Abstract
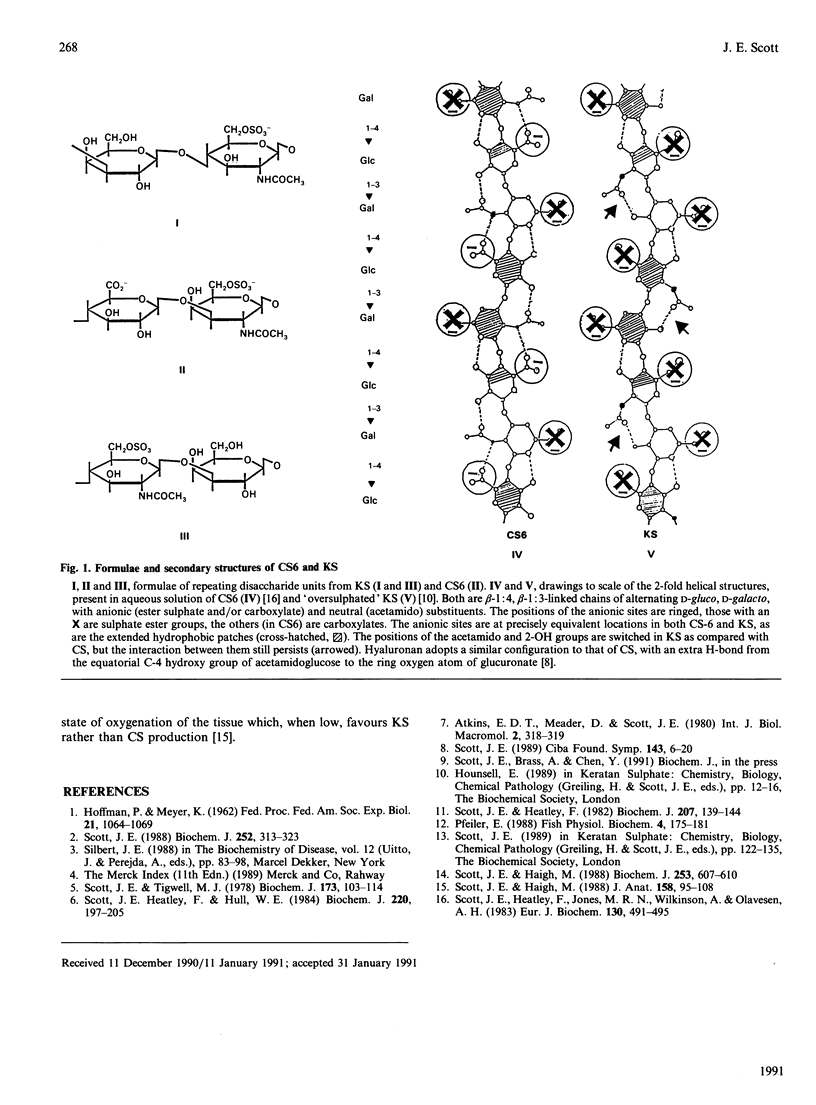
Keratan sulphate and chondroitin sulphate (KS and CS) in the 2-fold helical configurations that are prevalent in solution are of very similar tacticity. The chiral centres, anionic sites and hydrophobic patches are in identical conformations. Only the position of the acetamido group varies from CS to KS, but part of its intramolecular H-bonding potential in CS is retained in KS. The formation of tertiary aggregates, observed in vitro and in tissues, is explicable on these bases. The proposal that KS may be a functional substitute for CS [Scott & Haigh (1988) J. Anat. 158, 95-108] under low-O2 conditions is relevant.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HOFFMAN P., MEYER K. Structural studies of mucopolysaccharides of connective tissues. Fed Proc. 1962 Nov-Dec;21:1064–1069. [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Identification of specific binding sites for keratan sulphate proteoglycans and chondroitin-dermatan sulphate proteoglycans on collagen fibrils in cornea by the use of cupromeronic blue in 'critical-electrolyte-concentration' techniques. Biochem J. 1988 Jul 15;253(2):607–610. doi: 10.1042/bj2530607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Keratan sulphate and the ultrastructure of cornea and cartilage: a 'stand-in' for chondroitin sulphate in conditions of oxygen lack? J Anat. 1988 Jun;158:95–108. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F. Detection of secondary structure in glycosaminoglycans via the H n.m.r. signal of the acetamido NH group. Biochem J. 1982 Oct 1;207(1):139–144. doi: 10.1042/bj2070139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Hull W. E. Secondary structure of hyaluronate in solution. A 1H-n.m.r. investigation at 300 and 500 MHz in [2H6]dimethyl sulphoxide solution. Biochem J. 1984 May 15;220(1):197–205. doi: 10.1042/bj2200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Jones M. N., Wilkinson A., Olavesen A. H. Secondary structure of chondroitin sulphate in dimethyl sulphoxide. Eur J Biochem. 1983 Feb 15;130(3):491–495. doi: 10.1111/j.1432-1033.1983.tb07177.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Secondary structures in hyaluronan solutions: chemical and biological implications. Ciba Found Symp. 1989;143:6-15; discussion 15-20, 281-5. doi: 10.1002/9780470513774.ch2. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Tigwell M. J. Periodate oxidation and the shapes of glycosaminoglycuronans in solution. Biochem J. 1978 Jul 1;173(1):103–114. doi: 10.1042/bj1730103. [DOI] [PMC free article] [PubMed] [Google Scholar]


