Abstract
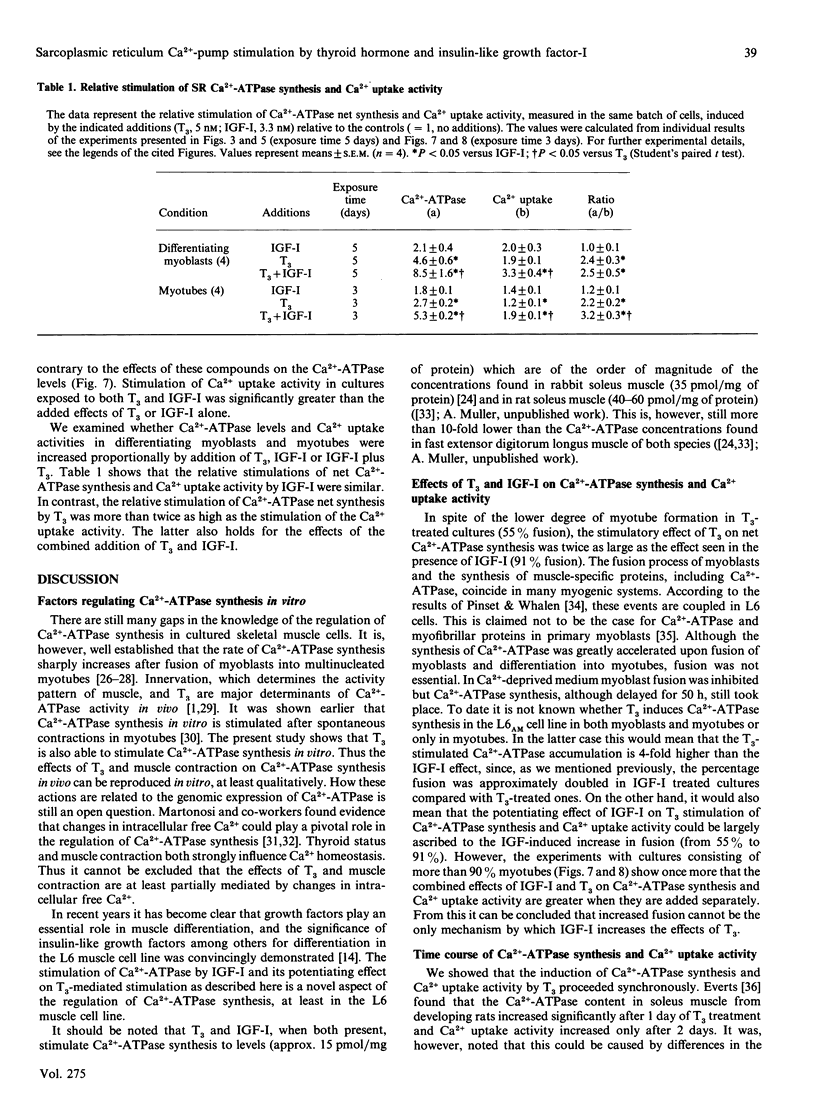
Net synthesis of the fast-type sarcoplasmic reticulum (SR) Ca2(+)-ATPase was studied in the muscle cell line L6AM using an immunochemical assay (e.l.i.s.a.). In addition, Ca2+ uptake by SR was monitored in muscle cell homogenates by a method employing the fluorescent Ca2+ indicator fura-2. Measurements were done both in differentiating myoblasts and in myotubes. Ca2(+)-ATPase levels were low (1 pmol/mg of protein) in undifferentiated myoblasts (controls) and only doubled over a period of 8 days in the absence of thyroid hormone (L-triiodothyronine; T3). This corresponded to a similar increase in Ca2+ uptake activity. Only half of the myoblasts fused under these conditions. Fusion was not increased in the presence of T3 (5 nM), but Ca2(+)-ATPase levels increased 4-fold and the Ca2+ uptake activity doubled compared with controls. In contrast, insulin-like growth factor-I (IGF-I) induced almost complete myotube formation (greater than 90% fusion), but only slightly stimulated (50%) net Ca2(+)-ATPase synthesis above control levels. However, the doubling of the Ca2+ uptake stimulation by IGF-I was comparable with that caused by T3. The effects of T3 plus IGF-I on Ca2(+)-ATPase levels and Ca2+ uptake activity were more than additive. Furthermore, the temporal relationship between the induction of Ca2(+)-ATPase net synthesis and Ca2+ uptake activity was identical with the two hormones. Qualitatively similar results were obtained when T3 and IGF-I were added to maximally fused cell cultures. The enhanced effect of T3 on Ca2(+)-ATPase net synthesis and Ca2+ uptake activity in the presence of IGF-I cannot therefore be explained by an increased myotube formation stimulated by the latter. In both differentiating myoblasts and myotubes the effect of T3 was more prominent on Ca2(+)-ATPase net synthesis than on Ca2+ uptake activity, whereas in myotubes the opposite was observed for IGF-I. This could imply complementary actions of the two agents in the development of a functional SR.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beekman R. E., van Hardeveld C., Simonides W. S. On the mechanism of the reduction by thyroid hormone of beta-adrenergic relaxation rate stimulation in rat heart. Biochem J. 1989 Apr 1;259(1):229–236. doi: 10.1042/bj2590229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman R. E., van Hardeveld C., Simonides W. S. Thyroid status and beta-agonistic effects on cytosolic calcium concentrations in single rat cardiac myocytes activated by electrical stimulation or high-K+ depolarization. Biochem J. 1990 Jun 15;268(3):563–569. doi: 10.1042/bj2680563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein P. J., Draznin B., Johnson C. J., Schalch D. S. The effect of hypothyroidism on growth, serum growth hormone, the growth hormone-dependent somatomedin, insulin-like growth factor, and its carrier protein in rats. Endocrinology. 1979 Apr;104(4):1107–1111. doi: 10.1210/endo-104-4-1107. [DOI] [PubMed] [Google Scholar]
- Charuk J. H., Holland P. C. Effect of tetrodotoxin relaxation of cultured skeletal muscle on the sarcoplasmic reticulum Ca2+-transport ATPase. Exp Cell Res. 1983 Mar;144(1):143–157. doi: 10.1016/0014-4827(83)90448-2. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F. The rate of tetanic relaxation is correlated with the density of calcium ATPase in the terminal cisternae of thyrotoxic skeletal muscle. Pflugers Arch. 1990 Jan;415(4):433–439. doi: 10.1007/BF00373620. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Everts M. E., Andersen J. P., Clausen T., Hansen O. Quantitative determination of Ca2+-dependent Mg2+-ATPase from sarcoplasmic reticulum in muscle biopsies. Biochem J. 1989 Jun 1;260(2):443–448. doi: 10.1042/bj2600443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts M. E. Effects of thyroid hormone on Ca2+ efflux and Ca2+ transport capacity in rat skeletal muscle. Cell Calcium. 1990 May;11(5):343–352. doi: 10.1016/0143-4160(90)90037-u. [DOI] [PubMed] [Google Scholar]
- Everts M. E., van Hardeveld C., Ter Keurs H. E., Kassenaar A. A. Force development and metabolism in skeletal muscle of euthyroid and hypothyroid rats. Acta Endocrinol (Copenh) 1981 Jun;97(2):221–225. doi: 10.1530/acta.0.0970221. [DOI] [PubMed] [Google Scholar]
- Fitts R. H., Winder W. W., Brooke M. H., Kaiser K. K., Holloszy J. O. Contractile, biochemical, and histochemical properties of thyrotoxic rat soleus muscle. Am J Physiol. 1980 Jan;238(1):C14–C20. doi: 10.1152/ajpcell.1980.238.1.C15. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Magri K. A. Effects of growth factors on myogenic differentiation. Am J Physiol. 1989 Apr;256(4 Pt 1):C701–C711. doi: 10.1152/ajpcell.1989.256.4.C701. [DOI] [PubMed] [Google Scholar]
- Holland P. C., MacLennan D. H. Assembly of the sarcoplasmic reticulum. Biosynthesis of the adenosine triphosphatase in rat skeletal muscle cell culture. J Biol Chem. 1976 Apr 10;251(7):2030–2036. [PubMed] [Google Scholar]
- James-Kracke M. R. Measurement of cytoplasmic free Ca2+ concentration in cultured muscle cells by aequorin and quin 2. Am J Physiol. 1986 Oct;251(4 Pt 1):C512–C523. doi: 10.1152/ajpcell.1986.251.4.C512. [DOI] [PubMed] [Google Scholar]
- Kim D. H., Witzmann F. A., Fitts R. H. Effect of thyrotoxicosis on sarcoplasmic reticulum in rat skeletal muscle. Am J Physiol. 1982 Sep;243(3):C151–C155. doi: 10.1152/ajpcell.1982.243.3.C151. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Smith R. J. L6 cells as a tissue culture model for thyroid hormone effects on skeletal muscle metabolism. J Clin Invest. 1985 Aug;76(2):878–881. doi: 10.1172/JCI112046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberer E., Pette D. Immunochemical quantification of sarcoplasmic reticulum Ca-ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles of defined fiber composition. Eur J Biochem. 1986 May 2;156(3):489–496. doi: 10.1111/j.1432-1033.1986.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Leijendekker W. J., van Hardeveld C., Elzinga G. Heat production during contraction in skeletal muscle of hypothyroid mice. Am J Physiol. 1987 Aug;253(2 Pt 1):E214–E220. doi: 10.1152/ajpendo.1987.253.2.E214. [DOI] [PubMed] [Google Scholar]
- Limas C. J. Calcium transport ATPase of cardiac sarcoplasmic reticulum in experimental hyperthyroidism. Am J Physiol. 1978 Dec;235(6):H745–H752. doi: 10.1152/ajpheart.1978.235.6.H745. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N., Dux L., Terjung R. L., Roufa D. Regulation of membrane assembly during development of sarcoplasmic reticulum: the possible role of calcium. Ann N Y Acad Sci. 1982;402:485–514. doi: 10.1111/j.1749-6632.1982.tb25771.x. [DOI] [PubMed] [Google Scholar]
- Martonosi A., Roufa D., Ha D. B., Boland R. The biosynthesis of sarcoplasmic reticulum. Fed Proc. 1980 May 15;39(7):2415–2421. [PubMed] [Google Scholar]
- Martonosi A. The development of sarcoplasmic reticulum membranes. Annu Rev Physiol. 1982;44:337–355. doi: 10.1146/annurev.ph.44.030182.002005. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985 Oct;8(8):676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Pinset C., Whalen R. G. Manipulation of medium conditions and differentiation in the rat myogenic cell line L6. Dev Biol. 1984 Apr;102(2):269–277. doi: 10.1016/0012-1606(84)90192-1. [DOI] [PubMed] [Google Scholar]
- Pontecorvi A., Tata J. R., Phyillaier M., Robbins J. Selective degradation of mRNA: the role of short-lived proteins in differential destabilization of insulin-induced creatine phosphokinase and myosin heavy chain mRNAs during rat skeletal muscle L6 cell differentiation. EMBO J. 1988 May;7(5):1489–1495. doi: 10.1002/j.1460-2075.1988.tb02967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer D., Dillmann W. H. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem. 1988 May 25;263(15):6941–6944. [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. Effects of the thyroid status on the sarcoplasmic reticulum in slow skeletal muscle of the rat. Cell Calcium. 1986 Jun;7(3):147–160. doi: 10.1016/0143-4160(86)90018-7. [DOI] [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. The effect of hypothyroidism on sarcoplasmic reticulum in fast-twitch muscle of the rat. Biochim Biophys Acta. 1985 Feb 21;844(2):129–141. doi: 10.1016/0167-4889(85)90083-7. [DOI] [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. The postnatal development of sarcoplasmic reticulum Ca2+ transport activity in skeletal muscle of the rat is critically dependent on thyroid hormone. Endocrinology. 1989 Mar;124(3):1145–1152. doi: 10.1210/endo-124-3-1145. [DOI] [PubMed] [Google Scholar]
- Suko J. The calcium pump of cardiac sarcoplasmic reticulum. Functional alterations at different levels of thyroid state in rabbits. J Physiol. 1973 Feb;228(3):563–582. doi: 10.1113/jphysiol.1973.sp010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E., MacDonald G., Phillips L., Jorgensen A. O., MacLennan D. H. Monoclonal antibodies to the Ca2+ + Mg2+-dependent ATPase of sarcoplasmic reticulum identify polymorphic forms of the enzyme and indicate the presence in the enzyme of a classical high-affinity Ca2+ binding site. J Bioenerg Biomembr. 1984 Dec;16(5-6):441–464. doi: 10.1007/BF00743238. [DOI] [PubMed] [Google Scholar]