Abstract
Background This study aimed to determine the proportion of organ donors suitable for donation after circulatory death and investigate the current process followed by critical care physicians for declaring circulatory death to establish organ donation. Methods This observational study involved potential organ donors who had recently died after discontinuation of life support. We conducted an online survey of intensivists to determine how these deaths were confirmed. Results Among the 177 patients who died after withdrawal of life-sustaining treatment across 19 intensive care units in 11 institutions, 49 (27.7%) were considered potential donors. According to general medical criteria for organ donation, 20 (11.3%) patients were identified as medically suitable donors. Notably, 116 (73.9%) patients exhibited a flat electrocardiogram within 5 min after the loss of pulse. In the survey, 90 physicians (59.2%) agreed to implement the concept of the 5-min no-touch period for the declaration of circulatory death. Conclusions This study found that 11.3% of the patients who died following the withdrawal of life-sustaining treatment in the intensive care units were identified as suitable donors after circulatory death. Most of critical care physicians agree with the concept of a 5-min no-touch period for the declaration of circulatory death.
Keywords: Organ transplantation, Withholding treatment, Epidemiology, Intensive care unit, Survey, Questionnaire
Subject terms: Epidemiology, Medical ethics, Quality of life, Organ transplantation
Introduction
Organ transplantation is among the major advances of contemporary medical science, and it helps extend the life expectancy of patients with acute or chronic organ failure by procuring healthy organs from living or deceased donors. However, despite the contributions from living donors and donors following brain death, the persistently increasing demand for organs for transplantation exceeds the available supply1,2. Over the past decade, an average of 450 donations after brain death (DBD) have been reported annually in South Korea, comprising approximately 1,500–2,300 organs per year since 2011. Although the number of patients who are awaiting organ transplantation are increasing constantly for five years (from 37,217 to 49,993 patients), actual number of transplanted organs are declining for 4 years (from 1,611 to 1,353 organs), and number of patients who have deceased while on waiting list are rising for five years (from 1,894 to 2,918)3. The overall number of deceased organ donors remains modest, with 8.56 per million individuals in 2021, which is significantly lower compared to other countries (e.g., USA, 41.88; Spain, 40.2; France, 24.68; UK, 20.12; Brazil, 13.8; and Argentina, 13.75 per million individuals)4.
For over five decades, the concept of brain death has served as a benchmark for legally certifying death and as the main method for DBD5,6. Conventionally, medical term of death has been associated with circulatory death. However, organs from patients with circulatory death exhibit ischemic constraints and are unsuitable for transplantation. Donors with brain death have historically been the preferred choice for achieving favorable transplant outcomes. Advancements in medical science have mitigated the challenges associated with organ ischemia. Consequently, some countries now endorse donation after circulatory death (DCD) as a viable alternative with favorable outcomes7–11, particularly given the concern regarding organ viability12–14.
DCD entails the retrieval of organs from patients whose death has been confirmed based on circulatory death criteria. The “Maastricht Classification of Donation after Circulatory Death”15 has been universally adopted over the past two decades to categorize patients with circulatory death based on distinct end-of-life circumstances16, into either I “Dead on arrival at the hospital,” II “Death with unsuccessful resuscitation,” III “Awaiting cardiac arrest, including withdrawal of life-sustaining treatments (LST),” or IV “Cardiac arrest during brain death”16. Notably, few studies have focused on DCD in South Korea, with the recent exception of death classified as category IV according to the Modified Maastricht Classification17.
The prerequisites to establish a DCD protocol include a collection of comprehensive epidemiological data related to the deceased patients, the proportion of patients classified under different categories according to the Modified Maastricht Classification, and the delineation of unambiguous objective criteria for the declaration of circulatory death. Other nations that have already adopted the DCD criteria to identify patients suitable for organ donation have deliberated extensively on the legal and ethical aspects18,19 and have advocated for a “5-min no-touch” interval as a criterion to ascertain circulatory death20–22.
This study aimed to identify potential DCD donors within the intensive care units (ICUs) of South Korea, with a focus on epidemiological considerations, and explore the current practices among critical care physicians for declaring the death of a patient. Our aims were as follows: (1) to assess the epidemiological data related to adult deaths in ICUs, particularly for those patients who fit into category III of the Modified Maastricht Classification; and (2) to execute an online survey targeting intensivists. We hypothesized that the use of this data would help establish reliable criteria for cardiac death declaration and unequivocally define circulatory death, eventually facilitating the formulation of new policy recommendations for DCD.
Results
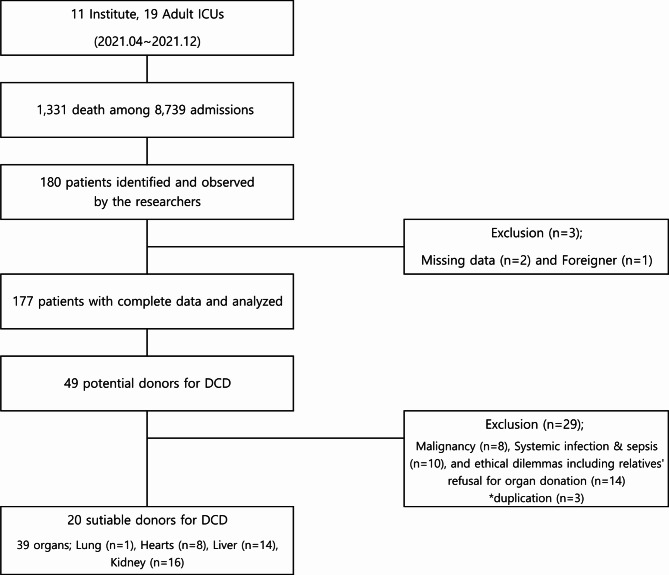
In total, 1,331 critically ill adults (15.2%) died in 19 ICUs equipped with 341 beds in 11 institutions from April to December 2021. Among those, 177 patients (128 [72.3%] males and 49 [27.7%] females), who were identified as medically futile, were comprehensively analyzed for this study (Fig. 1). The mean age of the included patients was 69.3 ± 13.3 years. The mean APACHE-II and SOFA scores upon ICU admission were 26 ± 7.1 and 9.8 ± 3.9, respectively. The average duration of hospital stay was 16.7 ± 22.3 days, and the mean ICU stay lasted 12.3 ± 18.1 days. The most prevalent diagnosis upon ICU admission was acute organ failure (58, 32.8%), followed by malignancy (31, 17.5%), and sepsis and septic shock (27, 15.3%) (Table 1). The three leading causes of death were organ failure (81, 45.8%), sepsis and septic shock (24, 13.6%), and malignancy (20, 11.3%). Among the observed individuals, 49 (27.7%) were identified as potential DCD donors based on the decision of the attending physician. The predominant causes of death among the potential DCD donors were organ failure (13, 26.5%), trauma (8, 16.3%), hypoxic brain damage (6, 12.2%), malignancy (6, 12.2%), and cerebrovascular diseases (6, 12.2%) (Table 1). Ultimately, 20 patients (11.3%) were considered suitable donors for DCD, following a comprehensive review by two senior critical care specialists. From these 20 suitable donors, the expected organ donations included 1 lung, 8 hearts, 14 livers, and at least 8 kidneys, assuming no warm ischemic time.
Figure 1.
Study flow chart.
Table 1.
Demographics and clinical characteristics of 177 observed patients.
| Characteristics | All patients† (n = 177) |
Potential DCD donors† (n = 49) |
|
|---|---|---|---|
| Age, years | 69.3 ± 13.3 | 65.1 ± 14.8 | |
| Sex | |||
| Male | 128 (72.3) | 39 (79.6) | |
| Female | 49 (27.7) | 10 (20.4) | |
| APACHE-II score | 26 ± 7.1 | 27 ± 6.1 | |
| SOFA score on day 1 | 9.8 ± 3.9 | 8.9 ± 3.3 | |
| DOS, hospital (day) | 16.7 ± 22.3 | 15 ± 14.8 | |
| DOS, ICU (day) | 12.3 ± 18.1 | 12 ± 13.6 | |
| Major diagnosis at admission, n (%) | |||
| Cardiovascular disease | 17 (9.6) | 5 (10.2) | |
| Cerebrovascular disease | 7 (4) | 5 (10.2) | |
| Sepsis or septic shock | 27 (15.3) | 3 (6.1) | |
| Suicide | 5 (2.8) | 3 (6.1) | |
| Malignancy | 31 (17.5) | 4 (8.2) | |
| Trauma | 15 (8.5) | 9 (18.4) | |
| Organ failure | 58 (32.8) | 13 (26.5) | |
| Cardiopulmonary arrest | 11 (6.2) | 6 (12.2) | |
| Other | 6 (3.4) | - | |
| Cause of death, n (%) | |||
| Cardiovascular disease | 17 (9.6) | 5 (8.2) | |
| Cerebrovascular disease | 7 (4) | 6 (12.2) | |
| Sepsis or septic shock | 24 (13.6) | 3 (6.1) | |
| Suicide | 2 (1.1) | 1 (2) | |
| Malignancy | 20 (11.3) | 6 (12.2) | |
| Trauma | 13 (7.3) | 8 (16.3) | |
| Organ failure | 81 (45.8) | 13 (26.5) | |
| Brain death | 1 (0.6) | 1 (2) | |
| Hypoxic brain damage | 12 (6.8) | 6 (12.2) | |
APACHE-II acute physiology and chronic health evaluation-II, SOFA sequential organ failure assessment, DOS duration of stay, ICU intensive care unit, DCD donation after circulatory death
† Values are presented as a number (%) or mean ± standard deviation
Regarding the death declaration practices among the 177 observed deaths, disruption to two or three vital parameters (61, 34.5%, and 50, 28.2%, respectively) was considered a prerequisite for the declaration of death. Notably, a flat ECG wave was the most common requirement (176, 99.4%), followed by loss of the arterial pulse and wave (151, 85.3%), low SpO2 oxygen saturation (68, 38.4%), diminished respiratory rate (61, 34.4%), and loss of pupil light reflex (46, 25.9%).
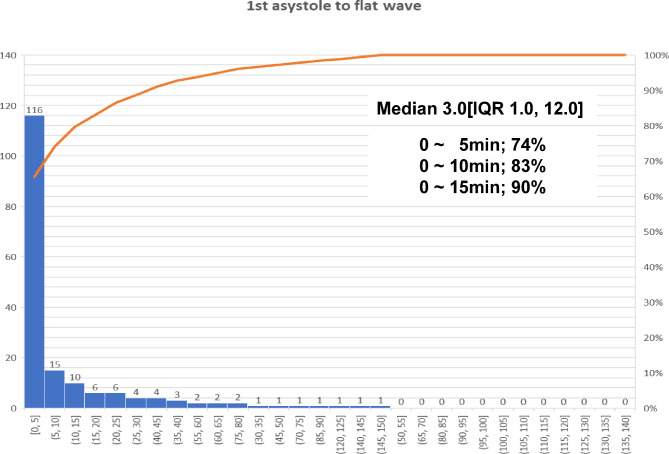
The time interval from withdrawing LST to asystole was 63.4 ± 105.7 min on average, with a median of 27 min and an IQR of 13.5–64 min. The duration from asystole to the ECG flat line was 13.3 ± 24.9 min on average, with a median of 3 min and an IQR of 1–12 min (Table 2). Death was conclusively declared within 5 min of asystole in 116 patients (73.9%), whereas the precise time for the declaration of death was not documented in 20 cases (Fig. 2).
Table 2.
Time to circulatory death in potential organ donors after withdrawal of life-sustaining treatment.
| Characteristics | Number† | Median‡ [min] |
IQR‡ [min] |
Mean ± SD‡ [min] |
Minimum, Maximum‡ [min] |
|---|---|---|---|---|---|
| WLST to loss of pulse§ (min) | |||||
| All patients | 177 | 27 | 13.5–64 | 63.4 ± 105.7 | 0, 951 |
| Potential donor | 49 | 16 | 10.5–52 | 55.8 ± 96.8 | 0, 430 |
| Non-potential donor | 128 | 29 | 14.2–74.8 | 66 ± 108.9 | 1, 951 |
| Loss of pulse to flat ECG¶ (min) | |||||
| All patients | 177 | 3 | 1–12 | 13.3 ± 24.9 | 0, 149 |
| Potential donor | 49 | 3 | 1–8 | 9.9 ± 18.6 | 0, 78 |
| Non-potential donor | 128 | 4 | 0.25–17 | 14.5 ± 26.8 | 0, 149 |
SD standard deviation, IQR interquartile range, WLST withdrawal of life-sustaining treatment
† Values are presented as numbers
‡ Values were obtained using the independent t-test or Mann–Whitney U test
§ Duration between LST discontinuation and pulse loss
¶ Duration between pulse loss and flat ECG
Figure 2.
Time from loss of pulse to flat electrocardiogram (ECG) wave.
The online survey was sent to a total of 829 Korean critical care specialists and fellows through email, and 152 responses (18.3%) were received. The majority of the participants (81, 53.3%) were affiliated with hospitals with 500- to 1,000-bed capacity, and 100 (65.8%) participants worked in tertiary referral hospitals. Additional details regarding the participants’ specialties, length of service, and frequency of declaring death within the previous year are presented in Table 3.
Table 3.
Demographics of the online survey respondents and their current practices and opinions on death declaration.
| Characteristics | Respondents† (n = 152) |
|---|---|
| Hospital bed capacity | |
| ≥ 1,000 beds | 54 (35.5) |
| 500–1,000 beds | 81 (53.3) |
| < 500 beds | 17 (11.2) |
| Hospital type | |
| Tertiary | 100 (65.8) |
| Secondary | 52 (34.2) |
| Hospital performing organ transplantation | |
| Yes | 128 (84.2) |
| No | 24 (15.8) |
| Specialties | |
| Internal medicine | 62 (40.8) |
| General surgery | 22 (14.5) |
| Anesthesiology | 18 (11.8) |
| Cardiothoracic surgery | 13 (8.6) |
| Neurosurgery | 13 (8.6) |
| Others | 24 (15.7) |
| Duration of service in specialty | |
| ≥10 years | 101 (66.4) |
| 5–10 years | 40 (26.3) |
| <5 years | 11 (7.2) |
| ICU attending physician | |
| Yes (critical care specialist) | 100 (65.8) |
| No (a trainee in critical care medicine) | 52 (34.2) |
| More than 10 death declarations within the last 1 year | |
| Yes | 84 (55.3) |
| No | 68 (44.7) |
| Number of parameters used for death declaration | |
| 1 parameter | 43 (28.3) |
| 2 parameters | 38 (25) |
| 3 parameters | 25 (16.4) |
| 4 parameters or more | 46 (30.3) |
| Use of ECG | |
| Yes | 147 (96.7) |
| No | 5 (3.3) |
| Use of pulse | |
| Yes | 103 (67.8) |
| No | 49 (32.2) |
| Use of respiratory rate | |
| Yes | 64 (42.1) |
| No | 88 (57.9) |
| Use of pupillary light reflex | |
| Yes | 56 (36.8) |
| No | 96 (63.2) |
| Use of SpO2 | |
| Yes | 24 (15.8) |
| No | 128 (84.2) |
| Time of death declaration | |
| Immediately after flat ECG | 63 (41.4) |
| 2 min after flat ECG | 40 (26.3) |
| 5 min after flat ECG | 30 (19.7) |
| 10 min after flat ECG | 10 (6.6) |
| Immediately after pulselessness | 1 (0.7) |
| 2 min after pulselessness | 1 (0.7) |
| 5 min after pulselessness | 3 (2) |
| 10 min after pulselessness | 4 (2.6) |
| 5-min no-touch period | |
| Agree | 90 (59.2) |
| Disagree | 62 (40.8) |
| Reason for disagreement | (n = 62) |
| 5 min is extremely long | 41 (66.1‡) |
| 5 min is not sufficiently long | 5 (8.1‡) |
| Need consideration for other physical situations | 5 (8.1‡) |
| Possible ethical issues | 5 (8.1‡) |
| Insufficient supporting evidence | 2 (3.2‡) |
| Other opinions | 4 (6.5‡) |
| Need for legislation | |
| Agree | 110 (72.4) |
| Disagree | 42 (27.6) |
ICU, intensive care unit; ECG, electrocardiogram; SpO2, peripheral capillary oxygen saturation.
† Values are presented as numbers (%)
‡ Values represent the percentage among the 62 physicians who disagreed
Regarding the number of parameters assessed for declaring the death of a patient (ECG, arterial pulse, respiratory rate, pupil light reflex, and oxygen saturation), 106 (69.7%) respondents reported using three or fewer parameters, whereas the remaining 46 (30.3%) respondents confirmed the use of more than four parameters. For verifying the occurrence of death, most of the physicians (147, 96.7%) relied on monitoring flat-line ECG waves. Specifically, 103 (67.8%) respondents checked for the absence of an arterial pulse, and 64 (42.1%) monitored the respiratory rate. In response to questions about the precise moment of death declaration as per comprehensive decision-making, 63 (41.4%) participants stated that they would pronounce cardiac death immediately upon observing a flat ECG wave. Furthermore, 40 (26.3%), 30 (19.7%), and 10 (6.6%) physicians reported declaring death at 2, 5, and 10 min after the flattening of the ECG wave, respectively. The remaining nine (5.9%) physicians indicated that they would declare death in the absence of an arterial pulse, irrespective of the flat ECG wave (Table 3).
Concerning the widely accepted “5-min no-touch” concept for DCD candidates, 90 (59.2%) physicians expressed their agreement with the concept. Among the 62 (40.8%) physicians who disagreed, 41 raised concerns that a 5-min duration was excessive, potentially unnecessarily resulting in organ ischemia. The remaining objectors stated that this standard is insufficient for confirming death (5, 8.1%) and requires additional medical and physiological considerations, whereas others stated the lack of sufficient evidence supporting this criteria (2, 3.2%) (Table 3).
Discussion
To determine the suitability for organ transplantation after circulatory death, confirming that potential donors have received LST with no apparent medical benefits is necessary. This process involves securing the patient’s and family’s consent for organ donation, withdrawing life support, declaring circulatory death through a clearly defined protocol, and procuring organs for transplantation. For each step, ethical considerations must be carefully identified and addressed. Notably, in this study, we did not conduct an in-depth investigation of the ethical issues of the participants. However, the wishes of the patients and their families regarding organ transplantation were rather simply confirmed.
The primary objective of this study was to obtain basic epidemiological data from Korean ICUs about the proportion of patients with potential and suitable DCD status among those who died after LST withdrawal. Our study revealed that, of the 177 patients who died after LST discontinuation, 20 (11.3%) were designated as suitable DCD donors, which is in line with the findings of previous studies12,22. For example, data from the potential donor audit courtesy of the NHS Blood and Transplant Service demonstrated that, of 3,825 potential DCD cases from October 2009 to December 2010, 397 (10.4%) resulted in actual organ donation or were categorized as suitable for organ donation22. The dataset defines potential DCD donors, eligible or medically suitable DCD donors, and actual DCD donors according to the guidelines published by the World Health Organization27. In contrast to the study by Manara et al., our study was not a nationwide survey; however, it prospectively analyzed the data of patients who were declared dead and screened possible DCD donors in multicenter ICUs. According to the medical and legal criteria, all participants died after withholding or withdrawing LST. A single-center study in a French ICU reported that among 76 patients who were categorized as having foreseeable circulatory death under any form of life support, 32 (42.1%) theoretically met the medical criteria for organ donation12. Notably, Lesieur et al. overestimated this proportion, as they included patients on LST rather than those who died after LST withdrawal. This indicates that the results of the present study might have reflected the real epidemiological data more accurately.
The key to successful organ transplantation is the reduction of warm ischemia time. A French study classified 32 patients of a total of 79 patients as eligible donors; however, only 3 (3.9%) patients died within 2 h of discontinuing life support, a timeframe considered compatible with organ viability12. The present study identified 20 medically suitable DCD donors according to the established medical criteria. Among those, 14 patients died within 60 min of progression to asystole after discontinuing life support, whereas 2 died between 60 and 120 min after LST discontinuation. The remaining patients experienced asystole for approximately > 2 h after discontinuing LST, a timeframe incompatible with organ donation. These results suggested that a standardized definition of medically eligible DCD donors, a universal procedure for withholding/withdrawing LST, and clear standard criteria for circulatory death are essential for reducing ischemic time in viable organs.
In contrast to the results of previous studies, the causes of death were notably different in the current study. For example, the proportion of patients with hypoxic brain damage (14.3%) was lower in our study than that reported by Lesieur et al. (post-cardiac arrest brain injury, 56%)12 and Manara et al. (hypoxic brain damage, 25%)22. These differences might be attributed to variations in disease prevalence, medical practices, social culture, and ethical and legal frameworks for withdrawing LST.
In this study, we also aimed to investigate the prevailing practices and criteria for death declaration in Korean ICUs. Notably, the majority of respondents declared the death of patients upon observing a flat ECG signal. Based on previous studies, several European countries also use the flat ECG signal as a parameter for declaring DCD, with some countries additionally requiring invasive arterial blood pressure measurement or echocardiography for death declaration. In particular, the code of practice in the United Kingdom suggests that circulatory death should be declared only after identifying the absence of a palpable pulse and audible heart sounds, which can be supplemented by the presence of a flat ECG signal, the absence of a pulse wave on invasive arterial blood pressure monitoring, or the absence of cardiac contraction as seen on echocardiography28.
Most physicians (87.5%) who participated in this survey reported declaring cardiac death immediately or within 5 min of a flat ECG signal. In addition, only 59.2% of the respondents agreed that a “5-minute no-touch” period was sufficient for declaring circulatory death. Nevertheless, among the physicians who disagreed with the “5-min no-touch” (66.1%), many believed that a 5-min duration was excessive. Meanwhile, 86.2% of the physicians believed that cardiac death could be declared in ≤ 5 min. Notably, among 18 European countries, 13 recognize the “5-min no-touch” period as either a national guideline or an expert opinion23.
In Europe, where DCD is widely accepted, several countries have established legally binding national legislation or non-legally binding national guidelines23. In this survey, 72.4% of the respondents agreed that legislation to define circulatory death and DCD was necessary.
To the best of our knowledge, this is the first multicenter prospective study that assessed DCD in South Korea and provided fundamental epidemiological evidence in this context. A total of 11 medical centers, where organ transplantation surgeries are performed, participated in this study. In particular, adult patients who fulfilled the Modified Maastricht Classification category III and were deemed medically futile by healthcare professionals were considered. Moreover, the study adhered to the general and precise medical eligibility criteria for organ transplantation, thereby ensuring accurate classification of the DCD donors.
Our study also has some limitations. As this was a multicenter study, the declaration of death was not consistently made by a single physician, particularly during nighttime hours. Moreover, not all deaths in the ICUs were examined. We could not enlist and monitor every single patient who were deceased after LST withdrawal in the ICU, since every candidate were not immediately notified by researchers. We could only access to the medical records of 180 deceased individuals who are classified as Category III of Maastricht Classification of Donation after Circulatory Death. Therefore, the number of patients who died after LST withdrawal or those eligible for DCD might be higher.
Conclusions
We identified medically suitable DCD cases among patients who died after withdrawing or withholding LST and were classified into category III according to the Modified Maastricht Classification. To reduce the warm ischemia time and thereby enhance the viability of the donated organs, establishing practical guidelines for LST withdrawal by the relevant medical societies and institutions is essential.
Methods
Study design and patient selection
We conducted a multicenter, prospective, observational study from April to December 2021. Overall, data from 19 adult ICUs from eight tertiary hospitals and three general hospitals was included in the study. The inclusion criteria were adult patients aged > 18 years who were categorized into category III of the Modified Maastricht Classification and were deemed medically futile by the healthcare team. Each ICU is supervised by critical care specialists with varying operational structures, encompassing semi-closed or open systems. In this study, “potential donors” were defined as patients with at least one core organ function, undergoing any form of LST, and expected to die based on the assessment of the institutional ICU physician. “Suitable donors” for DCD were identified based on their fulfillment of specific medical criteria, including the absence of current solid or hematologic malignancies, active systemic infections, or any ethical issues. Advanced age was not regarded as a contraindication for organ donation. Foreigners and patients with brain death who have donated organs were excluded. Patients who were discharged without death or unexpectedly experienced cardiac arrest prior to the cessation of LST were also excluded. Critically ill patients with COVID-19 and pregnant women were not considered within the scope of this study.
Data collection
In the ICUs, the researchers monitored patients who underwent discontinuation of LST until an official declaration of death by a physician. Subsequently, the researchers collected comprehensive data from the electronic medical records, including demographic data (such as age and sex) and admission information (such as the reasons for ICU admission, primary diagnoses, surgical history, and admission and discharge dates for both the hospital and the ICU). Additional data including disease-related information, such as prior functional status as measured using the Eastern Cooperative Oncology Group (ECOG) performance status scale, comorbidities, disease severity scores (sequential organ failure assessment [SOFA] and acute physiology and chronic health evaluation-II [APACHE-II] scores) at the time of ICU admission, 24 h after admission, and 24 h before death, and infection source and type based on a positive culture result, were also collected. Vital sign data were recorded automatically at 1- or 5-min intervals, from withholding LST to the loss of the arterial pulse and from losing the pulse to flat electrocardiogram (ECG) waves and declaration of death. The modalities and parameters used for the declaration of death were also documented.
Online survey
An online survey was sent to Korean critical care physicians to investigate the contemporary practices of death declaration. The survey consisted of a set of semi-structured questionnaires covering the clinical practices of death declaration. In addition, it inquired about their opinions on the widely recognized “5-min no-touch” period concept applied in other countries23–26. Basic information about the participating physicians, including hospital type and bed capacity, clinical careers in critical care fields, major specialties, and involvement in caring for deceased patients within the previous year, was also collected. The survey was distributed through email to the members of the Korean Society of Critical Care Medicine on three occasions in March 2022, with assistance from the society secretariat.
Ethical considerations and statistical analysis
The protocol for this multicenter, observational study was independently approved by the Institutional Review Board of each participating institution (Korea University Anam Hospital, Chungnam University Sejong Hospital, Korea University Guro Hospital, Pusan National University Yangsan Hospital, National Medical Center, Chonnam National University Hospital, Chungnam National University Hospital, Dong-A University Hospital, Ulsan University Hospital, Inha University Hospital, and Jeju National University Hospital). The declaration of death and the overall process of withholding and withdrawing LST adhered to the domestic legal regulations of the hospitals. All procedures were performed in compliance with the principles outlined in the 1964 Declaration of Helsinki and its subsequent revisions, as well as the relevant guidelines. In addition, this study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Statistical analyses were performed using SPSS (version 23.0; SPSS, Inc., Chicago, IL, USA). Continuous variables were presented as the means ± standard deviation (SD) or medians with interquartile ranges (IQR), and categorical variables were presented as numbers and percentages.
Ethics approval and consent to participate
The protocol for this multicenter, observational study was independently approved by the Institutional Review Board of each participating institution. The declaration of death and the overall process of withholding and withdrawing LST adhered to the domestic legal regulations of the hospitals. All procedures were performed in compliance with the principles outlined in the 1964 Declaration of Helsinki and its subsequent revisions, as well as the relevant guidelines. In addition, this study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Patients participated in the clinical trial after receiving an explanation of the study and providing informed consent. In cases where patients were deemed to be in vulnerable circumstances, making it challenging to provide voluntary consent, consent was obtained from their legal representatives before proceeding with the trial.
Acknowledgements
The authors wish to thank Hyojeong Kim, research associate, and Hyeri Yun, M.S., Youjin Lee, M.S., critical care nurses, from Chungnam National Univ. Sejong Hospital for their helpful support of technical assistance.
Abbreviations
- DBD
Donation after Brain Death
- DCD
Donation after Circulatory Death
- LST
Life-Sustaining Treatments
- ICU
Intensive Care Unit
- ECOG
Eastern Cooperative Oncology Group
- ECG
Electrocardiogram
- SD
Standard Deviation
- IQR
Interquartile Range
- SOFA
Sequential Organ Failure Assessment
- APACHE-II
Acute Physiology and Chronic Health Evaluation-II
Author contributions
Conceptualization; Jae Young Moon, Jae-myeong Lee. Data curation; Jae Young Moon, Jae-myeong Lee. Formal analysis; Jae Young Moon, Jae-myeong Lee, Han Young Lee. Funding acquisition; Jae Young Moon, Jae-myeong Lee. Investigation; Choon Hak Lim, Young Seok Lee, Taehwa Kim, Joohae Kim, Dong Hun Lee, Hong Joon Ahn, Dong Hyun Lee, Byung Ju Kang, Ah Jin Kim, and Gil Myeong Seong. Methodology; Jae Young Moon, Jae-myeong LeeWriting - original draft; Han Young LeeWriting - review & editing; Han Young LeeAll authors reviewed the manuscript.
Funding
This study was supported by a 2019-research grant from the Korean Society of Critical Care Medicine.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Consent for publication
Consent for publication was obtained from all patients at the time of research enrollment and registration. All authors of this manuscript have agreed for publication to the Scientific Reports.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the name of the author Dong Hun Lee, which was incorrectly given as Dong Hyun Lee.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/10/2024
A Correction to this paper has been published: 10.1038/s41598-024-81890-1
Contributor Information
Jae-myeong Lee, Email: ljm3225@hanmail.net.
Jae Young Moon, Email: diffable@hanmail.net.
References
- 1.Lesieur, O., Genteuil, L. & Leloup, M. A few realistic questions raised by organ retrieval in the intensive care unit. Ann. Transl Med.5 (4), S44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manyalich, M., Nelson, H. & Delmonico, F. L. The need and opportunity for donation after circulatory death worldwide. Curr. Opin. Organ. Transpl.23 (1), 136–141 (2018). [DOI] [PubMed] [Google Scholar]
- 3.The Korean National Institute of Organ. Tissue and Blood management, https://www.konos.go.kr/board/boardListPage.do?page=sub4_2_1&boardId=30 [Accessed 25 August 2024].
- 4.International registry in organ donation. and transplantation, https://www.irodat.org/?p=database; [Accessed 25 October 2023].
- 5.Conference of Medical Royal Colleges and their faculties in the United Kingdom Diagnosis of brain death. Br. Med. J.2 (6045), 1187–1188 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 205 (6), 337–340 (1968). [PubMed] [Google Scholar]
- 7.Miñambres, E., Rubio, J. J., Coll, E. & Domínguez-Gil, B. Donation after circulatory death and its expansion in Spain. Curr. Opin. Organ. Transpl.23 (1), 120–129 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Nemes, B. et al. Extended criteria donors in liver transplantation part I: reviewing the impact of determining factors. Expert Rev. Gastroenterol. Hepatol.10 (7), 827–839 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Nemes, B. et al. Extended-criteria donors in liver transplantation part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev. Gastroenterol. Hepatol.10 (7), 841–859 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Croome, K. P. et al. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transpl.23 (3), 342–351 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Laing, R. W. et al. Liver transplantation using grafts from donors after circulatory death: a propensity score-matched study from a single center. Am. J. Transpl.16 (6), 1795–1804 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Lesieur, O. et al. Eligibility of patients withheld or withdrawn from life-sustaining treatment to organ donation after circulatory arrest death: epidemiological feasibility study in a French Intensive Care Unit. Ann. Intensive Care. 3 (1), 36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg, D. S. et al. Interpreting outcomes in DCDD liver transplantation: first report of the multicenter IDOL consortium. Transplantation. 101 (5), 1067–1073 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Dubbeld, J. et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg.97 (5), 744–753 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Kootstra, G., Daemen, J. H. & Oomen, A. P. Categories of non-heart beating organ donors. Transplant Proc. 27 (5), 2893–4. (1995). [PubMed]
- 16.Thuong, M. et al. New classification of donation after circulatory death donors definitions and terminology. Transpl. Int.29 (7), 749–759 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Jeong, E. et al. First organ donation after circulatory death following withdrawal of life-sustaining treatment in Korea: a case report. J. Korean Med. Sci.36 (23), e171 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalle Ave, A. L. & Shaw, D. M. Controlled donation after circulatory determination of death. J. Intensive Care Med.32 (3), 179–186 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Gries, C. J. et al. Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: ethical and policy considerations in organ donation after circulatory determination of death. Am. J. Respir Crit. Care Med.88 (1), 103–109 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Wind, J., Faut, M., van Smaalen, T. C. & van Heurn, E. L. Variability in protocols on donation after circulatory death in Europe. Crit. Care. 17 (5), R217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrissey, P. E. & Monaco, A. P. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 97 (3), 258–264 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Manara, A. R., Murphy, P. G. & O’Callaghan, G. Donation after circulatory death. Br. J. Anaesth.108 (1), i108–i121 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Lomero, M. et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl. Int.33 (1), 76–88 (2020). [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. (2012). https://www.who.int/patientsafety/montreal-forum-report.pdf; [Accessed 7 May 2021].
- 25.American Society of Anesthesiologists. (2017). https://www.asahq.org/standards-and-guidelines/statement-on-controlled-organ-donation-aftercirculatory-death; [Accessed 7 May 2021].
- 26.Domínguez-Gil, B. et al. The critical pathway for deceased donation: reportable uniformity in the approach to deceased donation. Transpl. Int. 24 (4), 373–378 (2011). [DOI] [PubMed]
- 27.World Health Organization. Clinical criteria for the determination of death, WHO Technical Expert Consultation, WHO Headquarters, Geneva, Switzerland, 22–23 September 2014, pp 5–12 (WHO, 2017).
- 28.Academy of Medical Royal Colleges. Oct. A code of practice for the diagnosis and confirmation of death, pp. 12–13. https://aomrc.org.uk/wp-content/uploads/2016/04/Code_Practice_Confirmation_Diagnosis_Death_1008-4.pdf (2008)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.