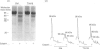Abstract
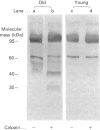
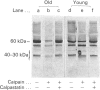
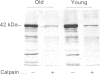
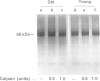
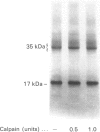
Band 3 protein is a major erythrocyte transmembrane glycoprotein. We compared the degradation of band 3 protein by calpain I (a cytoplasmic, micromolar-Ca2(+)-requiring thiol proteinase) in the cells from old individuals (greater than 70 years old) to that in the cells from young ones (20-30 years old). In the young, little degradation of band 3 protein occurred in calpain-treated erythrocyte ghosts. In the old, significant band 3 protein degradation was found in erythrocyte ghosts treated similarly. The difference between young and old in the susceptibility of band 3 protein to calpain was retained in membrane vesicles (membranes stripped of peripheral proteins by NaOH) and in chymotrypsin-generated 60 kDa fragment (CH-60). The isolated N-terminal cytoplasmic 43 kDa fragment was degraded by calpain to a similar extent in old and in young. The separated 17 kDa membrane domain of the CH-60 and the trypsin-generated C-terminal 55 kDa membrane-spanning fragment were not degraded by calpain I in the young, nor in the old. Thus the N-terminal cytoplasmic domain is the domain degraded by calpain I. Enhanced sensitivity in the old is observed in intact band 3 protein and in CH-60, the isolated cytoplasmic domain being equally susceptible in young and old. The observed age-related enhanced sensitivity to calpain is consistent with the presence of modifications in band 3 protein and alterations in the association with the calpain-calpastatin system. Band 3 protein has several important functions, with modifications in the protein having implications for altered cell behaviour in the old individual.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boivin P. Role of the phosphorylation of red blood cell membrane proteins. Biochem J. 1988 Dec 15;256(3):689–695. doi: 10.1042/bj2560689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman G. J., Kay M. M. Erythrocyte aging: a comparison of model systems for simulating cellular aging in vitro. Blood Cells. 1988;14(1):19–46. [PubMed] [Google Scholar]
- Cousin J. L., Motais R. Inhibition of anion transport in the red blood cell by anionic amphiphilic compounds. I. Determination of the flufenamate-binding site by proteolytic dissection of the band 3 protein. Biochim Biophys Acta. 1982 May 7;687(2):147–155. doi: 10.1016/0005-2736(82)90540-5. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerwiński M., Waśniowska K., Steuden I., Duk M., Wiedłocha A., Lisowska E. Degradation of the human erythrocyte membrane band 3 studied with monoclonal antibody directed against an epitope on the cytoplasmic fragment of band 3. Eur J Biochem. 1988 Jul 1;174(4):647–654. doi: 10.1111/j.1432-1033.1988.tb14147.x. [DOI] [PubMed] [Google Scholar]
- Danon D., Marikovsky Y. The aging of the red blood cell. A multifactor process. Blood Cells. 1988;14(1):7–18. [PubMed] [Google Scholar]
- Davis L., Lux S. E., Bennett V. Mapping the ankyrin-binding site of the human erythrocyte anion exchanger. J Biol Chem. 1989 Jun 5;264(16):9665–9672. [PubMed] [Google Scholar]
- Gershon H., Gershon D. Altered enzyme function and premature sequestration of erythrocytes in aged individuals. Blood Cells. 1988;14(1):93–101. [PubMed] [Google Scholar]
- Glaser T., Kosower N. S. Calpain-calpastatin and fusion. Fusibility of erythrocytes is determined by a protease-protease inhibitor [calpain-calpastatin] balance. FEBS Lett. 1986 Sep 29;206(1):115–120. doi: 10.1016/0014-5793(86)81351-5. [DOI] [PubMed] [Google Scholar]
- Glaser T., Kosower N. S. Fusion of rat erythrocytes by membrane-mobility agent A2C depends on membrane proteolysis by a cytoplasmic calpain. Eur J Biochem. 1986 Sep 1;159(2):387–392. doi: 10.1111/j.1432-1033.1986.tb09880.x. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Beswick H. T., Ajiboye R., Huby R., Blakytny R., Rixon K. C. Non-enzymic post-translational modification of proteins in aging. A review. Mech Ageing Dev. 1989 Oct;50(1):7–16. doi: 10.1016/0047-6374(89)90054-7. [DOI] [PubMed] [Google Scholar]
- Inomata M., Hayashi M., Nakamura M., Saito Y., Kawashima S. Properties of erythrocyte membrane binding and autolytic activation of calcium-activated neutral protease. J Biol Chem. 1989 Nov 5;264(31):18838–18843. [PubMed] [Google Scholar]
- Jennings M. L., Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonate. Biochim Biophys Acta. 1979 Jul 5;554(2):498–519. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Structure and function of the red blood cell anion transport protein. Annu Rev Biophys Biophys Chem. 1989;18:397–430. doi: 10.1146/annurev.bb.18.060189.002145. [DOI] [PubMed] [Google Scholar]
- Kapprell H. P., Goll D. E. Effect of Ca2+ on binding of the calpains to calpastatin. J Biol Chem. 1989 Oct 25;264(30):17888–17896. [PubMed] [Google Scholar]
- Kay M. M., Flowers N., Goodman J., Bosman G. Alteration in membrane protein band 3 associated with accelerated erythrocyte aging. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5834–5838. doi: 10.1073/pnas.86.15.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Lee B. S., Simmons D. M., Lindsey A. E., Morgans C. W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989 Dec 1;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Korsgren C., Cohen C. M. Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J Biol Chem. 1988 Jul 25;263(21):10212–10218. [PubMed] [Google Scholar]
- Kuboki M., Ishii H., Kazama M. Procalpain is activated on the plasma membrane and the calpain acts on the membrane. Biochim Biophys Acta. 1987 Jul 6;929(2):164–172. doi: 10.1016/0167-4889(87)90172-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lowenson J., Clarke S. Does the chemical instability of aspartyl and asparaginyl residues in proteins contribute to erythrocyte aging? The role of protein carboxyl methylation reactions. Blood Cells. 1988;14(1):103–118. [PubMed] [Google Scholar]
- Lutz H. U., Fasler S., Stammler P., Bussolino F., Arese P. Naturally occurring anti-band 3 antibodies and complement in phagocytosis of oxidatively-stressed and in clearance of senescent red cells. Blood Cells. 1988;14(1):175–203. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren R. L. Calcium-dependent proteases: an enzyme system active at cellular membranes? FASEB J. 1987 Aug;1(2):110–115. doi: 10.1096/fasebj.1.2.2886390. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L., Lane R. D., Mericle M. T. The binding of large calpastatin to biologic membranes is mediated in part by interaction of an amino terminal region with acidic phospholipids. Biochim Biophys Acta. 1989 Nov 9;999(1):71–77. doi: 10.1016/0167-4838(89)90032-0. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S. The calpains. Trends Neurosci. 1989 Nov;12(11):438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Melloni E., Salamino F., Sparatore B., Michetti M., Pontremoli S. Characterization of the single peptide generated from the amino-terminus end of alpha- and beta-hemoglobin chains by the Ca2+-dependent neutral proteinase. Biochim Biophys Acta. 1984 Jul 17;788(1):11–16. doi: 10.1016/0167-4838(84)90291-7. [DOI] [PubMed] [Google Scholar]
- Morrison M., Grant W., Smith H. T., Mueller T. J., Hsu L. Catabolism of the anion transport protein in human erythrocytes. Biochemistry. 1985 Oct 22;24(22):6311–6315. doi: 10.1021/bi00343a041. [DOI] [PubMed] [Google Scholar]
- Murachi T. Calcium-dependent proteinases and specific inhibitors: calpain and calpastatin. Biochem Soc Symp. 1984;49:149–167. [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol. 1986;103:61–203. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Passow H., Raida M., Wendel J., Legrum B., Bartel D., Lepke S., Furuto-Kato S. Anion transport systems in the mouse erythrocyte: kinetic studies in situ and after expression of mouse erythroid band 3 protein in oocytes of Xenopus laevis. Biochem Soc Trans. 1989 Oct;17(5):812–815. doi: 10.1042/bst0170812. [DOI] [PubMed] [Google Scholar]
- Sakai K., Hayashi M., Kawashima S., Akanuma H. Calcium-induced localization of calcium-activated neutral proteinase on plasma membranes. Biochim Biophys Acta. 1989 Oct 2;985(1):51–54. doi: 10.1016/0005-2736(89)90102-8. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imajoh S., Emori Y., Kawasaki H., Minami Y., Ohno S. Calcium-activated neutral protease and its endogenous inhibitor. Activation at the cell membrane and biological function. FEBS Lett. 1987 Aug 17;220(2):271–277. doi: 10.1016/0014-5793(87)80828-1. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willardson B. M., Thevenin B. J., Harrison M. L., Kuster W. M., Benson M. D., Low P. S. Localization of the ankyrin-binding site on erythrocyte membrane protein, band 3. J Biol Chem. 1989 Sep 25;264(27):15893–15899. [PubMed] [Google Scholar]