Abstract
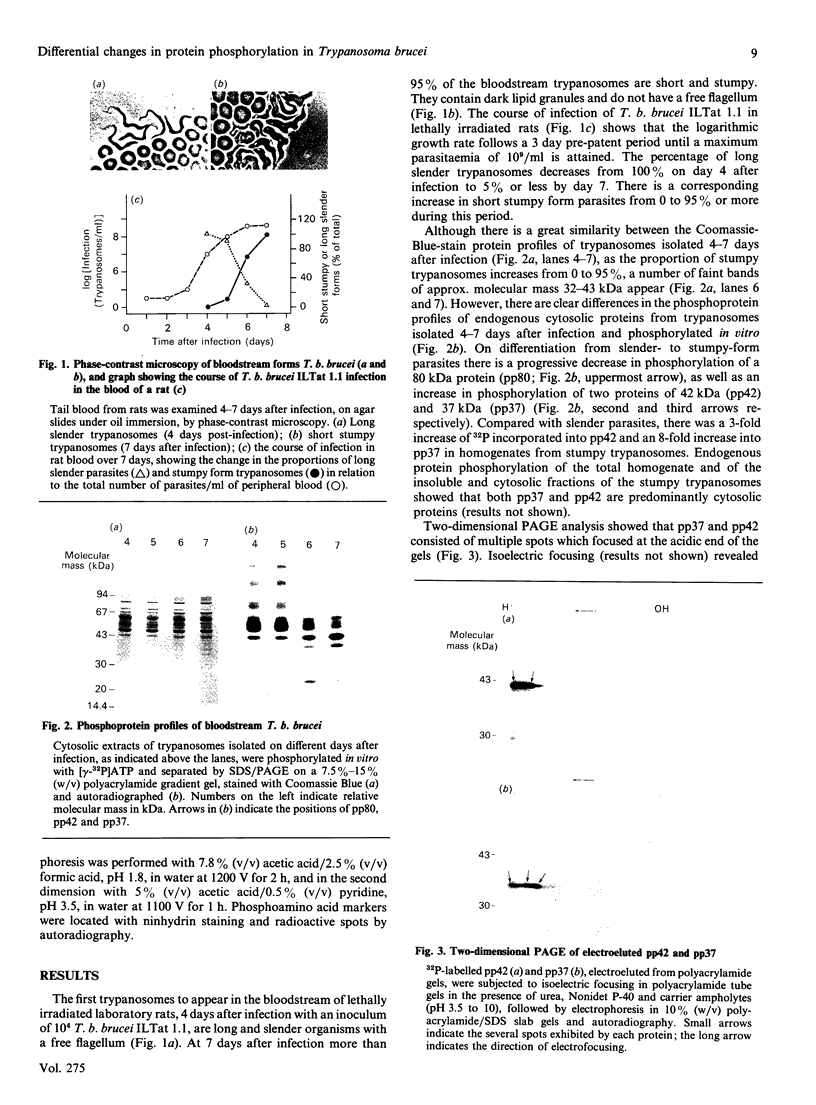
Early in an infection the bloodstream forms of the African trypanosome Trypanosoma brucei brucei are long, slender and rapidly dividing. Later, non-dividing, short, stumpy forms may be found. In this report we described biochemical differences between the two parasite populations in the phosphorylation of their proteins in vitro. Compared with the slender populations, the non-dividing stumpy forms of the parasites exhibit decreased phosphorylation of an 80 kDa protein and enhanced phosphorylation of 37 kDa and 42 kDa proteins (pp37 and pp42). These changes occurred regardless of whether the stumpy trypanosomes were generated naturally during the course of the infection or induced by difluoromethylornithine treatment. The phosphorylation of pp37 and pp42 occurs on serine and threonine residues and is totally dependent upon the presence of Mn2+ or Mg2+. However, excess Mn2+ or Mg2+ inhibits phosphorylation. Maximal phosphorylation of pp42 occurs with 1 mm-Mn2+ or 10 mm-Mg2+, whereas that of pp37 occurs with 50 mM-Mn2+ or greater than 100 mm-Mg2+. The phosphorylation of pp37 is greatly enhanced by KCl, whereas that of pp42 is only slightly increased by this salt. Ca2+, calmodulin, phospholipids and cyclic AMP have no discernible effect upon the phosphorylation of pp42 or pp37 in vitro, whereas heparin, suramin, polylysine, polyarginine and polyamines all inhibit phosphorylation. Thus the enzymes that phosphorylate pp42 and pp37 have properties similar to, but distinct from, those of mammalian casein kinase II. Since the casein-kinase-like activity is higher in the slender than in the stumpy forms, the enhanced phosphorylation of pp42 and pp37 in the non-dividing parasites is probably a result of the enhanced synthesis of these acidic proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C. J., Garofalo J., Mockenhaupt D., McCann P. P., Diekema K. A., Pegg A. E., Nathan H. C., Mullaney E. A., Chunosoff L., Sjoerdsma A. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol. 1983 Mar;7(3):209–225. doi: 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- Bar-Zvi D., Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J Biol Chem. 1986 Jul 25;261(21):9614–9621. [PubMed] [Google Scholar]
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D., Moran E. Synthesis of p34, the mammalian homolog of the yeast cdc2+/CDC28 protein kinase, is stimulated during adenovirus-induced proliferation of primary baby rat kidney cells. Oncogene. 1988 Jun;2(6):553–557. [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Flynn I. W., Bowman I. B. The metabolism of carbohydrate by pleomorphic African trypanosomes. Comp Biochem Physiol B. 1973 May 15;45(1):25–42. doi: 10.1016/0305-0491(73)90281-2. [DOI] [PubMed] [Google Scholar]
- Garofalo J., Bacchi C. J., McLaughlin S. D., Mockenhaupt D., Trueba G., Hutner S. H. Ornithine decarboxylase in Trypanosoma brucei brucei: evidence for selective toxicity of difluoromethylornithine. J Protozool. 1982 Aug;29(3):389–394. doi: 10.1111/j.1550-7408.1982.tb05418.x. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Grab D. J., Bwayo J. J. Isopycnic isolation of African trypanosomes on Percoll gradients formed in situ. Acta Trop. 1982 Dec;39(4):363–366. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Interaction of polyamines and magnesium with casein kinase II. Arch Biochem Biophys. 1984 Aug 15;233(1):133–138. doi: 10.1016/0003-9861(84)90609-x. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Tuazon P. T., Traugh J. A. Casein kinase I. Methods Enzymol. 1983;99:308–317. doi: 10.1016/0076-6879(83)99066-3. [DOI] [PubMed] [Google Scholar]
- Hensey C. E., Boscoboinik D., Azzi A. Suramin, an anti-cancer drug, inhibits protein kinase C and induces differentiation in neuroblastoma cell clone NB2A. FEBS Lett. 1989 Nov 20;258(1):156–158. doi: 10.1016/0014-5793(89)81639-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Brown M. S., Slaughter C. A., Goldstein J. L. Phosphorylation of serine 833 in cytoplasmic domain of low density lipoprotein receptor by a high molecular weight enzyme resembling casein kinase II. J Biol Chem. 1987 Jan 25;262(3):1344–1351. [PubMed] [Google Scholar]
- Krebs E. G. The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture. Biochem Soc Trans. 1985 Oct;13(5):813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- Labbe J. C., Lee M. G., Nurse P., Picard A., Doree M. Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cdc2+. Nature. 1988 Sep 15;335(6187):251–254. doi: 10.1038/335251a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lee M., Nurse P. Cell cycle control genes in fission yeast and mammalian cells. Trends Genet. 1988 Oct;4(10):287–290. doi: 10.1016/0168-9525(88)90171-0. [DOI] [PubMed] [Google Scholar]
- Manai M., Cozzone A. J. Two-dimensional separation of phosphoamino acids from nucleoside monophosphates. Anal Biochem. 1982 Jul 15;124(1):12–18. doi: 10.1016/0003-2697(82)90213-5. [DOI] [PubMed] [Google Scholar]
- Martensen T. M. Chemical properties, isolation, and analysis of O-phosphates in proteins. Methods Enzymol. 1984;107:3–23. doi: 10.1016/0076-6879(84)07003-8. [DOI] [PubMed] [Google Scholar]
- Meggio F., Flamigni F., Caldarera C. M., Guarnieri C., Pinna L. A. Phosphorylation of rat heart ornithine decarboxylase by type-2 casein kinase. Biochem Biophys Res Commun. 1984 Aug 16;122(3):997–1004. doi: 10.1016/0006-291x(84)91190-2. [DOI] [PubMed] [Google Scholar]
- Meggio F., Pinna L. A. Subunit structure and autophosphorylation mechanism of casein kinase-TS (type-2) from rat liver cytosol. Eur J Biochem. 1984 Dec 17;145(3):593–599. doi: 10.1111/j.1432-1033.1984.tb08598.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay N. K., Shome K., Saha A. K., Hassell J. R., Glew R. H. Heparin binds to Leishmania donovani promastigotes and inhibits protein phosphorylation. Biochem J. 1989 Dec 1;264(2):517–525. doi: 10.1042/bj2640517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics. 1980 Nov;96(3):627–637. doi: 10.1093/genetics/96.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana M., Guasch M. D., Itarte E. Effect of bivalent cations on rat liver cytosol casein (glycogen synthase) kinases 1 and 2. Influence of the protein substrate. Biochem J. 1985 Aug 15;230(1):69–74. doi: 10.1042/bj2300069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Naessens J., Liesegang B., Moloo S. K., Magondu J. Analysis by flow cytometry of DNA synthesis during the life cycle of African trypanosomes. Acta Trop. 1984 Dec;41(4):313–323. [PubMed] [Google Scholar]
- Shenolikar S. Protein phosphorylation: hormones, drugs, and bioregulation. FASEB J. 1988 Sep;2(12):2753–2764. doi: 10.1096/fasebj.2.12.2842213. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Bingham E. W., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Site-specific phosphorylation of casein variants. Eur J Biochem. 1979 Mar;94(2):497–504. doi: 10.1111/j.1432-1033.1979.tb12918.x. [DOI] [PubMed] [Google Scholar]
- Ui N. Isoelectric points and conformation of proteins. I. Effect of urea on the behavior of some proteins in isoelectric focusing. Biochim Biophys Acta. 1971 Mar 23;229(3):567–581. [PubMed] [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985 Apr;41(2):105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Polymorphism and mitochondrial activity in sleeping sickness trypanosomes. Nature. 1965 Nov 20;208(5012):762–766. doi: 10.1038/208762a0. [DOI] [PubMed] [Google Scholar]
- WIJERS D. J., WILLETT K. C. Factors that may influence the infection rate of Glossina palpalis with Trypanosoma gambiense. II. The number and morphology of the trypano-somes present in the blood of the host at the time of the infected feed. Ann Trop Med Parasitol. 1960 Oct;54:341–350. [PubMed] [Google Scholar]
- Walter R. D., Ebert F. Effect of polyamines on protein kinases activities from Trypanosoma cruzi. Tropenmed Parasitol. 1979 Mar;30(1):9–12. [PubMed] [Google Scholar]










