Abstract
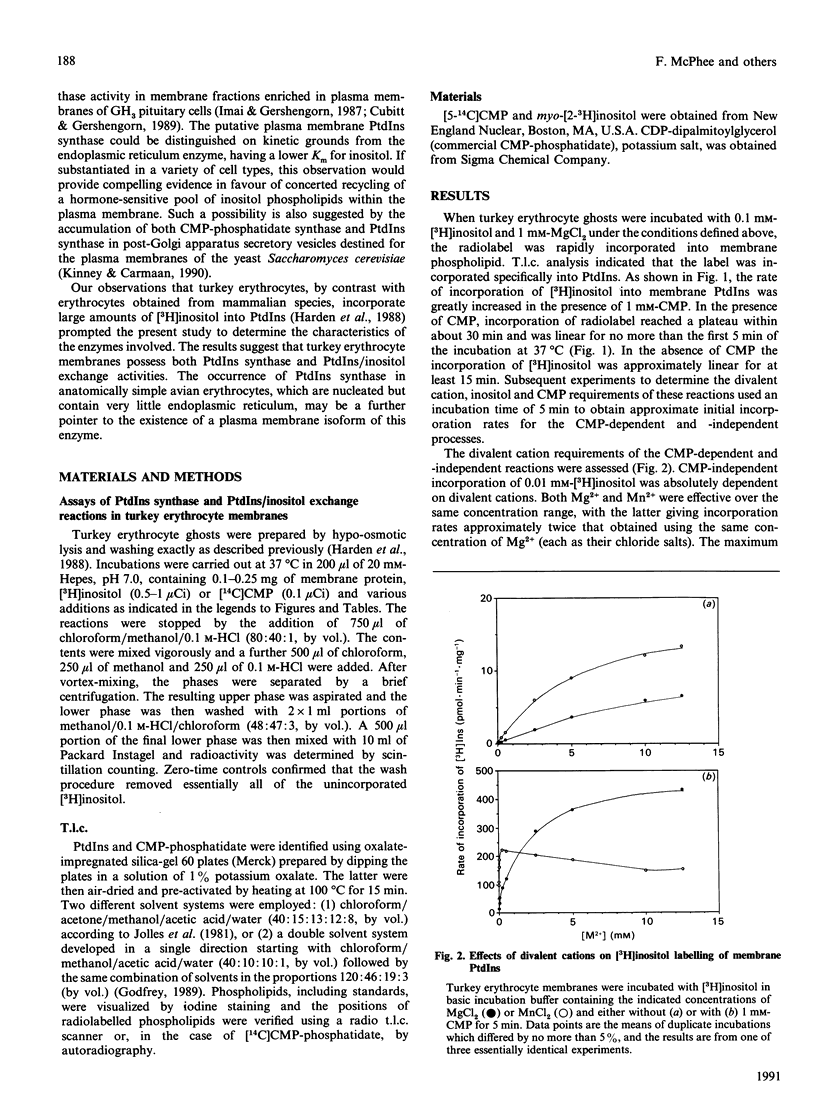
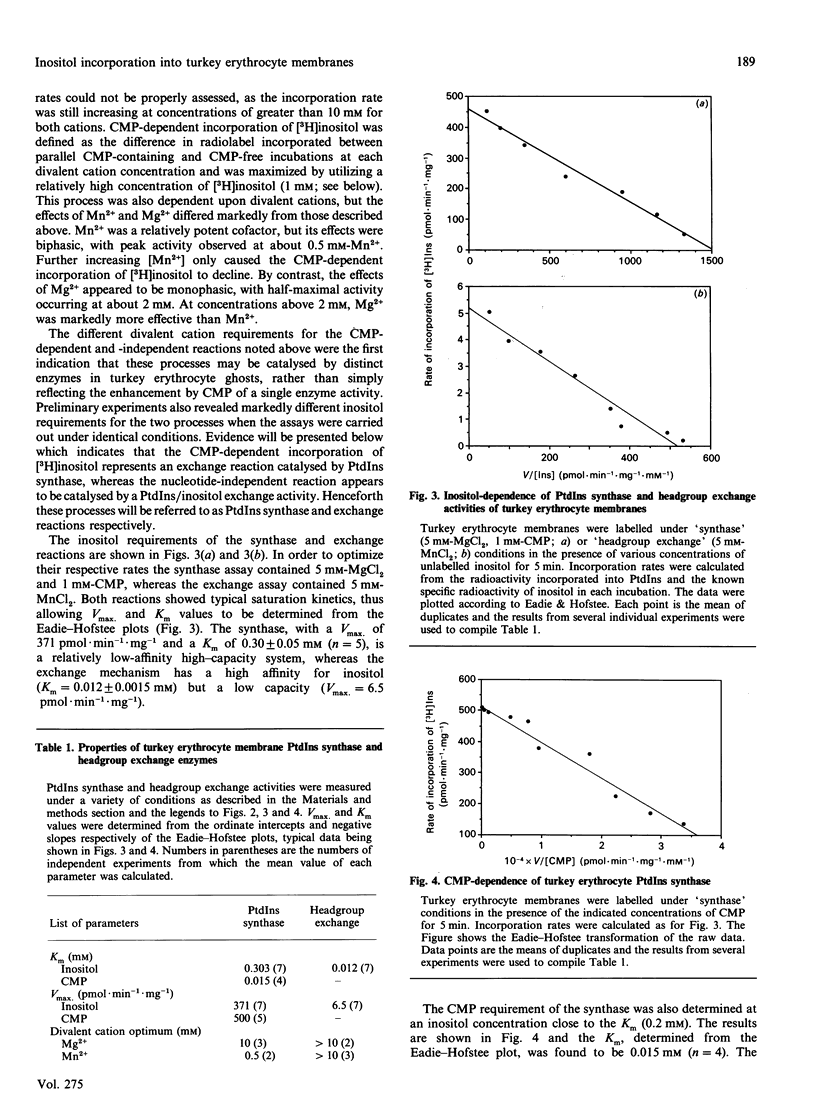
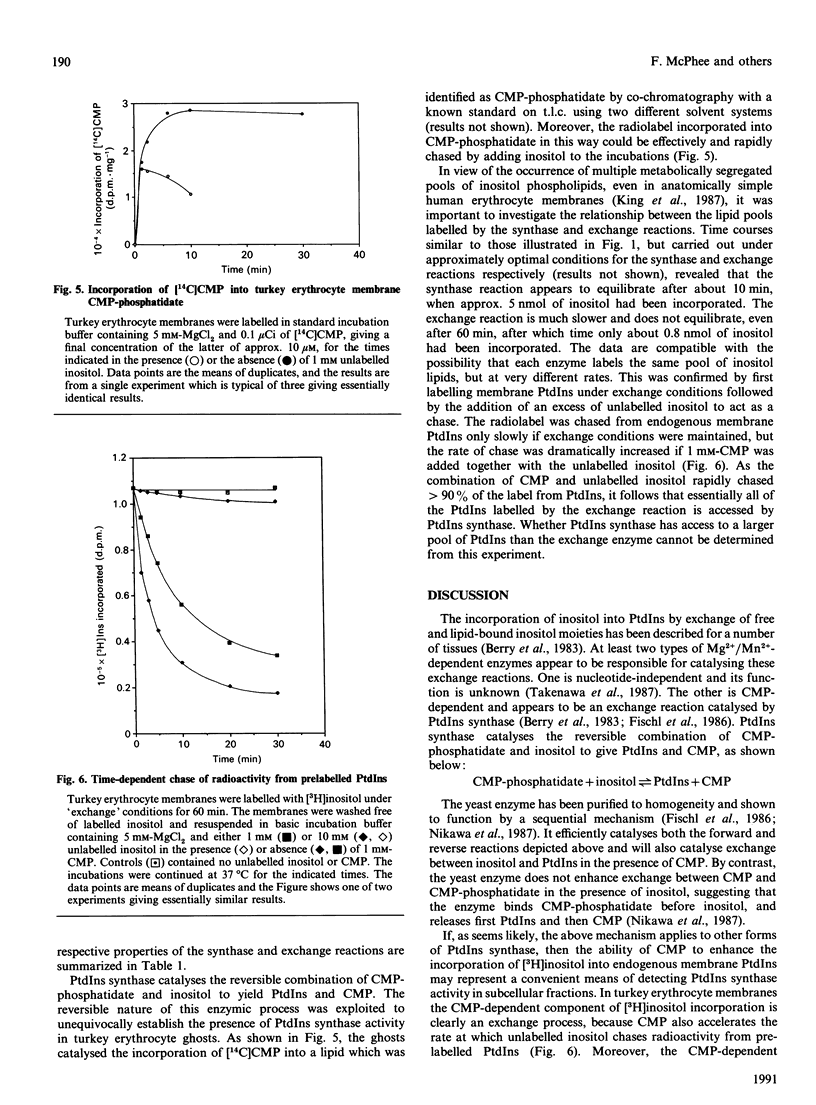
Unlike human erythrocytes, those from avian species, such as turkeys and chicks, rapidly incorporate myo-[3H]inositol into membrane phospholipids. The mechanisms regulating [3H]Ins labelling of phosphatidylinositol have been investigated using turkey erythrocyte membranes. In the absence of added nucleotides, [3H]inositol incorporation appears to proceed via phosphatidylinositol/inositol exchange, with a Km for inositol of 0.01 mM. The reaction was dependent upon divalent cations, either Mg2+ or Mn2+, with the latter metal ion being the more effective. [3H]Inositol incorporation was accelerated by CMP, especially when the concentration of Ins was greater than the Km for the exchange reaction. CMP-dependent labelling of PtdIns had a Km for inositol of 0.3 mM and for CMP of 0.015 mM. Divalent cations were also required for this reaction: activity peaked at 0.5 mM-Mn2+ and declined at higher concentrations. At relatively high concentrations, Mg2+ was more effective than Mn2+, with peak activity being achieved above 10 mM. CMP-dependent incorporation of [3H]inositol appears to reflect an exchange reaction catalysed by PtdIns synthase. Definitive evidence for the occurrence of PtdIns synthase in turkey erythrocyte membranes was obtained by demonstrating the formation of [14C]CMP-phosphatidate from [14C]CMP. The radioactivity could be efficiently chased from [14C]CMP-phosphatidate in the presence of unlabelled inositol. The detection of PtdIns synthase activity in morphologically simple turkey erythrocytes should help to clarify the subcellular distribution of this important component of the phosphatidylinositol cycle.
Full text
PDF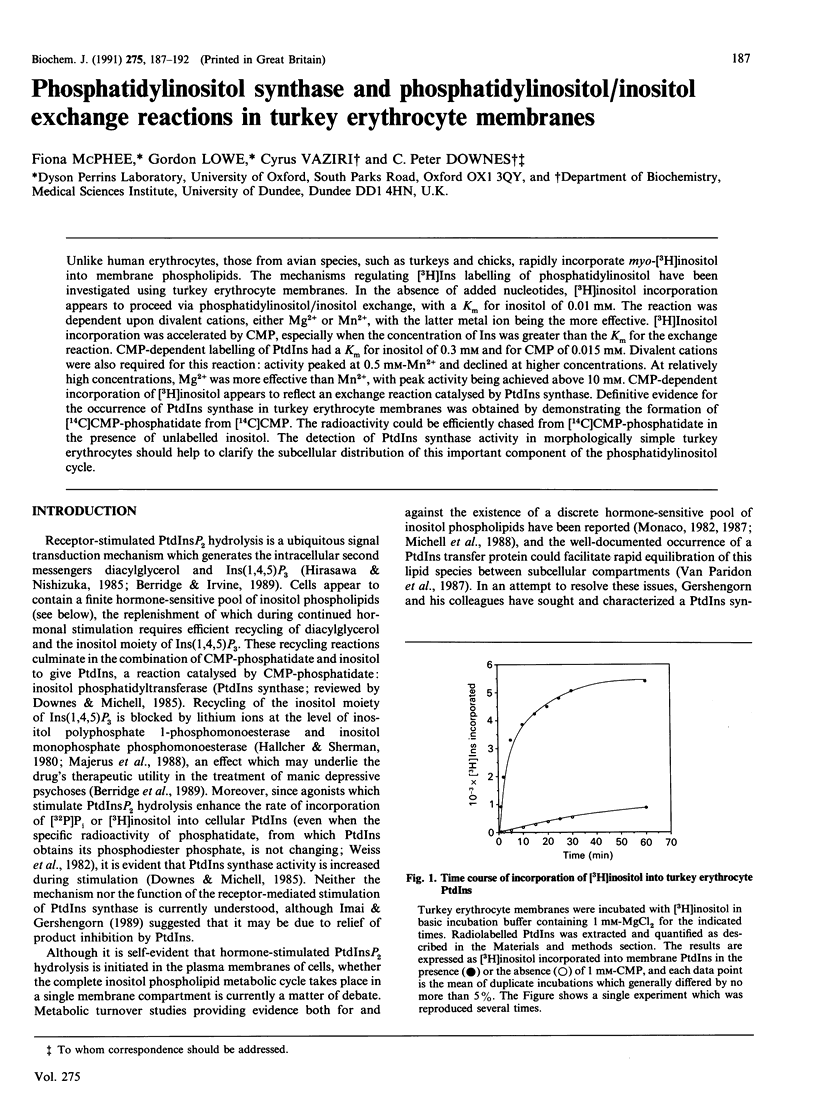


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Alper S. L., Palade G. E., Greengard P. Hormonally regulated phosphoprotein of turkey erythrocytes: localization to plasma membrane. J Cell Biol. 1979 Oct;83(1):1–15. doi: 10.1083/jcb.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berry G., Yandrasitz J. R., Segal S. CMP-dependent phosphatidylinositol:myo-inositol exchange activity in isolated nerve-endings. Biochem Biophys Res Commun. 1983 May 16;112(3):817–821. doi: 10.1016/0006-291x(83)91690-x. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Downes C. P., Harden T. K. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989 Jan 15;264(2):884–890. [PubMed] [Google Scholar]
- Cubitt A. B., Zhang B., Gershengorn M. C. Analysis by base exchange of thyrotropin-releasing hormone responsive and unresponsive inositol lipid pools in rat pituitary tumor cells. J Biol Chem. 1990 Jun 15;265(17):9707–9714. [PubMed] [Google Scholar]
- Fischl A. S., Homann M. J., Poole M. A., Carman G. M. Phosphatidylinositol synthase from Saccharomyces cerevisiae. Reconstitution, characterization, and regulation of activity. J Biol Chem. 1986 Mar 5;261(7):3178–3183. [PubMed] [Google Scholar]
- Ghalayini A., Eichberg J. Purification of phosphatidylinositol synthetase from rat brain by CDP-diacylglycerol affinity chromatography and properties of the purified enzyme. J Neurochem. 1985 Jan;44(1):175–182. doi: 10.1111/j.1471-4159.1985.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Godfrey P. P. Potentiation by lithium of CMP-phosphatidate formation in carbachol-stimulated rat cerebral-cortical slices and its reversal by myo-inositol. Biochem J. 1989 Mar 1;258(2):621–624. doi: 10.1042/bj2580621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Harden T. K., Hawkins P. T., Stephens L., Boyer J. L., Downes C. P. Phosphoinositide hydrolysis by guanosine 5'-[gamma-thio]triphosphate-activated phospholipase C of turkey erythrocyte membranes. Biochem J. 1988 Jun 1;252(2):583–593. doi: 10.1042/bj2520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Hokin-Neaverson M., Sadeghian K., Harris D. W., Merrin J. S. Synthesis of CDP-diglyceride from phosphatidylinositol and CMP. Biochem Biophys Res Commun. 1977 Sep 9;78(1):364–371. doi: 10.1016/0006-291x(77)91263-3. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Regulation by phosphatidylinositol of rat pituitary plasma membrane and endoplasmic reticulum phosphatidylinositol synthase activities. A mechanism for activation of phosphoinositide resynthesis during cell stimulation. J Biol Chem. 1987 May 15;262(14):6457–6459. [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. E., Stephens L. R., Hawkins P. T., Guy G. R., Michell R. H. Multiple metabolic pools of phosphoinositides and phosphatidate in human erythrocytes incubated in a medium that permits rapid transmembrane exchange of phosphate. Biochem J. 1987 May 15;244(1):209–217. doi: 10.1042/bj2440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney A. J., Carman G. M. Enzymes of phosphoinositide synthesis in secretory vesicles destined for the plasma membrane in Saccharomyces cerevisiae. J Bacteriol. 1990 Jul;172(7):4115–4117. doi: 10.1128/jb.172.7.4115-4117.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Maccallum S. H., Hunt P. A. Inositol lipids: receptor-stimulated hydrolysis and cellular lipid pools. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):239–246. doi: 10.1098/rstb.1988.0074. [DOI] [PubMed] [Google Scholar]
- Monaco M. E. Inositol metabolism in WRK-1 cells. Relationship of hormone-sensitive to -insensitive pools of phosphoinositides. J Biol Chem. 1987 Sep 25;262(27):13001–13006. [PubMed] [Google Scholar]
- Monaco M. E. The phosphatidylinositol cycle in WRK-1 cells. Evidence for a separate, hormone-sensitive phosphatidylinositol pool. J Biol Chem. 1982 Mar 10;257(5):2137–2139. [PubMed] [Google Scholar]
- Nikawa J., Kodaki T., Yamashita S. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem. 1987 Apr 5;262(10):4876–4881. [PubMed] [Google Scholar]
- Parries G. S., Hokin-Neaverson M. Phosphatidylinositol synthase from canine pancreas: solubilization by n-octyl glucopyranoside and stabilization by manganese. Biochemistry. 1984 Sep 25;23(20):4785–4791. doi: 10.1021/bi00315a039. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Egawa K. CDP-diglyceride:inositol transferase from rat liver. Purification and properties. J Biol Chem. 1977 Aug 10;252(15):5419–5423. [PubMed] [Google Scholar]
- Takenawa T., Saito M., Nagai Y., Egawa K. Solubilization of the enzyme catalyzing CDP-diglyceride-independent incorporation of myo-inositol into phosphatidyl inositol and its comparison to CDP-diglyceride:inositol transferase. Arch Biochem Biophys. 1977 Jul;182(1):244–250. doi: 10.1016/0003-9861(77)90304-6. [DOI] [PubMed] [Google Scholar]
- Tijburg L. B., Houweling M., Geelen M. J., Van Golde L. M. Inhibition of phosphatidylethanolamine synthesis by glucagon in isolated rat hepatocytes. Biochem J. 1989 Feb 1;257(3):645–650. doi: 10.1042/bj2570645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Paridon P. A., Gadella T. W., Jr, Somerharju P. J., Wirtz K. W. On the relationship between the dual specificity of the bovine brain phosphatidylinositol transfer protein and membrane phosphatidylinositol levels. Biochim Biophys Acta. 1987 Sep 18;903(1):68–77. doi: 10.1016/0005-2736(87)90156-8. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Regulation of phosphatidate synthesis by secretagogues in parotid acinar cells. Biochem J. 1982 May 15;204(2):587–592. doi: 10.1042/bj2040587. [DOI] [PMC free article] [PubMed] [Google Scholar]


