Pedersen J, Uren E, Locarnini S. Long-term ganciclovir chemotherapy for congenital duck hepatitis B virus infection in vivo: effect on intrahepatic-viral DNA, RNA, and protein expression. Hepatology. 1996;24:766–773. doi: 10.1053/jhep.1996.v24.pm0008855174. [DOI] [PubMed] [Google Scholar]
- 29.Magliano D, Marshall J A, Bowden D S, Vardaxis N, Meanger J, Lee J Y. Rubella virus replication complexes are virus-modified lysosomes. Virology. 1998;240:57–63. doi: 10.1006/viro.1997.8906. [DOI] [PubMed] [Google Scholar]
- 30.Mano Y, Ishii M, Kisara N, Kobayashi Y, Ueno Y, Kobayashi K, Hamada H, Toyota T. Duct formation by immortalized mouse cholangiocytes: an in vitro model for cholangiopathies. Lab Investig. 1998;78:1467–1468. [PubMed] [Google Scholar]
- 31.Newbold J E, Xin H, Tencza M, Sherman G, Dean J, Bowden S, Locarnini S. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69:3350–3357. doi: 10.1128/jvi.69.6.3350-3357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nibbering C P, Carey M C. Sphingomyelins of rat liver: biliary enrichment with molecular species containing 16:0 fatty acids as compared to canalicular-enriched plasma membranes. J Membr Biol. 1999;167:165–171. doi: 10.1007/s002329900480. [DOI] [PubMed] [Google Scholar]
- 33.Nicoll A, Locarnini S, Chou S T, Smallwood R, Angus P. Effect of nucleoside analogue therapy on duck hepatitis B viral replication in hepatocytes and bile duct epithelial cells in vivo. J Gastroenterol Hepatol. 2000;15:304–310. doi: 10.1046/j.1440-1746.2000.02079.x. [DOI] [PubMed] [Google Scholar]
- 34.Nicoll A J, Angus P W, Chou S T, Luscombe C A, Smallwood R A, Locarnini S A. Demonstration of duck hepatitis B virus in bile duct epithelial cells: implications for pathogenesis and persistent infection. Hepatology. 1997;25:463–469. doi: 10.1002/hep.510250235. [DOI] [PubMed] [Google Scholar]
- 35.Nicoll A J, Colledge D L, Toole J J, Angus P W, Smallwood R A, Locarnini S A. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl)adenine, an acyclic phosphonate nucleoside analogue. Antimicrob Agents Chemother. 1998;42:3130–3135. doi: 10.1128/aac.42.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parola M, Cheeseman K H, Biocca M E, Dianzani M U, Slater T F. Isolation and characterization of biliary epithelial cells from normal rat liver. J Hepatol. 1988;6:175–186. doi: 10.1016/s0168-8278(88)80029-1. [DOI] [PubMed] [Google Scholar]
- 37.Shaw T, Amor P, Civitico G, Boyd M, Locarnini S. In vitro antiviral activity of penciclovir, a novel purine nucleoside, against duck hepatitis B virus. Antimicrob Agents Chemother. 1994;38:719–723. doi: 10.1128/aac.38.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw T, Locarnini S A. Combination chemotherapy for HBV: the way forward. Drugs. 2000;60:517–531. doi: 10.2165/00003495-200060030-00001. [DOI] [PubMed] [Google Scholar]
- 39.Shaw T, Luscombe C A, Locarnini S A. Animal models for hepatitis B infections—duck hepatitis. In: Zak O, Sande M A, editors. Handbook of animal models of infection. New York, N.Y: Academic Press; 1999. [Google Scholar]
- 40.Sirica A E, Gainey T W. A new rat bile ductular epithelial cell culture model characterized by the appearance of polarized bile ducts in vitro. Hepatology. 1997;26:537–549. doi: 10.1002/hep.510260302. [DOI] [PubMed] [Google Scholar]
- 41.Strain A J, Wallace L, Joplin R, Daikuhara Y, Ishii T, Kelly D A, Neuberger J M. Characterization of biliary epithelial cells isolated from needle biopsies of human liver in the presence of hepatocyte growth factor. Am J Pathol. 1995;146:537–545. [PMC free article] [PubMed] [Google Scholar]
- 42.Tuttleman J S, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 43.Venetianer A, Schiller D L, Magin T, Franke W W. Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature. 1983;305:730–733. doi: 10.1038/305730a0. [DOI] [PubMed] [Google Scholar]
- 44.Vroman B, LaRusso N F. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Investig. 1996;74:303–313. [PubMed] [Google Scholar]
- 45.Walter E, Teubner K, Blum H E, Offensperger W B, Offensperger S, Gerok W. Duck hepatitis B virus infection of non-hepatocytes. Liver. 1991;11:53–62. doi: 10.1111/j.1600-0676.1991.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Luscombe C, Bowden S, Shaw T, Locarnini S. Inhibition of duck hepatitis B virus DNA replication by antiviral chemotherapy with ganciclovir-nalidixic acid. Antimicrob Agents Chemother. 1995;39:556–558. doi: 10.1128/aac.39.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu T T, Coates L, Aldrich C E, Summers J, Mason W S. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 48.Yokosuka O, Omata M, Imazeki F, Ito Y, Okuda K. Hepatitis B virus RNA transcripts and DNA in chronic liver disease. N Engl J Med. 1986;315:1187–1192. doi: 10.1056/NEJM198611063151903. [DOI] [PubMed] [Google Scholar]