Abstract
Introduction:
This study aims to investigate the efficacy and tolerance of biologic disease-modifying anti-rheumatic drug (bDMARDs) in the current management of rheumatoid arthritis (RA) by identifying the retention time and survival rate of bDMARDs.
Materials and Methods:
We conducted a retrospective cohort study including Tunisian patients initiating bDMARD treatment between 2016 and 2018 whose data were collected from the National Health Insurance Fund (NHIF). The NHIF is the national office which organises and centralises patients under bDMARDs from all over the country. Retention and survival rate of bDMARDs at 48 months were analysed using Kaplan-Meier survival curves and compared using the log-rank test. Survival factor analysis was performed using Cox regression.
Results:
Three hundred seventy-four patients, aged 55.5±12.5years [20–90], (87.2%women), were included. The mean duration of RA was 11.7±6.7 years [2–41]. The mean disease activity score (DAS)28 at initiation of the first bDMARD was 6.01±0.89 [5.37–6.5]. This first bDMARD induced low disease activity (LDA) in 55% of cases. Remission was observed in 28% of patients. The highest LDA and remission rates were observed with Tocilizumab (70.8% and 33.3% of cases, respectively). LDA and remission were achieved within a mean of 45 weeks [26–88] and 72 weeks [31–117] respectively. The 48-month first-line survival rate was 55.9%. Retention time was 41.7 months, 95%CI [39.47–43.91]. Presence of rheumatoid factors, co-prescription of methotrexate, and good initial therapeutic response were factors influencing better survival of bDMARDs (p<0.01). Glucocorticoid use predicted poorer survival (p<10-3). The first bDMARD was interrupted in 39% of cases. Ineffectiveness was the most common cause of treatment cessation (52.7%).
Conclusion:
This real-life study of the Tunisian population allowed us to establish the factors that can influence the survival and retention rates of bDMARDs.
Keywords: rheumatoid arthritis, biologic drugs, retention, survival rate, bDMARDs
INTRODUCTION
The emergence of biologic disease-modifying anti-rheumatic drugs (bDMARDs) in the late 1990s revolutionised the management of rheumatoid arthritis (RA).1 These therapies can control symptoms, reduce inflammation, limit joint destruction, induce clinical remission, prevent functional impairment2 and thereby reduce the morbidity and mortality associated with RA.3 Despite the evidence for the efficacy and safety of bDMARDs from several randomised therapeutic trials, the validity of these studies in real life is significantly reduced due to inclusion and exclusion criteria.4 For these reasons, national registries have been established to study the efficacy and safety of bDMARDs in a real-world setting. The retention time and survival rate of bDMARDs varies considerably depending on several factors that deserve clarification.
Indeed, the survival of a drug can be considered an indicator of its efficacy, safety, and tolerability. The latter two are the most common reasons for discontinuation of bDMARDs,5 however, drug survival can also be influenced by other factors, mainly related to patient characteristics.6,7
As many factors affect the survival of bDMARDs, the results of international studies could not be extrapolated to our population. Therefore, we conducted this study to determine i) the retention time and survival rate of bDMARDs in the Tunisian population, ii) the predictive factors of bDMARDs failure or discontinuation.
MATERIALS AND METHODS
Data Source
This was a retrospective cohort study (conducted from 2016 to 2020) of bDMARDs claims made for 374 Tunisian patients at the National Health Insurance Fund (NHIF) (office that centralises all patients on bDMARDs, in order to operate them nationwide).
The study protocol was reviewed and approved by our local ethics committee. Informed consent was obtained.
Cohort Selection
We included RA patients over 18 years old, in whom the diagnosis of RA (code 17M05 of the NHIF) was established according to the ACR/EULAR2010 criteria and who started the first line bDMARD between January 2016 and December 2018. The index date was defined as the date of the first intake of this bDMARD. The identified bDMARDs were those with a marketing authorisation in Tunisia during this period: TNF inhibitors (TNFi): Infliximab (IFX), Adalimumab (ADA), Etanercept (ETN), and Certolizumab Pegol (CZP); IL6 receptor inhibitor (IL6Ri): Tocilizumab (TCZ); and CD20 inhibitor (CD20i): Rituximab (RTX). The choice of bDMARD is made according to international and national recommendations and contraindications to the prescription of any of the available bDMARDs. In case of inefficiency or side effects, the treatment is changed to another class or molecule.
Unusable records and those of patients with bDMARDs agreement without follow-up were excluded. Patients without regular follow-up, non-adherent to treatment and whose doses of conventional disease-modifying anti-rheumatic drug (csDMARDs) and/or corticosteroids were modified were not included.
Outcome
The main outcome was bDMARDs efficacy and tolerance by specifying their survival rate and retention time. The second outcome was the failure or drop-out of bDMARDs by specifying factors influencing treatment survival rate.
Study Parameters
Retention time for bDMARDs has been defined as the time from initiation of bDMARDs to discontinuation, which may be due to ineffectiveness, adverse effects, or other reasons.8 bDMARDs drug survival at a given time point is the rate of patients who retained the same bDMARD at this time.
Data Gathering
The included records were analysed. Missing data were collected by telephone interviews with patients.
We collected epidemiological data of the study population: age, gender, comorbidities; and RA characteristics: disease duration, presence of rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPA) and antinuclear antibodies (ANA), and extra-articular manifestations. Structural lesions were not specified because of the lack of data necessary for evaluation by a validated score such as the Sharp score.
We have also detailed the treatments used in these patients: symptomatic treatment including corticosteroids, csDMARDs [methotrexate (MTX), sulfasalazine (SLZ), synthetic antimalarial (SA), leflunomide (LEF)], and bDMARDs.
The time from index date to discontinuation (or study end date) was calculated for each patient and defined as the drug retention time. We also specified the time needed to reach low disease activity (LDA) or remission, and the time needed to increase bDMARDs injection/infusion intervals.
RTX discontinuation was considered when the interruption lasted more than 1 year.
RA activity was assessed using the Disease Activity Score 28 (DAS28). Follow-up at 3 months after the index date and then every 3 or 6 months, depending on disease activity, was performed in all patients. Assessment of therapeutic response was based on the change in DAS28 (delta DAS28) according to the EULAR response criteria.9
Statistical Analysis
Data were analysed using IBM SPSS Statistics v.26 software. We used frequencies, means±standard deviation, or medians for the study of the population characteristics. Survival information was studied by constructing survival curves using the Kaplan Meier method. Prognostic factors for survival were studied in univariate analysis (factor by factor) by comparing survival curves with the log-rank test. The risk factors associated with each event were identified via multivariate analysis using descending stepwise Cox regression. The significance level was set at a p-value<0.05.
RESULTS
The study included 374 patients, of which 77.8% (n=291) were younger than 65 years. RF and ACPA data were reported in 324 (86%) and 267 (71%) patient records, respectively.
ETA was the most prescribed TNFi (54%) followed by ADA (14%), CZP (13%), and IFX (6%). The clinical, biological, and therapeutic characteristics of patients are presented in Table 1.
Table 1.
Clinical, biological, and therapeutic characteristics of patients at bDMARDs initiation.
| Characteristics | Value | |
|---|---|---|
| Age (mean±SD [min-max]) years | 55±12.54 [20–90] | |
| Male/female (n (%)) | 48 (12.8)/326 (87.2) | |
| Disease duration (mean±SD [min–max]) years | 11.7±6.76 [2–41] | |
| FR+ (n (%)) | 296 (79) | |
| ACPA+ (n (%)) | 270 (72) | |
| Sjögren’s syndrome (n (%)) | 23 (6.14) | |
| Pulmonary involvement (n (%)) | 19 (5.08) | |
| Rheumatoid nodule (n (%)) | 5 (1.33) | |
| Hip involvement (n (%)) | 24 (4.01) | |
| Atlantoaxial dislocation (n (%)) | 15 (4) | |
| DAS28 (mean±SD [min–max]) | 6.02±0.82 [5.37–6.5] | |
| Methotrexate (n (%)) | 365 (97.6) | |
| Methotrexate dose (mean±SD [min–max]) mg/week | 19±2.84 [10–25] | |
| Methotrexate+Sulfasalazine (n (%)) | 219 (58.6) | |
| Methotrexate+Leflunomide (n (%)) | 147 (31.3) | |
| Methotrexate+Synthetic antimalarial (n (%)) | 83 (22.2) | |
| Corticosteroids (n (%)) | 214 (57.2) | |
| Mean dose corticosteroids (mean±SD [min–max]) (mg/day) | 6.20±2.34 [2.5–20] | |
| TNFi (n (%)) | 323 (86.4) | |
| IFX (n (%)) | 23 (6.1) | |
| ADA (n (%)) | 52 (13.9) | |
| ETN (n (%)) | 202 (54) | |
| CZP (n (%)) | 46 (12.3) | |
| Methotrexate+TNFi (n (%)) | 223 (59.6) | |
| Methotrexate dose (mean±SD [min–max]) mg/week | 15.1±4.2 [7.5–25] | |
| Oral/intramuscular route ((n (%)/n (%)) | 185 (83)/38 (17) | |
| TCZ (n (%)) | 24 (6.4) | |
| RTX (n (%)) | 27 (7.2) | |
SD: standard deviation; min: minimum; max: maximum; FR+: positive rheumatoid factor; ACPA+: positive anti-citrullinated protein antibodies; DAS: disease activity score; TNFi: TNF inhibitor; IFX: Infliximab; ADA: Adalimumab; ETN: Etanercept; CZP: Certolizumab Pegol; TCZ: Tocilizumab; RTX: Rituximab.
Response to the First bDMARD
At 3 months, the response was good in 77.2% of cases and moderate in 11.1% of cases. However, 11.7% of patients had not responded. ETN was associated with the highest response rate (77.8%).
The first bDMARD induced LDA in 55% of cases. Remission occurred in 28% of patients.
The highest LDA and remission rates were observed with Tocilizumab (70.8% and 33.3% of cases, respectively).
The mean time to LDA and remission was 45 weeks [26–88] and 72 weeks [31–117], respectively.
The survival rate of the first bDMARD was 55.9% at 4 years of treatment. The first bDMARD was retained for a mean duration of 41.7 months, 95%CI [39.47–43.91].
The time to LDA and remission, drug survival rates and retention time for each bDMARD are detailed in Table 2.
Table 2.
Response to the first-line bDMARDs.
| Time to LDA (weeks) [min–max] | Time to remission (weeks) [min–max] | Drug survival (%) | Drug retention (months) [CI 95%] | ||||
|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 36 months | 48 months | ||||
| ETA | 70.60 [8.7–220.1] | 89.50 [8.7–199.9] | 87.13 | 70.30 | 60.04 | 48.40 | 41.78 [38.78–44.78] |
| ADA | 38.20 [10.1–78.7] | 57.17 [10.5–113.6] | 86.27 | 66.60 | 53.64 | 48.40 | 37.51 [31.86–43.15] |
| CTZ | 44.00 [11.1–107.6] | 46.70 [11.1–120.6] | 87.5 | 76.75 | 71.64 | - | 30.96 [27.82–34.11] |
| IFX | 61.50 [18.3–145.4] | 94.80 [29.7–160] | 81.82 | 54.17 | 49.24 | 28.14 | 32.3 [24.83–39.24] |
| TCZ | 49.90 [8.9–106.6] | 78.90 [26.9–171.9] | 95.83 | 83.33 | 77.38 | – | 40.07 [35.24–44.89] |
| RTX | 30.10 [17.4–42.9] | 28.20 [17.4–42.9] | 66.67 | 62.75 | 58.26 | 58.26 | 37.57 [30.05–45.08] |
LDA: Low disease activity; min: minimum; max: maximum; CI: confidence interval; TNFi: TNF inhibitor; ETA: Etanercept; ADA: Adalimumab; CTZ: Certolizumab; IFX: Infliximab; TCZ: Tocilizumab; RTX: Rituximab.
bDMARDs intervals were increased in 100 patients (27%) due to sustained remission. This interval increase involved, in most cases, patients on TNFi. The first interval increase occurred after a mean time of 104.6±46.4 weeks [52–162.3]. With a mean DAS28 before interval increase of 2.8±0.8 [2.1–3.2] and after interval increase of 2.9±0.57 [2.5–3.4]. The mean difference in DAS28 of interval increase was 0.86±0.2 (p=0.364). IFX doses were optimised to 5mg/kg in three patients without efficacy. The first bDMARD was discontinued in 39% of cases for various reasons (Table 3). Switching to a second treatment was made in 79 patients (21.1%). Fifty-four patients received TNFi, 19 received TCZ, and 6 received RTX. CZP was the most prescribed second-line bDMARD (41.8%).
Table 3.
Causes of bDMARDs discontinuation.
| Number of patients (n=146) | ||
|---|---|---|
| Inefficiency | Primary | 32 |
| Secondary | 45 | |
| Intolerance | Cutaneous | 8 |
| Respiratory | 4 | |
| Hepatic | 2 | |
| Digestive | 1 | |
| Neoplasm/Haemopathy | Breast cancer | 3 |
| Adenocarcinoma of the upper rectum | 1 | |
| Bladder carcinoma | 1 | |
| Severe Infection | Intestinal amoebiasis | 1 |
| Peritonitis | 1 | |
| Tuberculosis | 1 | |
| Fungal sepsis | 1 | |
| Other | Patient lost to follow-up | 13 |
| Remission/LDA not obtained | 12 | |
| Not justified | 9 | |
| Lack of social security coverage | 5 | |
| Death | 3 | |
| Social fund decision | 2 | |
| Lupus | 1 | |
Factors Affecting First-Line bDMARD Retention Time and Survival Rate
We assessed drug survival based on therapeutic response three months after the index date and found a statistically significant difference between “no response,” “moderate response,” and “good response” (p<10-3) with a higher rate for patients with moderate and good response.
Regarding disease activity, we found that the higher the delta DAS28 at 3 months of treatment, the lower the risk of discontinuing bDMARDs, as reflected by the Hazard Ratio (HR:0.709, 95%CI [0.321–0.937], p<10-3).
Age did not influence bDMARDs survival (HR=0.997, 95%CI [0.763–1.122], p=0.667).
Male gender appeared to be a predictor of bDMARDs discontinuation (HR=1.442, 95%CI [1.252–1.941]), however this result was not statistically significant (p=0.107).
RF positivity was predictive of better survival of bDMARDs (HR=0.643, 95%CI [0.321–0.845], p=0.023). This was not similar for ACPA positivity (HR=1.004, 95%CI [0.982–1.123], p=0.987).
The combination of RA and Sjögren’s syndrome could increase the risk of discontinuation of bDMARDs (HR=1.236). However, this risk was not significant (p=0.519).
The combination of MTX with bDMARDs reduced the risk of discontinuation (HR=0.506, p<10-3) in contrast to corticosteroid therapy, which significantly increased this risk (HR=1.781, 95%CI [0.531–1.982], p=0.001).
The various clinical, immunological, and therapeutic factors and their influence on the survival and retention time of bDMARDs are detailed in Tables 4 and 5.
Table 4.
Factors influencing the bDMARDs survival rate.
| Survival rate at 12 months (%) | Survival rate at 24 months (%) | Survival rate at 36 months (%) | Survival rate at 48 months (%) | p | ||
|---|---|---|---|---|---|---|
| Age | <65 years | 87.29 | 70.33 | 59.46 | 54.41 | 0.521 |
| ≥65 years | 81.71 | 69.54 | 65.30 | 60.94 | ||
| Gender | Female | 86.50 | 72.04 | 62.11 | 56.62 | 0.102 |
| Male | 81.25 | 55.80 | 51.05 | 51.05 | ||
| Immunological status | RF - | 86.76 | 63.17 | 42.79 | 35.50 | 0.020 |
| RF + | 85.16 | 69.90 | 61.91 | 58.80 | ||
| ACPA - | 90.67 | 69.26 | 58.01 | 52.05 | 0.986 | |
| ACPA + | 84.37 | 67.13 | 58.02 | 49.21 | ||
| Sjogren’s Syndrome | SS - | 85.75 | 70.85 | 61.05 | 56.13 | 0.514 |
| SS + | 86.96 | 56.52 | 56.52 | 56.52 | ||
| RA treatment | MTX - | 81.46 | 59.52 | 47.38 | 42.89 | <103 |
| MTX + | 88.79 | 77.04 | 69.71 | 64.90 | ||
| Corticosteroid therapy - | 87.50 | 76.76 | 70.65 | 68.10 | <103 | |
| Corticosteroid therapy + | 84.58 | 64.90 | 53.04 | 45.96 | ||
| Nonresponse to bDMARDs | 71.79 | 51.28 | 39.89 | 28.49 | <103 | |
| Moderate and good response to bDMARDs | 92.83 | 76.41 | 66.45 | 63.87 |
RF: Rheumatoid Factor; ACPA: Anti-Citrullinated Protein Antibodies; SS: Sjögren’s syndrome; RA: Rheumatoid Arthritis; MTX: Methotrexate; bDMARDs: biologic disease modifying antirheumatic drugs.
Table 5.
Drug retention time, according to clinical, immunologic, and therapeutic features.
| Mean duration (months) | CI 95% | ||
|---|---|---|---|
| Age | <65 years | 41.43 | [38.93–43.93] |
| ≥65 years | 42.21 | [37.51–46.92] | |
| Gender | male | 42.3 | [39.98–44.46] |
| female | 36.31 | [30.08–42.53] | |
| Immunological status | RF - | 34.97 | [30.06–39.89] |
| RF + | 42.15 | [39.43–44.87] | |
| ACPA - | 40.05 | [35.30–44.80] | |
| ACPA + | 40.05 | [36.87–43.24] | |
| Sjogren’s Syndrome | SS + | 41.83 | [39.54–44.12] |
| SS - | 39.83 | [31.26–48.40] | |
| RA treatment | MTX - | 34.86 | [31.55–38.17] |
| MTX + | 45.46 | [42.74–48.18] | |
| Corticosteroid therapy - | 45.34 | [42.25–48.43] | |
| Corticosteroid therapy + | 38.32 | [35.31–41.33] | |
| Nonresponse to bDMARDs | 28.55 | [22.41–34.69] | |
| Moderate and good response to bDMARDs | 45.35 | [43.06–47.64] |
CI: Confidence interval; RF: Rheumatoid Factor; ACPA: Anti-Citrullinated Protein Antibodies; SS: Sjögren’s syndrome; RA: Rheumatoid Arthritis; MTX: Methotrexate; bDMARDs: biologic disease modifying antirheumatic drugs.
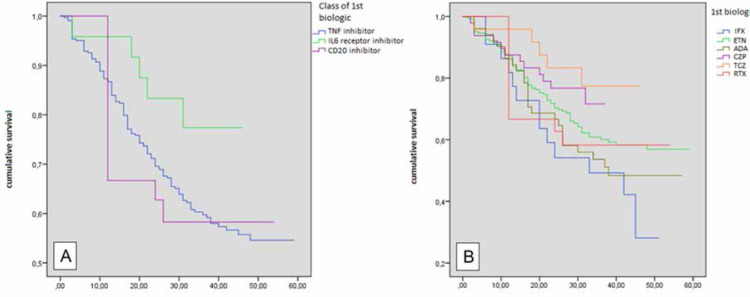
Impact of bDMARDS on their Survival Rate and Retention Time We found no significant difference between the survival curves according to the class of the bDMARD (TNFi, IL6Ri and CD20i) (p=0.208). Similarly, when studying the curves of all bDMARDs separately (IFX, ADA, ETN, CZP, TCZ and RTX), no difference was detected between survival curves (p=0.109) (Figure 1). Compared to IFX, the risk of discontinuation was statistically lower for CZP (HR=0.443, p=0.037) and TCZ (HR=0.304, p=0.024), respectively.
Figure 1.
Survival curves according to (A): the class of the first bDMARD; (B): each bDMARD separately.
Multivariate Analysis
Multivariate analysis concluded that the combination of corticosteroids with bDMARDs doubles the risk of bDMARD failure (HR=2.127, p<10-3).
In addition, RF positivity is associated with better drug survival (HR=0.558, p=0.006). Similarly, the combination with MTX was associated with a reduced risk of bDMARDs discontinuation of up to 48% (HR=0.522, p<10-3).
Moreover, therapeutic response at 3 months was a key factor in bDMARDs survival. A moderate or good initial response was associated with better bDMARDs survival by reducing the risk of discontinuation by 66% (HR=0.434, p<10-3).
In contrast, female gender and IFX were associated with an increased risk of discontinuation (HR=1.186, HR=1.682, respectively).
DISCUSSION
This study of RA patients on bDMARDs showed an average response to first-line bDMARDs after four years of follow-up. Indeed, LDA was observed in half of our patients and remission was achieved in about a quarter. The four-year first-line survival rate was 55.9% and the bDMARD was maintained for approximately four years. The most effective bDMARD was TCZ. Among TNFi, the highest rate of good response was observed under ETN (77.83%). RF positivity, MTX combination, and good initial therapeutic response were factors influencing the survival of bDMARDs. Corticosteroids use predicted a lower survival rate.
Factors Influencing Survival and Therapeutic Retention
Demographic factors
Some studies had not found an association between age and treatment retention,10–12 which is consistent with our results, in contrast to Seung Min et al. who found that elderly RA patients were more likely to discontinue bDMARDs within 24 months.13 On the other hand, Jino S et al. had studied Japanese patients with elderly-onset RA treated with TNFi and CTZ and found that drug retention was significantly higher with CTZ. Moreover, discontinuation due to lack of efficacy was significantly less frequent in CTZ while discontinuations due to adverse event or achievement of clinical remission were similar between the two groups.14
The mean duration of RA was 11.7 years. The literature data were divergent on this subject. Indeed, the different screening and treatment strategies could induce this disparity in the disease duration. In addition, the RA duration induced poor therapeutic response, especially for bDMARDs and was a risk factor for discontinuation, much more for reasons of side effects than inefficacy.15,16 These patients are more sensitive to the properties of these immunosuppressive molecules and are at higher risk of side effects.
Immunological biomarkers
There is conflicting opinion regarding the impact of RF and ACPA on survival of bDMARDs.17 Lv et al.18 concluded that immunological status did not influence the response to TNFi. However, Ogawa et al. noted that RF positivity was associated with a higher rate of TNFi discontinuation, whereas ACPA had no effect on drug survival. This is explained by the fact that IgM has a greater potential to activate complement, causing greater inflammation, which may explain the more frequent cessation of TNFi in the presence of IgM isotype RF. However, ACPA, being IgG, are associated with less discontinuation.17,19,20
On the other hand, in line with our results, Ching-Tsai Lin et al. found that IgM isotype RF positivity was negatively associated with drug survival (HR=0.48, 95%CI[0.27–0.85], p=0.013).21 Mulligen et al. also agreed with these results.22 This could be explained by the fact that RF- and ACPA-negative RA generally show less bone erosion, structural damage, and disease progression.23 Thus, the presence of these autoantibodies could be associated with a more aggressive disease responding less to bDMARDs.
This discrepancy could be due in part to the different genetic and ethnic characteristics of populations, the definition of the outcomes, and statistical methods.
Disease activity
The mean DAS28 on initiation of bDMARDs was 6.02±0.82 [5.37–6.5] in our study and between 3.95 and 6.3 in the literature.24,25
The initial DAS28 was an indicator of survival of bDMARDs, as suggested by Hetland et al.26 and Gabay et al.27 who had shown that a high DAS28 at initiation of bDMARDs allowed better retention. These results were not in agreement with the Spanish study by Leon et al. who showed that a DAS28 score>5.1 was not associated with subsequent survival of bDMARDs.28
We did not evaluate the association of initial DAS28 with survival rates in our study. In fact, all patients had an initial DAS28>5.1.
This was the condition for granting bDMARDs via NHIF.
RA Treatments
Concomitant use of corticosteroids was observed 57.2% in our study and between 43.5 and 75% in the literature.29–31 Its impact on the survival of bDMARDs has been studied by several authors who had identified an increased risk of discontinuation.16,32,33 Souto et al.33 explained these findings by having more severe RA requiring corticosteroid use with a greater risk of treatment failure and discontinuation. These results were consistent with our results. However, no effect of corticosteroids on the efficacy and safety of bDMARDs was found in a study that pooled data from four double-blind randomised controlled trials in RA (AMBITION, ACT-RAY, AD-ACTA, and FUNCTION).34
Although EULAR recommendations suggest the combination of TNFi with MTX in RA,35 up to one-third of patients with RA are treated with TNFi as monotherapy.36 MTX-TNFi combination was prescribed in 59.6% of our patients. These results were lower than those of Kobayakawa et al.30 Ebina et al.29 and Ramiro et al.31 who reported this combination in 75, 68.6 and 69% of cases, respectively. This disparity could be explained by a lower adherence to the oral route due to poor tolerance. This combination was predictive of better survival of bDMARDs in our study and other studies.5,15,29,33,37
Choice of bDMARDs
To date, there are no validated criteria for selecting the first-line bDMARD. This depends on comorbidities, systemic manifestations, and complications, as well as national and international recommendations.38–40 Several studies had not shown significant differences between these products in terms of clinical, functional or structural efficacy.41–44 TNFi was the most commonly prescribed first-line bDMARD.41,42
Among the TNFi in our study, CZP allowed a faster achievement of LDA (44 weeks [11.1–107.6]) and remission (46.7 weeks [11.1–120.6]) compared to the others. This was in agreement the PREDICT, RAPID-1, and RAPID-2 studies, which showed that CZP reduced disease activity and improved functional indices, fatigue, and pain in patients from the 12th week of treatment.45–47 More interestingly, Nakayama et al. had shown that RA patients with high serum RF (>166IU/ml) treated with TNFi without Fc fragment had significantly lower DAS28-ESR at 12 months of treatment compared with those treated with TNFi with Fc.48
Furthermore, among all bDMARDs in our study, TCZ had the highest LDA (70.8%) and remission rate (33.3%).
Our remission rate was lower than those in the literature (ranging from 45 to 86%).49–51 This could be explained by a longer duration of RA and the low percentage of men in our cohort. Indeed, remission is less frequent in women than in men.52
In addition, the retention time of the first bDMARD was 41.7 months [39.4–43.9]. This result was close to those reported by Ramiro et al. (49.2 months) and Brodszky et al. (42.8 months).31,53 Furthermore, the ANSWER cohort study showed that Abatacept and TCZ had higher retention, and TCZ had lower rates of discontinuation due to inefficacy compared with IFX. In contrast, IFX had a higher rate of discontinuation for remission than Abatacept, ETN, Golimumab, and TCZ in adjusted modeling.29
Hishitani et al.54 had found a retention time for TCZ, IFX, ETN and ADA of 2.5, 1.9, 2.9, and 1.3 years, respectively.
The authors of the Danish DANBIO registry,55 concluded that the median survival rate was 45 months for ETA and 29 months for IFX and found low remission rates despite long drug survival. However, Soubrier et al. found that there was no difference in retention between ADA, ETA, and IFX. Twenty-five percent of patients continued treatment for 15 years.32
Furthermore, in a recent systematic literature review including 170 publications, the survival rate of ETN was higher than that of the other TNFi after more than one year of follow-up.56 On the other hand, in a cohort study by Choi S et al. patients who received first-line TCZ, Tofacitinib or abatacept had a higher survival rate compared to first-line ETN.57 These discrepancies in retention time and survival rates could be explained by variations in patient recruitment methods, disease duration, activity at baseline, changes in global assessment, etc.58
Initial therapeutic response may predict better bDMARDs survival rates. Wei et al.59 had shown that the survival rate is better for patients who had initially responded well to treatment. Indeed, as the progression index (CDAI) decreases, the therapeutic survival improves. This was in agreement with our study.
To our knowledge, this study is one of the few to address the retention and survival of bDMARDs in an Arab and African population. It investigates the real-life survival of bDMARDs and highlights their value in the treatment of RA. Identification of factors that may affect survival would allow optimisation of treatment outcomes by acting on modifiable factors among them. However, our study has some limitations, mainly because of its open-label design, which is more prone to bias compared with double-blind controlled trials. In addition, the cohort size was relatively limited. Moreover, because of the “real-life” study design, the continued use of other medications, such as glucocorticoids and MTX, might affect the results. Another limitation of this study is that the data were collected from the NHIF database; recruitment bias may exist in the selection of patients to receive bDMARDs. Furthermore, given the significant disparity in the number of patients on different classes of bDMARDs and the fact that most of our patients were on TNFi (86.4%). The conclusions drawn from our comparative study remain to be validated by other, larger studies with similar proportions of bDMARDs classes.
CONCLUSION
The overall management of RA has improved significantly in recent decades thanks to bDMARDs. These treatments, not devoid of risk and not always effective, may be discontinued for one reason or another, hence the interest in studying their survival. The literature on the subject was divergent. Our study identified factors influencing biological survival, including RF positivity, MTX-TNFi combination, corticosteroid use and good initial response to treatment. Action on modifiable factors could improve bDMARDs survival, enabling better disease control. Larger-scale African and Arab studies would be needed to support our results.
AUTHOR CONTRIBUTIONS
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
S Boussaid: research conception/design, data acquisition, data analysis/interpretation, manuscript preparation, final approval
H Tbini: research conception/design, data analysis/interpretation, manuscript preparation
S Rekik: final approval
S Khaled: research conception/design, data acquisition, data analysis/interpretation
S Rahmouni: final approval
K Zouaoui: final approval
S Riahi: final approval
H Sahli: final approval
M Elleuch: final approval
CONFLICT OF INTEREST
The authors declare no conflict of interest.
RESEARCH FUNDING
None declared.
ETHICAL APPROVAL
Our locally appointed ethics committee has approved the study.
INFORMED CONSENT
Informed consent was obtained from all individuals included in this study.
AVAILABILITY OF DATA AND MATERIAL
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Kim G, Barner JC, Rascati K, Richards K. Factors associated with the initiation of biologic disease-modifying antiRheumatic drugs in Texas Medicaid patients with Rheumatoid arthritis. J Manag Care Spec Pharm 2015;21:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis JR, Singh JA. Use of biologics in Rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 2011;33:679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A, et al. Mortality in Rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFalpha inhibitors and rituximab. Ann Rheum Dis 2015;74:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe F, Michaud K, Dewitt EM. Why results of clinical trials and observational studies of antitumour necrosis factor (anti-TNF) therapy differ: methodological and interpretive issues. Ann Rheum Dis 2004;63 Suppl 2:ii13–ii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favalli EG, Pregnolato F, Biggioggero M, Becciolini A, Penatti AE, Marches-oni A, et al. Twelve-Year Retention rate of First-Line Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis: Real-Life Data From a Local Registry. Arthritis Care Res (Hoboken) 2016;68:432–9. [DOI] [PubMed] [Google Scholar]
- 6.Naffaa ME, Hassan F, Golan-Cohen A, Merzon E, Green I, Saab A, et al. Factors associated with drug survival on first biologic therapy in patients with Rheumatoid arthritis: a population-based cohort study. Rheumatol Int 2021. Nov;41:1905–13. [DOI] [PubMed] [Google Scholar]
- 7.Egeberg A, Rosenø NAL, Aagaard D, Lørup EH, Nielsen ML, Nymand L, et al. Drug survival of biologics and novel immunomodulators for Rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis - A nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum 2022; 53:151979. [DOI] [PubMed] [Google Scholar]
- 8.Cerveró AD, Cardona G, Morales A, Carrascosa M, Ferrandiz C, Guomundsdottir F, et al. 4CPS-041 Drug survival of biologic therapies for the treatment of psoriasis. Eur J Hosp Pharm 2018; 25:A59–A60. [Google Scholar]
- 9.van Gestel AM, Prevoo ML, van ‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996. Jan;39(1):34–40. [DOI] [PubMed] [Google Scholar]
- 10.Hilliquin P, Barnetche T, Baillet A, Flipo RM, Lespessailles E, Roux C, et al. Real-World 1-Year Retention rate of Subcutaneous Tocilizumab Treatment in Patients with Moderate to Severe Active Rheumatoid Arthritis: TANDEM Study. Rheumatol Ther 2021;8:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki S, Watanabe R, Harigae H, Fujii H. Better Retention of Abatacept Is Associated with High Rheumatoid Factor: A Five-Year Follow-Up Study of Patients with Rheumatoid Arthritis. Tohoku J Exp Med 2020;250:153–9. [DOI] [PubMed] [Google Scholar]
- 12.Ebina K, Hirano T, Maeda Y, Yamamoto W, Hashimoto M, Murata K, et al. Drug retention of secondary biologics or JAK inhibitors after tocilizumab or abatacept failure as first biologics in patients with Rheumatoid arthritis -the ANSWER cohort study. Clin Rheumatol 2020. Sep; 39:2563–2572. [DOI] [PubMed] [Google Scholar]
- 13.Seung MJ, Sang-Won L, Jungsik Song J, Sung-Hwan P, Yong-Beom P. Drug Survival of Biologic Therapy in Elderly Patients With Rheumatoid Arthritis Compared With Nonelderly Patients: Results From the Korean College of Rheumatology Biologics Registry. J Clin Rheumatol 2022. Jan 1; 28:e81–e88. [DOI] [PubMed] [Google Scholar]
- 14.Jinno S, Onishi A, Dubreuil M, Hashimoto M, Yamamoto W, Murata K, et al. Comparison of the drug retention and reasons for discontinuation of tumor necrosis factor inhibitors and interleukin-6 inhibitors in Japanese patients with elderly-onset rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther 2021. Apr 15;23:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Reino JJ, Rodriguez-Lozano C, Campos-Fernandez C, Montoro M, Descalzo MA, Carmona L, et al. Change in the discontinuation pattern of tumour necrosis factor antagonists in Rheumatoid arthritis over 10 years: data from the Spanish registry BIOBADASER 2.0. Ann Rheum Dis 2012;71:382–5. [DOI] [PubMed] [Google Scholar]
- 16.Markenson JA, Gibofsky A, Palmer WR, Keystone EC, Schiff MH, Feng J, et al. Persistence with anti-tumor necrosis factor therapies in patients with Rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol 2011;38:1273–81. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa Y, Takahashi N, Kaneko A, Hirano Y, Kanayama Y, Yabe Y, et al. Association between seropositivity and discontinuation of tumor necrosis factor inhibitors due to ineffectiveness in Rheumatoid arthritis. Clin Rheumatol 2019;38:2757–63. [DOI] [PubMed] [Google Scholar]
- 18.Lv Q, Yin Y, Li X, Shan G, Wu X, Liang D, et al. The status of Rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-TNFalpha agent treatment in patients with Rheumatoid arthritis: a meta-analysis. PLOS ONE 2014;9:e89442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurlander RJ, Rosse WF, Logue GL. Quantitative influence of antibody and complement coating of red cells on monocyte-mediated cell lysis. J Clin Invest 1978. May;61(5):1309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper NR. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol 1985;37:151–216. [DOI] [PubMed] [Google Scholar]
- 21.Lin CT, Huang WN, Tsai WC, Chen JP, Hung WT, Hsieh TY, et al. Predictors of drug survival for biologic and targeted synthetic DMARDs in rheumatoid arthritis: Analysis from the TRA Clinical Electronic Registry. PLoS One. 2021. Apr 30;16(4):e0250877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Mulligen E, Ahmed S, Weel AEAM, Hazes JMW, van der Helm-van Mil AHM, de Jong PHP. Factors that influence biological survival in rheumatoid arthritis: results of a real-world academic cohort from the Netherlands. Clin Rheumatol 2021. Jun;40:2177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmström V, Catrina AI, Klareskog L. The immunopathogenesis of sero-positive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol 2017. Jan;17(1):60–75. doi: 10.1038/nri.2016.124. Epub 2016 Dec 5. Erratum in: Nat Rev Immunol. 2022 Jul;22(7):459. PMID: 27916980. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos CG, Gartzonikas IK, Pappa TK, Markatseli TE, Migkos MP, Voulgari PV, et al. Eight-year survival study of first-line tumour necrosis factor alpha inhibitors in Rheumatoid arthritis: real-world data from a university centre registry. Rheumatol Adv Pract 2019;3:rkz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narongroeknawin P, Chevaisrakul P, Kasitanon N, Kitumnuaypong T, Mahakkanukrauh A, Siripaitoon B, et al. Drug survival and reasons for discontinuation of the first biological disease modifying antiRheumatic drugs in Thai patients with Rheumatoid arthritis: Analysis from the Thai Rheumatic Disease Prior Authorization registry. Int J Rheum Dis 2018;21:170–8. [DOI] [PubMed] [Google Scholar]
- 26.Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with Rheumatoid arthritis treated with adalimumab, etanercept, or IFXliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010; 62:22–32. [DOI] [PubMed] [Google Scholar]
- 27.Gabay C, Riek M, Scherer A, Finckh A, physicians Sc. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in Rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology (Oxford) 2015;54:1664–72. [DOI] [PubMed] [Google Scholar]
- 28.Leon L, Rodriguez-Rodriguez L, Rosales Z, Gomez A, Lamas JR, Pato E, et al. Long-term drug survival of biological agents in patients with Rheumatoid arthritis in clinical practice. Scand J Rheumatol 2016; 45:456–60. [DOI] [PubMed] [Google Scholar]
- 29.Ebina K, Hashimoto M, Yamamoto W, Ohnishi A, Kabata D, Hirano T, et al. Drug retention and discontinuation reasons between seven biologics in patients with Rheumatoid arthritis -The ANSWER cohort study. PLOS ONE 2018;13:e0194130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayakawa T, Kojima T, Takahashi N, Hayashi M, Yabe Y, Kaneko A, et al. Drug retention rates of second biologic agents after switching from tumor necrosis factor inhibitors for Rheumatoid arthritis in Japanese patients on low-dose methotrexate or without methotrexate. Mod Rheumatol 2015;25:251–6. [DOI] [PubMed] [Google Scholar]
- 31.Ramiro S, Landewe R, van der Heijde D, Harrison D, Collier D, Michaud K. Discontinuation rates of biologics in patients with Rheumatoid arthritis: are TNF inhibitors different from non-TNF inhibitors? RMD Open 2015; 1:e000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soubrier M, Pereira B, Frayssac T, Fan A, Couderc M, Malochet-Guinamand S, et al. Retention rates of adalimumab, etanercept and IFXliximab as first-line biotherapy agent for Rheumatoid arthritis patients in daily practice - Auvergne experience. Int J Rheum Dis 2018; 21:1924–32. [DOI] [PubMed] [Google Scholar]
- 33.Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in Rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford) 2016;55:523–34. [DOI] [PubMed] [Google Scholar]
- 34.Safy-Khan M, Jacobs JWG, de Hair MJH, Welsing PMJ, Edwardes MD, Teitsma XM, et al. Effect on efficacy and safety trial outcomes of also enrolling patients on ongoing glucocorticoid therapy in Rheumatoid arthritis clinical trials of tocilizumab or adalimumab or methotrexate monotherapy. Ann Rheum Dis 2020;79:460–3. [DOI] [PubMed] [Google Scholar]
- 35.Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of Rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 36.Emery P, Pope JE, Kruger K, Lippe R, demasi R, Lula S, et al. Efficacy of monotherapy with biologics and JAK inhibitors for the treatment of Rheumatoid arthritis: a systematic review. Adv Ther 2018;35:1535–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.l’Ami MJ, Kneepkens EL, Nurmohamed MT, Krieckaert CLM, Visman IM, Wolbink GJ. Long-term treatment response in Rheumatoid arthritis patients starting adalimumab or etanercept with or without concomitant methotrexate. Clin Exp Rheumatol May–Jun 2017;35:431–7. [PubMed] [Google Scholar]
- 38.Eddaoudi M, Rostom S, Hmamouchi I, El Binoune I, Amine B, Abouqal R, et al. The first biological choice in patients with Rheumatoid arthritis: data from the Moroccan register of biotherapies. Pan Afr Med J 2021;38:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monti S, Klersy C, Gorla R, Sarzi-Puttini P, Atzeni F, Pellerito R, et al. Factors influencing the choice of first- and second-line biologic therapy for the treatment of Rheumatoid arthritis: real-life data from the Italian LOHREN Registry. Clin Rheumatol 2017;36:753–61. [DOI] [PubMed] [Google Scholar]
- 40.Cantini F, Goletti D, Benucci M, Foti R, Damiani A, Niccoli L. Tailored first-line biologic and targeted synthetic disease modifying anti-rheumatic drugs therapy in patients with rheumatoid arthritis: 2021 updated ITABIO statements Expert Opin Drug Saf 2021;1–11. [DOI] [PubMed] [Google Scholar]
- 41.Ebina K. Drug efficacy and safety of biologics and Janus kinase inhibitors in elderly patients with Rheumatoid arthritis. Mod Rheumatol 2022;32:256–62. [DOI] [PubMed] [Google Scholar]
- 42.Shukla R, Emery P, Buch MH. Efficacy of tumor-necrosis factor-inhibitor in moderate disease activity rheumatoid arthritis: sub-analysis of the “VEDERA” trial. Rheumatology (Oxford) 2022;61:868–9. [DOI] [PubMed] [Google Scholar]
- 43.Lauper K, Nordstrom DC, Pavelka K, Hernandez MV, Kvien TK, Kristianslund EK, et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the panEuropean TOCERRA register collaboration. Ann Rheum Dis 2018;77:1276–82. [DOI] [PubMed] [Google Scholar]
- 44.Di Sanzo L, Scrivo R, Roberto Soriano E, Citera G, Mysler E, Cheng-Chung W, et al. Editorial: Drug Survival: Treatment of Rheumatic Diseases in the Biologic Era. Front Med (Lausanne) 2022;9:858817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keystone E, Landewe R, van Vollenhoven R, Combe B, Strand V, Mease P, et al. Long-term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5-year results from the RAPID 1 trial and open-label extension. Ann Rheum Dis 2014;73:2094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bessette L, Haraoui B, Chow A, Fortin I, Dixit S, Khraishi M, et al. Effectiveness and safety of certolizumab pegol in Rheumatoid arthritis patients in Canadian practice: 2-year results from the observational FalphasT-CAN study. Ther Adv Musculoskelet Dis 2019;11:1759720X19831151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayama Y, Watanabe R, Murakami K, Murata K, Tanaka M, Ito H, et al. Differential efficacy of TNF inhibitors with or without the immunoglobulin fragment crystallizable (Fc) portion in Rheumatoid arthritis: the ANSWER cohort study. Rheumatol Int 2022. Mar 10. Doi: 10.1007/s00296-021-05086-w.Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Sadeq A, Elnour AA, Ramadan A, Yousif El Khidir I, Don J, Al Amoodi A, et al. Randomized clinical trials on the efficacy and safety of tocilizumab in subjects with Rheumatoid arthritis: a systematic review. Curr Rev Clin Exp Pharmacol 2022. Feb 2. Doi: 10.2174/2772432817666220202115623.Online ahead of print. PMID: 35114930. [DOI] [PubMed] [Google Scholar]
- 50.Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Kelman A, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomized controlled trial. Ann Rheum Dis 2016;75:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bijlsma JWJ, Welsing PMJ, Woodworth TG, Middelink LM, Pethö-Schramm A, Bernasconi C, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multi-centre, randomised, double-blind, double-dummy, strategy trial. The Lancet. 2016; 388:343–55. [DOI] [PubMed] [Google Scholar]
- 52.Svensson B, Andersson ML, Bala SV, Forslind K, Hafstrom I, BARFOT study group . Long-term sustained remission in a cohort study of patients with rheumatoid arthritis: choice of remission criteria. BMJ Open 2013;3:e003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brodszky V, Biro A, Szekanecz Z, Soos B, Baji P, Rencz F, et al. Determinants of biological drug survival in Rheumatoid arthritis: evidence from a Hungarian Rheumatology center over 8 years of retrospective data. Clinicoecon Outcomes Res 2017;9:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hishitani Y, Ogata A, Shima Y, Hirano T, Ebina K, Kunugiza Y, et al. Retention of tocilizumab and anti-tumour necrosis factor drugs in the treatment of Rheumatoid arthritis. Scand J Rheumatol 2013;42:253–9. [DOI] [PubMed] [Google Scholar]
- 55.Ostergaard M, Unkerskov J, Linde L, Krogh NS, Ravn T, Ringsdal VS, et al. Low remission rates but long drug survival in Rheumatoid arthritis patients treated with infliximab or etanercept: results from the nationwide Danish DANBIO database. Scand J Rheumatol 2007;36:151–4. [DOI] [PubMed] [Google Scholar]
- 56.Emery P, Vlahos B, Szczypa P, Thakur M, Jones HE, Woolcott J, et al. Longterm Drug Survival of Tumor Necrosis Factor Inhibitors in Patients with Rheumatoid Arthritis. J Rheumatol 2020. Apr;47:493–501. [DOI] [PubMed] [Google Scholar]
- 57.Choi S, Ghang B, Jeong S, Choi D, Lee JS, Park SM, et al. Association of first, second, and third-line bDMARDs and tsDMARD with drug survival among seropositive rheumatoid arthritis patients: Cohort study in A real world setting. Semin Arthritis Rheum 2021. Aug;51:685–91. [DOI] [PubMed] [Google Scholar]
- 58.Lauper K, Mongin D, Alpizar-Rodriguez D, Codreanu C, Iannone F, Kristianslund EK, et al. Drug retention of biological DMARD in rheumatoid arthritis patients: the role of baseline characteristics and disease evolution. Rheumatology (Oxford) 2019. Dec1;58:2221–9. [DOI] [PubMed] [Google Scholar]
- 59.Wei W, Knapp K, Wang L, Chen CI, Craig GL, Ferguson K, et al. Treatment Persistence and Clinical Outcomes of Tumor Necrosis Factor Inhibitor Cycling or Switching to a New Mechanism of Action Therapy: Real-world Observational Study of Rheumatoid Arthritis Patients in the United States with Prior Tumor Necrosis Factor Inhibitor Therapy. Adv Ther 2017;34:1936–52.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.