Abstract
In well-differentiated human airway epithelia, the coxsackie B and adenovirus type 2 and 5 receptor (CAR) resides primarily on the basolateral membrane. This location may explain the observation that gene transfer is inefficient when adenovirus vectors are applied to the apical surface. To further test this hypothesis and to investigate requirements and barriers to apical gene transfer to differentiated human airway epithelia, we expressed CAR in which the transmembrane and cytoplasmic tail were replaced by a glycosyl-phosphatidylinositol (GPI) anchor (GPI-CAR). As controls, we expressed wild-type CAR and CAR lacking the cytoplasmic domain (Tailless-CAR). All three constructs enhanced gene transfer with similar efficiencies in fibroblasts. In airway epithelia, GPI-CAR localized specifically to the apical membrane, where it bound adenovirus and enhanced gene transfer to levels obtained when vector was applied to the basolateral membrane. Moreover, GPI-CAR facilitated gene transfer of the cystic fibrosis transmembrane conductance regulator to cystic fibrosis airway epithelia, correcting the Cl− transport defect. In contrast, when we expressed wild-type CAR it localized to the basolateral membrane and failed to increase apical gene transfer. Only a small amount of Tailless-CAR resided in the apical membrane, and the effects on apical virus binding and gene transfer were minimal. These data indicate that binding of adenovirus to an apical membrane receptor is sufficient to mediate effective gene transfer to human airway epithelia and that the cytoplasmic domain of CAR is not required for this process. The results suggest that targeting apical receptors in differentiated airway epithelia may be sufficient for gene transfer in the genetic disease cystic fibrosis.
The first steps in adenovirus infection involve primarily two proteins in the viral capsid: fiber and penton base (9, 11, 12). The adenovirus fiber protein forms a trimer which binds to the cell via a high-affinity receptor, the coxsackie B and adenovirus type 2 and 5 receptor (CAR) (3, 29). Recent structural and genetic studies support a model in which the lateral cleft between two neighboring knob domains on fiber interact with the extracellular amino-terminal immunoglobulin V domain of CAR (4, 8, 26). Interestingly, adenovirus-meditated gene transfer to lymphocyte and CHO cell lines does not require the transmembrane or cytoplasmic domains of CAR, suggesting that the interaction between fiber-knob and CAR mediates primarily attachment to the cell surface (18, 30, 37). In addition to the fiber-CAR interaction, the penton base interacts with αvβ3 and αvβ5 integrins, facilitating receptor-mediated endocytosis of adenovirus (12, 21, 40). Thus, CAR is required for binding and infection, and αvβ integrins act as coreceptors.
Human airway epithelia are a target for gene transfer in the genetic disease cystic fibrosis (CF) (27, 38). Earlier works showed that adenovirus infection and adenovirus-mediated gene transfer to differentiated airway epithelia are inefficient due to lack of CAR and integrins in the apical membrane (2, 10, 13, 15, 23–25, 35, 41, 42). Thus, lack of fiber-knob binding to the apical membrane may be the rate-limiting step for adenovirus-mediated gene transfer to airway epithelia. Despite its absence on the apical membrane, CAR is present on the basolateral membrane (25, 35). Consequently, adenovirus infects airway epithelia from the basolateral surface in a fiber-dependent manner (35).
These results raised the question of whether CAR localized in the apical membrane would be sufficient for adenovirus-mediated gene transfer from the apical surface. Answering this question is important for understanding the molecular mechanisms of adenovirus entry into human airway epithelia. The answer may also impact the development of targeted gene delivery of the cystic fibrosis transmembrane conductance regulator (CFTR) for CF. To address this question we studied adenovirus-mediated gene transfer in differentiated human airway epithelia expressing recombinant wild-type CAR and two modified CAR proteins: CAR lacking the cytoplasmic domain (Tailless-CAR) and CAR lacking the cytoplasmic and transmembrane domains but modified with a glycosyl-phosphatidylinositol (GPI) anchor signal sequence (GPI-CAR) to target the apical membrane (18, 30, 37). Recently, similar modifications in CAR (Tailless- and GPI-CAR) were found to localize to the apical membrane in a canine renal epithelial cell line (MDCK) (24). However, they did not facilitate adenovirus infection until the MDCK cells were treated with neuraminidase to remove sialic acid from the glycocalyx. To learn whether apically localized CAR facilitates gene transfer to the airways and to investigate the mechanisms involved, we studied primary cultures of well-differentiated human airway epithelia.
MATERIALS AND METHODS
Cells and culture.
NIH 3T3 cells were cultured on 100-mm-diameter plates (Corning Costar, Corning, N.Y.) in Eagle's minimum essential medium (EMEM) (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% fetal calf serum (Sigma Chemical Co.), 1% nonessential amino acids, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Airway epithelial cells were obtained from trachea and bronchi of lungs removed for organ donation. Cells were isolated by enzyme digestion as previously described (16, 43). Freshly isolated cells were seeded at a density of 5 × 105 cells/cm2 onto collagen-coated, 0.6-cm2 area Millicell polycarbonate filters (Millipore Corp., Bedford, Mass.). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and air. Twenty-four hours after plating, the mucosal medium was removed and the cells were grown at the air-liquid interface (16, 43). The culture medium consisted of a 1:1 mix of DMEM-Ham's F-12, 5% Ultroser G (Biosepra SA, Cergy-Saint-Christophe, France), penicillin (100 U/ml), streptomycin (100 μg/ml), 1% nonessential amino acids, and insulin (0.12 U/ml). Airway epithelia reached confluence and developed a transepithelial electrical resistance, indicating the development of tight junctions and an intact barrier. Epithelia were allowed to differentiate by culturing for at least 14 days after seeding, and the presence of a ciliated surface was tested by scanning electron microscopy (43).
Flag-tagged CAR constructs and recombinant adenoviruses.
cDNAs encoding three CAR constructs were kindly provided by J. M. Bergelson (Division of Immunologic and Infectious Diseases, Children's Hospital of Philadelphia, Philadelphia, Pa.): (i) full-length CAR (wt-CAR), (ii) CAR which lacks the cytoplasmic domain (Tailless-CAR), and (iii) CAR which has the decay-accelerating factor signal for GPI modification in place of the transmembrane and cytoplasmic domains (GPI-CAR) (37). All CAR constructs were modified with the Flag epitope tag consisting of amino acids DYKDDDDK, inserted downstream of the NH2-terminal hydrophobic leader signal sequence, as described previously (30).
We cloned the three Flag-tagged CARs into adenovirus vectors. Recombinant adenovirus vectors expressing the Flag-tagged CAR constructs (Ad5/wt-CAR, Ad5/Tailless-CAR, and Ad5/GPI-CAR), and β-galactosidase (β-Gal) (Ad2/βGal) were prepared by the University of Iowa Gene Transfer Vector Core at titers of ∼1010 infectious units/ml (determined by plaque assay) as previously described (39). A recombinant adenovirus vector expressing green fluorescent protein (GFP), Ad2/GFP, and CFTR (Ad2/CFTR-16) were a gift of Sam Wadsworth (Genzyme, Framingham, Mass.).
Expression of CAR.
NIH 3T3 cells, which normally express low levels of CAR, were infected with control adenovirus (Ad2/βGal), or adenovirus expressing either one of the three CAR constructs using Ad-CaPi coprecipiates. This method bypasses the need for the CAR receptor on target cells (6, 33). Briefly, Ad-CaPi coprecipitates were formed by adding CaCl2 to adenovirus particles in EMEM to achieve a final Ca2+ concentration of 5.8 mM. Cells were then infected with Ad-CaPi coprecipitates for 30 min, rinsed three times with EMEM, and evaluated for susceptibility to Ad2/GFP infection.
In airway epithelia, the CAR constructs were expressed by pretreating epithelia with 8 mM EGTA delivered in H2O to transiently disrupt the tight junctions. This technique results in reversible disruption of the tight junctions and allows apically administered adenovirus to access its endogenous receptor on the basolateral membrane (35, 36). Immediately after treatment, epithelia were infected with control adenovirus or adenovirus (multiplicity of infection [MOI], 10) expressing either of the three CAR constructs. Two days after infection with CAR-expressing adenoviruses, epithelial integrity was measured with an ohmmeter (EVOM; World Precision Instrument Inc., Sarasota, Fla.). The transepithelial resistance values for all infected epithelia were >300 Ω · cm2. Epithelia were then evaluated or studied as described below.
Analysis of Flag-tagged CAR protein expression.
Expression of the adenovirus-encoded CAR constructs was evaluated by Western blot analysis of airway epithelia 2 days after infection with CAR-expressing adenoviruses. Protein was extracted from epithelia by incubation for 1 h at 4°C with lysis buffer (1% Triton X-100; 10 mM Tris-HCl, pH 7.4; 150 mM NaCl), supplemented with protease inhibitors (10 μg each of leupeptin, aprotinin, and pepstatin A per ml). The lysates were diluted in Laemmli sample buffer, and equal amounts were subjected to Western blotting. Flag-tagged CAR proteins on nitrocellulose membranes were detected by incubation with a 1:500 dilution of horseradish peroxidase-conjugated anti-Flag M2 monoclonal antibody (Sigma Chemical Co.) in 10 mM TBS (Tris-HCl, pH 7.4; 150 mM NaCl; 10 mM EDTA) containing 5% nonfat milk, and visualized after chemiluminescence by exposure for 1 to 5 min to X-Omat film (Eastman Kodak, Rochester, N.Y.).
Cell surface distribution of Flag-tagged CAR constructs.
Apical localization of Flag-tagged CAR was evaluated by immunocytochemistry in epithelia 2 days after gene transfer with CAR-expressing adenovirus. Epithelia were coinfected with Ad2/GFP at the time of adenovirus-mediated CAR gene transfer to allow visualization of epithelial cells. Epithelia were fixed with 4% paraformaldehyde for 15 min at 23°C. Unless otherwise noted, SuperBlock (Pierce, Rockford, Ill.) was used to wash between incubations and also to dilute reagents. After cells were washed twice for 10 min each time, mouse anti-Flag monoclonal antibody (1:600; Sigma Chemical Co.) was placed on the apical surface of epithelia for 1 h at 37°C. Then, cells were rinsed twice for 5 min each and incubated with donkey anti-mouse immunoglobulin G conjugated with Texas Red fluorophore (1:500; Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) for 1 h at 37°C. The epithelia were rinsed twice with phosphate-buffered saline (PBS) for 5 min each then mounted onto glass slides using Vectashield (Vector Laboratories Inc., Burlingame, Calif.). Apical staining was evaluated by laser scanning confocal microscopy (model MRC-1024 microscope; Bio-Rad, Hercules, Calif.) at 60X magnification; images are shown as stacked XZ series.
Binding of iodinated mouse 125I-anti-Flag antibody, prepared with IODO-GEN reagent (Pierce), to airway epithelia was performed for quantitative analysis of the cell surface distribution of the Flag-tagged CAR constructs. Two days after infection, epithelia were washed with binding buffer (EMEM containing 1% bovine serum albumin and 10 mM HEPES, pH 7.3) and incubated for 2 h at 4°C with 2 μg of 125I-anti-Flag antibody per ml (2 × 105 to 4 × 106 dpm/μg). Antibody was added either to the apical or the basolateral surface of airway epithelia. After three washes with binding buffer for 10 min at 4°C, cell-associated 125I label was solubilized for 30 min at 23°C in 10% sodium dodecyl sulfate, followed by quantification in a gamma counter. Nonspecific binding of anti-Flag antibody was measured in parallel filters by adding a 100-fold excess (200 μg/ml) of unlabeled antibody along with 125I-labeled antibody. Specific binding was calculated by subtracting nonspecific binding from total binding. In all cases, nonspecific binding amounted to 20 to 30% of the total signal.
Binding of fluorescent adenovirus.
CAR constructs were expressed in airway epithelia as described above. Two days after gene transfer with CAR-expressing adenovirus, Cy3-labeled Ad5/βGal (MOI, 50) in 100 μl of EMEM was added to the apical surface of epithelia maintained at 4°C. Adenovirus was covalently labeled with the carbocyanine dye Cy3 (Amersham Pharmacia Biotech, Piscataway, N.J.) (19). The labeling procedure decreased the infectious unit/particle ratio by 5 to 35% (33). After a 30-min incubation the virus was removed, and epithelia were rinsed twice with EMEM. Cultures were fixed with 4% paraformaldehyde at 23°C for 10 min and then rinsed three times with PBS. The cells were stained with a 1:500 dilution of DAPI (4′,6′-dianidino-2-phenylindole) in PBS (Molecular Probes, Eugene, Oreg.) for 20 min at 23°C, rinsed, and then mounted on glass slides with Vectashield (Vector Laboratories Inc.). Binding of adenovirus to the epithelia was assessed by fluorescence microscopy (19, 33).
Gene transfer assays.
CAR-expressing NIH 3T3 cells were evaluated by detecting GFP expression 1 day after gene transfer with Ad2/GFP. Cells were dissociated with 0.05% trypsin and 0.53 mM EDTA, and fluorescence from 50,000 individual cells was analyzed using fluorescence-activated cell analysis (FACScan, Lysys II software; Becton Dickinson, San Jose, Calif.). The percentage of cells positive for GFP was assessed by determining the percentage of highly fluorescent cells in each group and subtracting the fluorescence of control cells.
To evaluate adenovirus-mediated gene transfer through the apical surface of human airway epithelia expressing CAR constructs, either Ad2/GFP, Ad2/βGal, or Ad2/CFTR 16 (MOI, 10) was delivered to the apical surface in 100 μl of EMEM and incubated for 30 min at 37°C. Epithelia were then rinsed twice with EMEM and assayed for gene transfer 2 days later. To assess GFP expression in airway epithelia, Ad2/GFP-infected epithelia were studied 2 days postinfection by fluorescence microscopy (34).
For analysis of β-Gal expression, total β-Gal activity was measured by a commercially available method (Galacto-Light; Tropix, Inc., Bedford, Mass.). Briefly, 2 days postinfection, epithelia were rinsed with PBS and incubated with lysis buffer (25 mM Tris-phosphate, pH 7.8; 2 mM dithiothreitol; 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid; 10% glycerol; and 1% Triton X-100) for 15 min. Light emission was quantified in a luminometer (Analytical Luminescence Laboratory, San Diego, Calif.).
To assess CFTR gene transfer, epithelia were mounted in modified Ussing chambers (Jim's Instruments, Iowa City, Iowa) as previously described (43). Epithelia were bathed on the submucosal surface with a Ringer's solution containing 135 mM NaCl, 2.4 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES, and 10 mM dextrose (pH 7.4). The mucosal solution was identical with the exception that NaCl was replaced with 135 mM sodium gluconate. Amiloride (10 μM) was added to the mucosal solution to inhibit Na+ channels and transepithelial Na+ transport. The cyclic AMP (cAMP) agonists, 10 μM forskolin and 100 μM isobutylmethylxanthine, were added to the mucosal and submucosal solutions to stimulate transepithelial Cl− current through CFTR Cl− channels. To assess total Cl− current, 100 μM bumetanide was added to the submucosal solution, and the change in current was measured.
Cell surface modifications.
Epithelia were biochemically treated to remove the glycocalyx from the apical surface. Two days after infection with CAR-expressing adenovirus, epithelia were rinsed with EMEM. Epithelia were then incubated with either 200 mU of bacterial sialidase NA Type III from Vibrio cholerae per ml, 10 mU of O-glycosidase from Streptococcus pneumoniae per ml, or 50 U of the peptide N-glycosidase F from Flavobacterium meningosepticum (Sigma Chemical Co.) per ml diluted in EMEM for 1 h at 37°C (2). Assay conditions for each enzyme were optimized by first testing a range of concentrations and determining the maximum concentration of enzyme that did not change the transepithelial resistance. To confirm removal of the glucocalyx, epithelia were fixed with 4% paraformaldehyde for 15 min at 23°C and then incubated with 0.1 μg of fluorescein isothiocyanate (FITC)-labeled wheat germ agglutinin (WGA) (Vector Laboratories Inc.) for 30 min at 23°C. WGA binding was quantitated by measuring average fluorescence intensity from histogram plots of confocal images (Confocal Assistant software). To assess gene transfer, enzyme-treated epithelia were washed with EMEM, infected with Ad2/βGal, and evaluated 2 days later for adenovirus-mediated gene transfer.
To evaluate the requirement for the GPI anchor on GPI-CAR, airway epithelia were treated with phosphatidylinositol-specific phospholipase C (PI-PLC) (from Bacillus cereus; Molecular Probes) for 2 h at 37°C followed by three washes with EMEM prior to infection.
RESULTS
GPI-CAR, Tailless, and wt-CAR mediate adenovirus gene transfer to 3T3 cells with similar efficiencies.
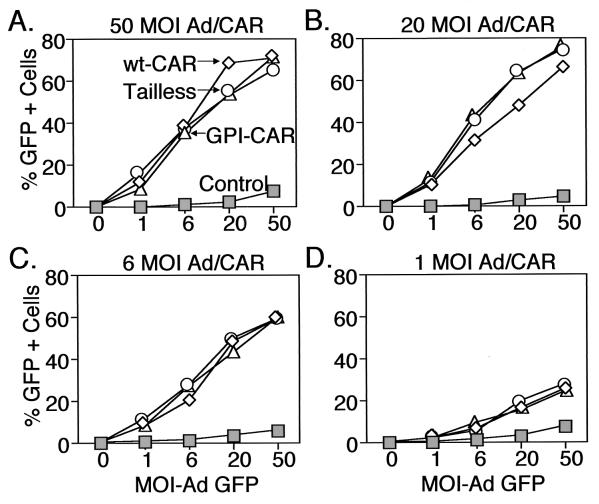
Adenovirus vectors were generated to express three different Flag-tagged CAR constructs: (i) full-length CAR (amino acids [aa] 1 to 365), (ii) CAR lacking the cytoplasmic domain (aa 1 to 260), and (iii) CAR with the cytoplasmic and transmembrane domains replaced by a GPI anchor signal sequence (aa 1 to 235). Earlier studies showed that similar constructs were capable of mediating adenovirus infection in cells lacking CAR; however, it is unknown whether they have similar efficiencies. To address this issue, we transduced NIH 3T3 cells with various amounts of CAR-expressing adenovirus to vary the amount of CAR receptor. Then, we applied increasing concentrations of Ad2/GFP and assessed the percentage of GFP-positive cells as a measure of gene transfer. In addition, we studied varying amounts of CAR because we were limited by not knowing how levels of recombinant CAR compare to endogenous CAR. In cells infected with a high MOI (MOI, 50) of Ad-CAR vectors, we observed a dose-dependent increase in adenovirus-mediated GFP gene transfer regardless of which CAR molecule was expressed (Fig. 1A). Moreover, the three CAR molecules seemed to function with similar efficiencies. Because differences in receptor efficiency may be more evident at lower levels of receptor (30), we also examined the dose-response of Ad2/GFP infection in cells expressing lower levels of CAR (Fig. 1B to D). As we reduced the amount of CAR expression by applying a lower MOI of the CAR-expressing virus, gene transfer with Ad2/GFP fell. However, we found similar reductions with all three CAR molecules. These observations are consistent with previous reports that all three CAR receptors can facilitate adenovirus-mediated gene transfer (18, 30, 37). In addition, these data suggest that their relative efficiencies are similar.
FIG. 1.
Effect of CAR expression on adenovirus-mediated gene transfer to NIH 3T3 cells. Cells were infected with varying MOIs of Ad-CaPi coprecipitates encoding wt-CAR (◊). Tailless-CAR (○), GPI-CAR (▵), or CFTR (□) as a control. One day later cells were infected with varying MOIs of Ad2/GFP. Data are the percentage of GFP-positive cells for cells infected with the Ad/CAR vectors at MOIs of 50 (A), 20 (B), 6 (C), and 1 (D).
Expression of modified CAR molecules in human airway epithelia.
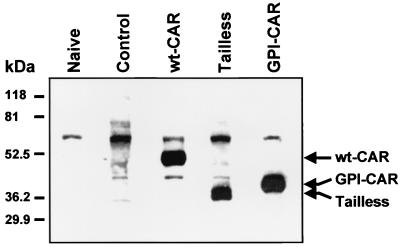
Given that these three receptors function with similar efficiencies in a cell line, we studied their expression, localization, and function in primary cultures of well-differentiated airway epithelia. We delivered the CAR-expressing adenovirus vectors to the basolateral membrane by transiently disrupting the tight junctions, as previously reported (35). Western blot analysis showed specific expression of each Flag-tagged CAR construct at approximately similar amounts (Fig. 2). Naive epithelia and epithelia expressing β-Gal did not show a specific band at the predicted molecular weight range for any of the CAR constructs, confirming that the anti-Flag antibody specifically detects the CAR constructs in human airway epithelia.
FIG. 2.
Expression of adenovirus-encoded CAR constructs in primary cultures of human airway epithelia. Differentiated human airway epithelia were mock infected (Naive), infected with Ad2/GFP (Control), or infected with adenovirus vectors encoding wt-CAR, Tailless-CAR, or GPI-CAR. Two days after infection, cellular lysates were assessed for expression of Flag-tagged CAR proteins by Western blot analysis using anti-Flag M2-HRP monoclonal antibody. Arrows indicate the observed migration profiles of full-length CAR, Tailless-CAR, and GPI-CAR; migration of the three bands was consistent with the predicted molecular masses. The band at approximately 70 kDa was nonspecific as it was also observed in naive and mock-infected cells.
GPI-CAR, Tailless, and wt-CAR show distinct patterns of cell surface distribution in differentiated airway epithelia.
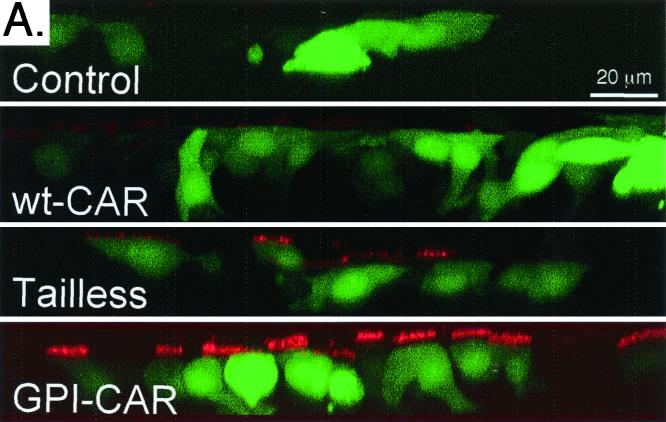
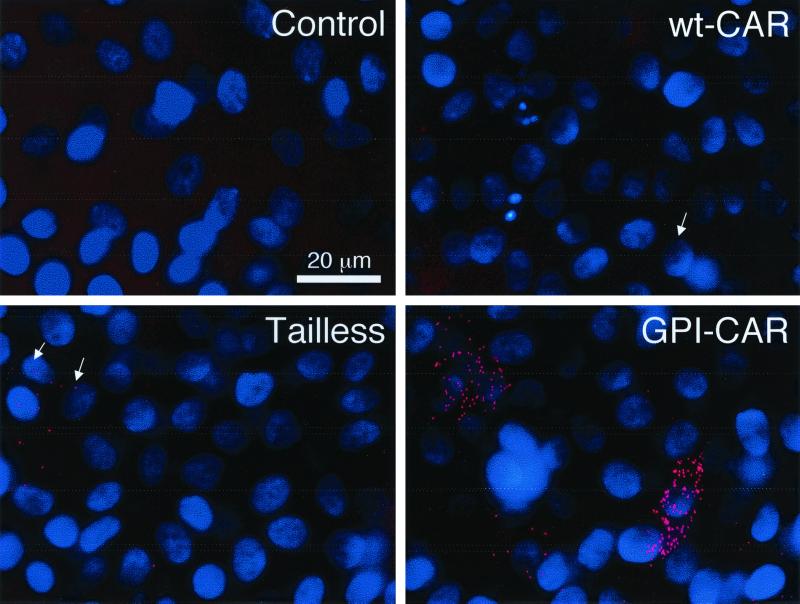
We analyzed apical expression of CAR proteins by applying anti-Flag antibody to the apical surface followed by immunocytochemistry. Neither the control nor epithelia expressing wt-CAR presented Flag-tagged CAR on the apical membrane (Fig. 3A). In contrast, epithelia expressing GPI-CAR showed substantial CAR on the apical membrane. Epithelia expressing Tailless-CAR showed a small amount of apical staining. These observations suggest the GPI modification targets CAR to the apical membrane, consistent with observations for GPI-anchored proteins in other epithelial cell types (20). In addition, the presence of Tailless-CAR on the apical surface suggests the cytoplasmic domain plays an essential role in exclusive basolateral localization of CAR in airway epithelia.
FIG. 3.
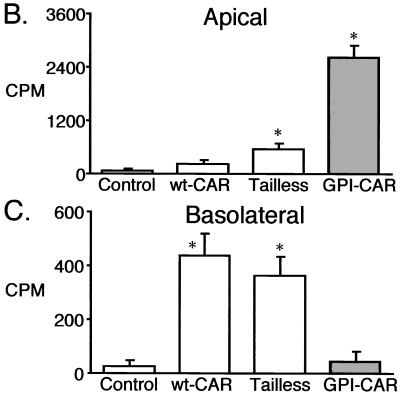
Cell surface distribution of modified CAR proteins expressed in human airway epithelia. (A) Apical localization of CAR molecules was evaluated in airway epithelia with immunocytochemistry. GFP-positive cells are shown in green, and apical Flag antibody binding is shown in red. Polarized surface distribution of CAR molecules was quantitated with a radioimmunoassay on the apical surface (B), or the basolateral surface (C). Data are means + standard errors of the means (error bars) (n = 6). ∗, P < 0.01 compared to control.
To obtain a more quantitative assessment of the polarized distribution of Flag-tagged CAR, we used a radioimmunoassay to measure specific binding of anti-Flag antibody to CAR-expressing airway epithelia. There was no specific binding of 125I-anti-Flag antibody to the apical surface of airway epithelia expressing wt-CAR (Fig. 3B). This result is consistent with the lack of endogenous CAR at the apical surface (25, 35). Consistent with the immunocytochemical localization, there was a large amount of apical binding in epithelia expressing GPI-CAR and a small amount in epithelia expressing Tailless-CAR. In contrast, at the basolateral membrane we observed specific binding of 125I-anti-Flag antibody in epithelia expressing wt-CAR and Tailless-CAR, but not GPI-CAR (Fig. 3C). Hence, airway epithelia specifically sequester wt-CAR in the basolateral membrane and GPI-CAR in the apical membrane. Tailless-CAR was distributed to both membrane domains.
Expression of GPI-CAR in airway epithelia enhances apical binding of adenovirus.
We also studied binding of Cy3-labeled adenovirus to the apical membrane of CAR-expressing airway epithelia. Consistent with previous observations, adenovirus did not bind to control epithelia expressing β-Gal (Fig. 4) (35). Moreover, there was little or no apical binding of adenovirus to epithelia expressing recombinant wt-CAR. This is consistent with basolateral localization of the protein (Fig. 3). However, adenovirus bound to the apical surface of epithelia expressing GPI-CAR and, to a lesser extent, the epithelia expressing Tailless-CAR. These data indicate that adenovirus can bind to the extracellular domain of CAR when it is present on the apical surface of differentiated epithelia.
FIG. 4.
Effect of CAR expression on adenovirus binding to the apical surface of CAR-expressing human airway epithelia. Data are en face projections of Cy3-labeled adenovirus (red) bound to the apical surface of control and CAR-expressing airway epithelia. DAPI-stained nuclei are blue.
Apical localization of CAR is sufficient for adenovirus-mediated gene transfer from the apical surface.
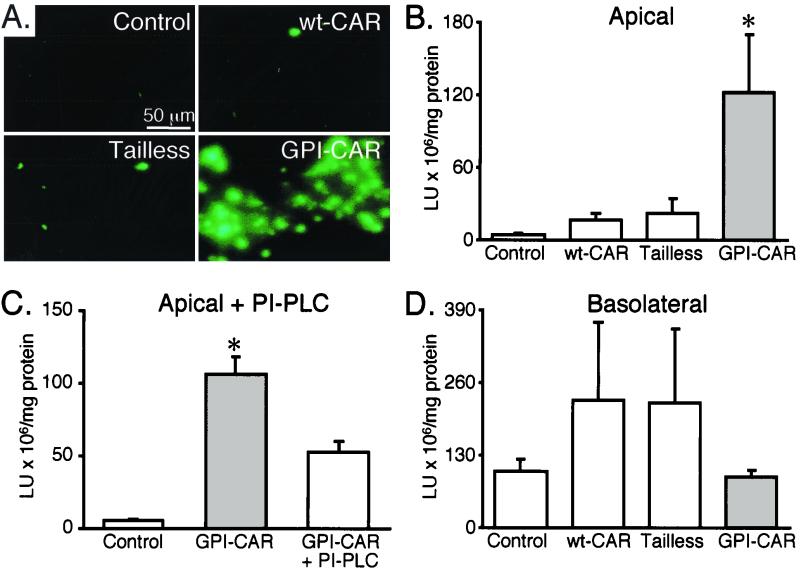
To determine if apical localization of CAR is sufficient for adenovirus infection, we investigated adenovirus-mediated gene transfer from the apical surface of epithelia expressing wt-CAR, Tailless-CAR, or GPI-CAR. Using Ad2/GFP we found minimal gene transfer in epithelia expressing wt-CAR and Tailless-CAR (Fig. 5A). However, GPI-CAR expression substantially increased gene transfer. These results indicate that expression of GPI-CAR in airway epithelia is sufficient for adenovirus-mediated gene transfer from the apical surface.
FIG. 5.
Effect of CAR expression on adenovirus-mediated gene transfer to airway epithelia. Two days after CAR gene transfer, airway epithelia were infected with Ad2/GFP from the apical surface (A), Ad2/βGal from the apical surface (B), Ad2/βGal from the apical surface following PI-PLC treatment (C), or Ad2/βGal from the basolateral surface (D) (each at an MOI of 10) for 30 min. Epithelia were studied 48 h later. β-Gal data are means + standard errors of the means (error bars) (n = 6). ∗, P < 0.01 compared to control. Lu, light units.
To obtain a more quantitative assessment of gene transfer, we measured β-Gal expression after apical application of Ad2/βGal. We observed a marginal increase in β-Gal activity in epithelia expressing either wt-CAR or Tailless-CAR as compared to control (Fig. 5B). In contrast, we found a marked increase in gene transfer from the apical surface of airway epithelia expressing GPI-CAR. Pretreatment of GPI-CAR expressing epithelia with PI-PLC inhibited gene transfer with Ad2/βGal, suggesting that gene transfer through GPI-CAR requires the GPI modification (Fig. 5C). We also measured gene transfer from the basolateral surface. All epithelia, including the controls, were readily infected with adenovirus, consistent with basolateral localization of the endogenous receptor (Fig. 5D) (25, 35). There was a slight although not statistically significant increase in gene transfer from the basolateral surface in epithelia overexpressing Tailless- and wt-CAR. Moreover, the absolute level of transgene expression after apical addition of vector to GPI-CAR expressing epithelia was similar to the transgene expression obtained after adding vector to the basolateral surface of control epithelia expressing only endogenous CAR (Fig. 5B and 5D).
Removing apical glycocalyx does not potentiate adenovirus infection in airway epithelia.
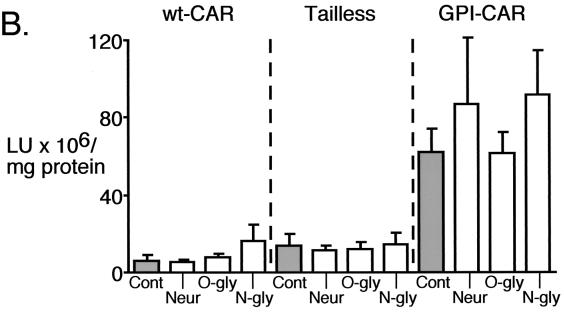
A previous study reported that expression of GPI-CAR was not sufficient for adenovirus-mediated gene transfer from the apical surface of a canine renal epithelial cell line (MDCK); rather, treatment with neuraminidase was also required (24). That study suggested that the glycocalyx, specifically sialic acid, was a barrier preventing apical gene transfer. Our finding that GPI-CAR efficiently rescues apical gene transfer suggests that the glycocalyx is not an absolute barrier in differentiated human airway epithelia. However, these data do not rule out the possibility that the glycocalyx constitutes a major but not absolute barrier to gene transfer. To test this possibility, we treated CAR-expressing epithelia with neuraminidase to remove sialic acid or with glycosidases to remove either N- or O-linked carbohydrates. The ability of the enzymes to remove carbohydrate from the apical surface was confirmed by a decrease in FITC-WGA binding to the apical membrane (Fig. 6A); WGA binds to sialic acid (32). Since disrupting the tight junctions allows adenovirus access to endogenous CAR on the basolateral membrane, we measured transepithelial resistance as an indication of epithelial integrity (35). None of the enzyme treatments altered transepithelial resistance (data not shown). Furthermore, the enzyme treatments of epithelia expressing wt-CAR or Tailless-CAR had no effect on gene transfer (Fig. 6B). Hence, enzyme treatments did not damage epithelial integrity and allow gene transfer through the basolateral membrane. In agreement with aforementioned results (Fig. 5), Fig. 6B shows that GPI-CAR facilitated adenovirus-mediated gene transfer from the apical surface. Importantly, removal of the glycocalyx with neuraminidase or glycosidases did not significantly enhance gene transfer compared to that seen with mock-treated epithelia.
FIG. 6.
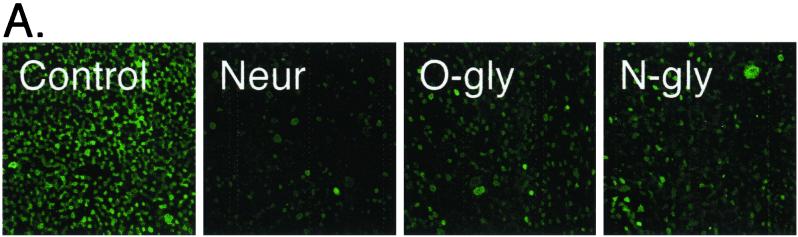
Effect of neuraminidase or glycosidase treatment on adenovirus-mediated gene transfer to CAR expressing airway epithelia. (A) Two days after CAR gene transfer, airway epithelia were pretreated with neuraminidase (Neur), O-glycosidase (O-gly), or N-glycosidase (N-gly) and stained with FITC-labeled WGA. Data are en face projections of WGA binding to the apical membrane of airway epithelia. WGA binding (in relative intensity units) decreased following treatment with neuraminidase (14.5 ± 1.9), O-glycosidase (21.0 ± 1.4), and N-glycosidase (20.3 ± 1.2) compared to control (29.5 ± 1.0) (n = 9 for each). (B) Treated epithelia were also assayed for gene transfer with Ad2/βGal. Fluorescence and β-Gal data are means + standard errors of the means (error bars) (n = 6).
GPI-CAR is sufficient for adenovirus-mediated gene transfer of CFTR from the apical surface of CF epithelia.
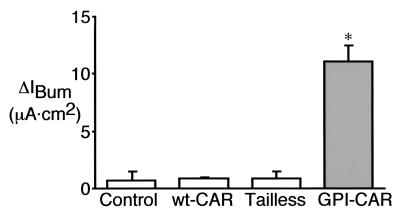
Airway epithelia are a target for gene transfer in CF. Therefore, we asked whether there are gene transfer barriers in CF epithelia not found in normal epithelia and whether gene transfer via the apical membrane can correct the Cl− transport defect. We measured transepithelial Cl− current in CAR-expressing CF epithelia following application of Ad2/CFTR. The Cl− current following CFTR gene transfer to wt-CAR- and Tailless-CAR-expressing epithelia was no different from that seen with the control (Fig. 7). In contrast, epithelia expressing GPI-CAR showed substantial correction of the Cl− current, approaching levels seen in normal epithelia (28 ± 1 μA · cm2). These results indicate that expression of GPI-CAR was sufficient to support adenovirus-mediated gene transfer from the apical surface of CF epithelia. Importantly, gene transfer occurred in cells which are capable of CFTR-dependent Cl− transport.
FIG. 7.
Effect of CAR expression on adenovirus-mediated CFTR expression by CF airway epithelia. Two days after CAR gene transfer, CF airway epithelia were infected with Ad2/CFTR-16 (MOI, 10) for 30 min from the apical surface. Forty-eight hours later the epithelia were studied in Ussing chambers. Data are changes in current (means + standard errors of the means [error bars]) after the addition of bumetanide to cyclic AMP-stimulated, amiloride-treated epithelia (ΔIBum) (n = 6). ∗, P < 0.01 compared to control.
DISCUSSION
These results show that placing a specific binding site for adenovirus vectors on the apical surface of airway epithelia facilitates gene transfer. In addition, the data show that similar treatment of CF epithelia also facilitated gene transfer and correction of the CF Cl− transport defect. This study has the advantage that it used well-differentiated human airway epithelia that retain many of the properties of the in vivo airways, including the resistance to adenovirus-mediated gene transfer. Nevertheless, although the in vitro model often predicts in vivo behavior, our study is limited in that there could be other barriers to gene transfer in vivo.
The apical membrane of airway epithelia contain few αvβ3 or αvβ5 integrins; nonetheless, GPI-CAR rescued both adenovirus binding and gene transfer from the apical membrane. Thus, these data indicate that the penton base-integrin interaction is not required for gene transfer. This conclusion is consistent with data obtained from in vivo studies in other systems (14). GPI-anchored proteins localize to lipid rafts (31), which may be involved in endocytic sorting (22). In addition, GPI-anchored proteins can be found in lipid raft domains in close proximity to caveolae or inside the caveolae (1, 28). Hence, the activity of GPI-CAR might be augmented by its location in areas of the apical membrane with active internalization (endocytosis or potocytosis), thereby masking the need for integrins. In either event, these results bring into question the need for coreceptors which can potentiate internalization, at least when the receptor is abundant or localized in an area of active membrane turnover. Therefore, targeting strategies to airway epithelia may not be hindered by the lack of coreceptors.
Previous work reported that the glycocalyx (specifically sialic acid) prevented gene transfer from the apical surface of MDCK cells (24). However, our data indicate that removing the glycocalyx did not potentiate gene transfer to airway epithelia. There are several possible explanations for these different observations. First, the difference may be due to structural differences between the MDCK cell lines and primary cultures of differentiated airway epithelia. Second, the two systems used to express CAR could have produced different levels of the receptor. Third, the glycocalyx could be a barrier in airway epithelia, and although the data show that apical carbohydrate was removed, we may not have removed sufficient glycocalyx to observe a difference in gene transfer. However, when we tested more prolonged enzyme treatments, transepithelial resistance fell, which would have allowed the vector to access basolateral receptors. Thus, although we cannot exclude the possibility that the glycocalyx interferes to some extent, this interference may be of secondary importance compared to the presence or absence of the receptor.
In conclusion, expressing CAR on the apical surface by GPI modification rescues adenovirus binding and gene transfer from the apical surface of airway epithelia. These data suggest that targeting binding sites on the apical surface will enhance gene transfer. This conclusion is consistent with other approaches to enhancing gene transfer. For example, increasing nonspecific apical binding of adenovirus by incorporation in CaPi coprecipitates or complexes including cationic lipids facilitated gene transfer (6, 7). Moreover, targeting adenovirus to another GPI-linked protein, the urokinase plasminogen activator receptor, or to P2Y receptors enhanced gene transfer (5, 17). Thus, perhaps numerous different methods of increasing binding may be sufficient to improve gene transfer. However, we predict that targeting a high-affinity receptor which is capable of internalization will result in the most efficient gene transfer to the airway epithelia.
ACKNOWLEDGMENTS
R.W.W. and W.V.H. contributed equally to this work.
We thank Michael Seiler, Janice Launspach, Tom Moninger, Phil Karp, Pary Weber, Tamara Nesselhauf, Theresa Mayhew, Rosanna Smith, and Michele Kadnar for excellent assistance. We especially appreciate the help of ISOPO and IIAM for providing the human lungs. We thank Sam Wadsworth, Genzyme, for the gift of Ad2/GFP and Ad2/CFTR and Jeffrey Bergelson, Children's Hospital of Philadelphia, for the gift of wt-CAR, Tailless-CAR, and GPI-CAR cDNAs. We appreciate the support of the In Vitro Cell Models Core and the University of Iowa Gene Transfer Vector Core.
This work was supported by NHLBI grants HL51670 (J.Z. and M.J.W.) and PO1 HL51746-08 (R.G.C.); the Cystic Fibrosis Foundation (W.V.H., J.Z., R.G.C., and M.J.W.); the Will Rogers Memorial Fund (R.G.C.); GenVec, Inc. (R.G.C.); the Parker B. Francis Foundation (W.V.H.); and the Roy J. Carver Charitable Trust (J.Z.). M.J.W. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Anderson R G. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 2.Arcasoy S M, Latoche J, Gondor M, Watkins S C, Henderson R A, Hughey R, Finn O J, Pilewski J M. MUCl and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie β viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 4.Bewley M C, Springer K, Zhang Y B, Freimuth P, Flanagan J M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–83. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 5.Drapkin P T, O'Riordan C R, Yi S M, Chiorini J A, Cardella J, Zabner J, Welsh M J. Targeting the urokinase plasminogen activator recéptor enhances gene transfer to human airway epithelia. J Clin Investig. 2000;105:589–596. doi: 10.1172/JCI8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasbender A, Lee J H, Walters R W, Moninger T O, Zabner J, Welsh M J. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Investig. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasbender A, Zabner J, Chillon M, Moninger T O, Puga A P, Davidson B L, Welsh M J. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 8.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg H S. The adenoviruses. New York, N.Y: Plenum Press; 1984. [Google Scholar]
- 10.Goldman M J, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greber U F, Singh I, Helenius A. Mechanisms of virus uncoating. Trends Microbiol. 1994;2:52–56. doi: 10.1016/0966-842x(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 12.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 13.Grubb B R, Pickles R J, Ye H, Yankaskas J R, Vick R N, Engelhardt J F, Wilson J M, Johnson L G, Boucher R C J. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 14.Hautala T, Grunst T, Fabrega A, Freimuth P, Welsh M J. An interaction between penton base and alpha v integrins plays a minimal role in adenovirus-mediated gene transfer to hepatocytes in vitro and in vivo. Gene Ther. 1998;5:1259–1264. doi: 10.1038/sj.gt.3300722. [DOI] [PubMed] [Google Scholar]
- 15.Knowles M R, Hohneker K W, Zhou Z, Olsen J C, Noah T L, Hu P C, Leigh M W, Engelhardt J F, Edwards L J, Jones K R, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. New Engl J Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Finkbeiner W E, Widdicombe J H. Simple technique for culture of highly differentiated cells from dog tracheal epithelium. Am J Physiol. 1991;261:L106–L117. doi: 10.1152/ajplung.1991.261.2.L106. [DOI] [PubMed] [Google Scholar]
- 17.Kreda S M, Pickles R J, Lazarowski E R, Boucher R C. G-protein-coupled receptors as targets for gene transfer vectors using natural small-molecule ligands. Nat Biotechnol. 2000;18:635–640. doi: 10.1038/76479. [DOI] [PubMed] [Google Scholar]
- 18.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 20.Lisanti M P, Caras I W, Davitz M A, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayor S, Sabharanjak S, Maxfield F R. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickles R J, Barker P M, Ye H, Boucher R C. Efficient adenovirus-mediated gene transfer to basal but not columnar cells of cartilaginous airway epithelia. Hum Gene Ther. 1996;7:921–931. doi: 10.1089/hum.1996.7.8-921. [DOI] [PubMed] [Google Scholar]
- 24.Pickles R J, Fahrner J A, Petrella J M, Boucher R C, Bergelson J M. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol. 2000;74:6050–6057. doi: 10.1128/jvi.74.13.6050-6057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roelvink P W, Mi Lee G, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–55. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 28.Schnitzer J E, McIntosh D P, Dvorak A M, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 29.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van't Hof W, Crystal R G. Manipulation of the cytoplasmic and transmembrane domains alters the cell surface levels of the coxsackie-adenovirus receptor and changes the efficiency of adenovirus infection. Hum Gene Ther. 2001;12:25–34. doi: 10.1089/104303401450933. [DOI] [PubMed] [Google Scholar]
- 31.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 32.Vierbuchen M J, Fruechtnicht W, Brackrock S, Krause K T, Zienkiewicz T J. Quantitative lectin-histochemical and immunohistochemical studies on the occurrence of alpha(2,3)- and alpha(2,6)-linked sialic acid residues in colorectal carcinomas. Relation to clinicopathologic features. Cancer. 1995;76:727–735. doi: 10.1002/1097-0142(19950901)76:5<727::aid-cncr2820760504>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Walters R, Welsh M. Mechanism by which calcium phosphate coprecipitation enhances adenovirus-mediated gene transfer. Gene Ther. 1999;6:1845–1850. doi: 10.1038/sj.gt.3301020. [DOI] [PubMed] [Google Scholar]
- 34.Walters R W, Duan D, Engelhardt J F, Welsh M J. Incorporation of adeno-associated virus in a calcium phosphate coprecipitate improves gene transfer to airway epithelia in vitro and in vivo. J Virol. 2000;74:535–540. doi: 10.1128/jvi.74.1.535-540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly D J, Davidson B L, McCray P B. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol. 2000;22:129–38. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Bergelson J M. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh M J, Ramsey B W, Accurso F, Cutting G R. Cystic fibrosis. In: Scriver C R, Beaudet A L, Sly W S, Valle D, Childs B, Vogelstein B, editors. The metabolic and molecular basis of inherited disease. 8th ed. New York, N.Y: McGraw-Hill; 2000. pp. 5121–5188. [Google Scholar]
- 39.Welsh M J, Zabner J, Graham S M, Smith A E, Moscicki R, Wadsworth S C. Adenovirus-mediated gene transfer for cystic fibrosis. Part A. Safety of dose and repeat administration in the nasal epithelium. Part B. Clinical efficacy in the maxillary sinus. Hum Gene Ther. 1995;6:205–218. doi: 10.1089/hum.1995.6.2-205. [DOI] [PubMed] [Google Scholar]
- 40.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 41.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zabner J, Ramsey B W, Meeker D P, Aitken M I, Balfour R P, Gibson R L, Launspach J, Moscicki R A, Richards S M, Standaert T A, Williams-WArren J, Wadsworth S C, Smith A E, Welsh M J. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Investig. 1996;97:1504–1511. doi: 10.1172/JCI118573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]