ABSTRACT
The cell cycle is a fundamental process involved in bacterial reproduction and cellular differentiation. For Sinorhizobium meliloti, cell cycle outcomes depend on its growth environment. This bacterium shows a tight coupling of DNA replication initiation with cell division during free-living growth. In contrast, it undergoes a novel program of endoreduplication and terminal differentiation during symbiosis within its host. While several DivK regulators at the top of its CtrA pathway have been shown to play an important role in this differentiation process, there is a lack of resolution regarding the downstream molecular activities required and whether they could be unique to the symbiosis cell cycle. The DivK kinase CbrA is a negative regulator of CtrA activity and is required for successful symbiosis. In this work, spontaneous symbiosis suppressors of ΔcbrA were identified as alleles of divL and cckA. In addition to rescuing symbiotic development, they restore wild-type cell cycle progression to free-living ΔcbrA cells. Biochemical characterization of the S. meliloti hybrid histidine kinase CckA in vitro demonstrates that it has both kinase and phosphatase activities. Specifically, CckA on its own has autophosphorylation activity, and phosphatase activity is induced by the second messenger c-di-GMP. Importantly, the CckAA373S suppressor protein of ΔcbrA has a significant loss in kinase activity, and this is predicted to cause decreased CtrA activity in vivo. These findings deepen our understanding of the CbrA regulatory pathway and open new avenues for further molecular characterization of a network pivotal to the free-living cell cycle and symbiotic differentiation of S. meliloti.
IMPORTANCE
Sinorhizobium meliloti is a soil bacterium able to form a nitrogen-fixing symbiosis with certain legumes, including the agriculturally important Medicago sativa. It provides ammonia to plants growing in nitrogen-poor soils and is therefore of agricultural and environmental significance as this symbiosis negates the need for industrial fertilizers. Understanding mechanisms governing symbiotic development is essential to either engineer a more effective symbiosis or extend its potential to non-leguminous crops. Here, we identify mutations within cell cycle regulators and find that they control cell cycle outcomes during both symbiosis and free-living growth. As regulators within the CtrA two-component signal transduction pathway, this study deepens our understanding of a regulatory network shaping host colonization, cell cycle differentiation, and symbiosis in an important model organism.
KEYWORDS: Sinorhizobium meliloti, cell cycle, symbiosis, CbrA, CtrA, CckA, DivL, alphaproteobacteria, Caulobacter crescentus, nitrogen fixation, Medicago sativa, bacteroid, Cell signaling
INTRODUCTION
The establishment of a symbiotic relationship between nitrogen-fixing rhizobia and leguminous plants contributes to the global nitrogen cycle in an ecologically sustainable manner. Within host root nodules, rhizobia reduce N2 into ammonia to support plant growth in exchange for photosynthates, making this symbiosis of significant agricultural interest (1). Sinorhizobium meliloti is one well-studied model bacterium that participates in this symbiosis. It forms a chronic intracellular infection with various legumes, including Medicago sativa and Medicago truncatula, within a plant-derived organ called a nodule that forms on host roots (2, 3). During nodule infection, actively growing bacteria are guided into the nodule via an infection thread and eventually deposited into host cells. This process of infection likely occurs using the typical S. meliloti cell cycle observed during free-living reproduction (4). Intracellular bacteria then terminally differentiate into bacteroid cells capable of nitrogen fixation (4). A critical aspect of the bacteroid differentiation program involves significant changes to its cell cycle compared to free-living cells.
For S. meliloti, terminal differentiation into a bacteroid includes endoreduplication, resulting in an increased genome content via repeated rounds of DNA replication without cell division (4). This approximately 24N bacteroid then permanently exits the cell cycle, enlarged and branched in morphology, as a terminally differentiated G0 non-reproductive cell. This cell cycle program is controlled in part by host peptides synthesized within nodules and imported into bacterial cells (5, 6).
Nodule-specific cysteine-rich peptides also induce endoreduplication and block cell division ex planta, however, through unknown mechanisms, and so the precise mechanisms underlying S. meliloti cell cycle outcomes either in planta or ex planta remain unclear (7, 8).
Caulobacter crescentus is a model among alphaproteobacterial for understanding how DNA replication initiation is limited to once-and-only-once per cell cycle coupled to asymmetric cell division. In C. cresecentus, a two-component signal transduction (TCS) pathway regulates CtrA and thereby contributes to effectuating these cell cycle outcomes (9–11). CtrA is a DNA-binding response regulator whose activity is modulated by a complex TCS pathway through its phosphorylation and stability. It functions as a transcription factor that coordinates the temporal expression of cell cycle-related genes throughout the course of cell cycle progression. At cell division, it also contributes to asymmetric daughter-cell differentiation by binding near the chromosome origin to block DnaA-mediated initiation of DNA replication in swarmer daughter cells to impose a G1 phase cell fate. However, CtrA activity is absent from stalked daughter cells. This causes these cells to be born directly into S phase and able to immediately initiate a new round of DNA replication.
S. meliloti also couples DNA replication initiation with cell division to effectuate a once-and-only-once round of genome replication per cell division (4), along with asymmetric cell division to produce daughter cells with distinct replicative developmental fates (12). While CtrA helps to couple DNA replication initiation with cell division in S. meliloti (13, 14), it remains unclear how asymmetric daughter cell fate is established as there are no CtrA-binding sites near the chromosome origin to repress DNA replication initiation in G1 daughter cells as observed in C. crescentus (11).
S. meliloti has orthologs of many components of the C. crescentus CtrA TCS (Fig. 1) (11). Along with the DivK phosphatase PleC, which is essential in S. meliloti (15), there are two DivK histidine kinases (HKs): DivJ and the novel CbrA regulator (15, 16). Either ΔdivJ or ΔcbrA is tolerated, however, the two genes are synthetically lethal (15), highlighting the critical role of regulating DivK phosphorylation for reproduction. The DivK/DivL/CckA phosphotransfer switch as well as the downstream target ChpT (11) are also present. Finally, while lacking PopA, S. meliloti does have CpdR1 and RcdA orthologs that regulate CtrA stability by mediating ClpXP proteolysis (13, 14, 17).
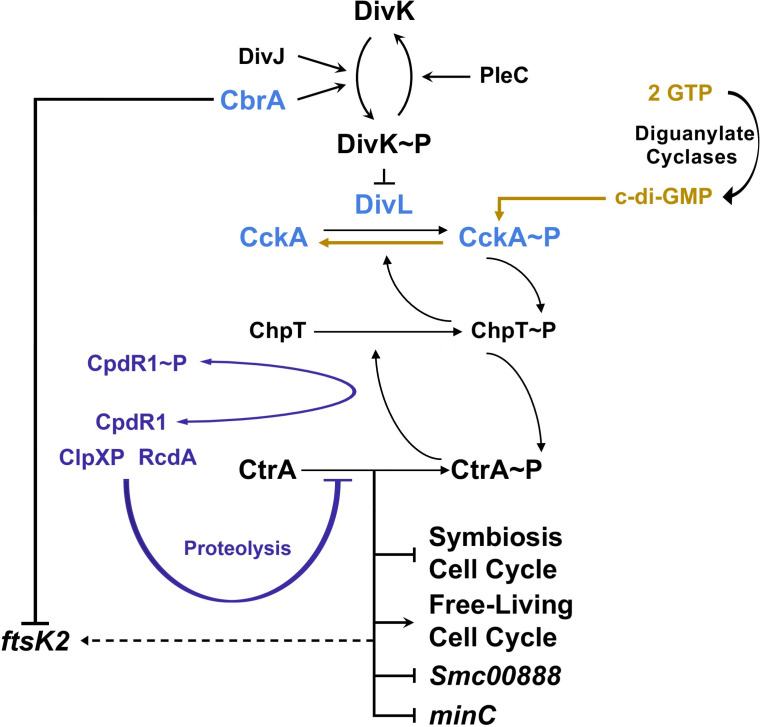
Fig 1.
Model for CtrA-dependent cell cycle regulation in S. meliloti. DivJ and CbrA have been shown to function as DivK kinases, while PleC is a DivK phosphatase (11, 15, 16). When phosphorylated, DivK~P likely inhibits the ability of DivL to bind CckA and activate its kinase activity, thereby leading to the loss of CtrA~P activity. This TCS pathway regulating CtrA phosphorylation status is required to couple DNA replication initiation with cell division during free-living reproduction as well as bacteroid cell cycle differentiation during symbiosis (15, 16). CpdR1 is known to promote the proteolysis of CtrA via RcdA (highlighted in purple) (13, 14, 17). In addition, CckA phosphatase activity is enhanced in the presence of high concentrations of c-di-GMP, and this likely reduces the activity of CtrA downstream (highlighted in gold, this study). At present, the identification of divL and cckA symbiosis suppressors of ΔcbrA strongly supports the model that DivL and CckA act as a kinase/phosphate switch in conjunction with DivK to control the downstream phosphorylation status of CtrA (highlighted in blue, this study) through the histidine phosphotransferase ChpT. More specifically, the CckAA373S suppressor protein is defective for kinase activity (this study), and it is, therefore, likely that repression of CtrA activity is critical to allowing symbiotic cell cycle differentiation. Downstream transcriptional changes to cell cycle and CtrA-regulated genes Smc00888 and minC may contribute to the modulation of c-di-GMP levels and the process of cell division, respectively (this study). While CtrA indirectly affects the expression of ftsk2 (14), and this cell cycle-regulated gene is transcriptionally repressed by CbrA in a manner independent of CtrA activity (this study).
In S. meliloti, excess CtrA activity in either ΔdivJ or ΔcbrA mutants results in cellular filamentation and polyploidy during free-living growth (15, 16). Importantly, these null mutants also have symbiotic defects that result in stunted plant growth. For ΔcbrA, suppression of this defect is observed with constitutive expression of non-phosphorylatable CpdR1D53A, which represses CtrA activity by promoting its proteolysis (Fig. 1) (13). Thus, it has been suggested that decreased CtrA activity may be important to allow bacteroid cell cycle differentiation.
While DivJ and CbrA components of the CtrA TCS are essential for symbiosis, the underlying mechanisms required to promote bacteroid cell cycle differentiation are not well understood. We utilized a forward-genetics approach by isolating spontaneous suppressors of the ΔcbrA mutant symbiosis defect, thus allowing the host to identify functions critical to bacteroid differentiation. Several suppressor alleles are located within either divL or cckA, whose activities are also able to suppress ΔcbrA free-living cell cycle defects and therefore represent universal functions within the S. meliloti TCS regulating cell cycle progression (Fig. 1). Importantly, the cckA suppressor mutation causes a significant loss of kinase activity in vitro and therefore represents the most direct evidence to date that downstream repression of CtrA activity is likely critical for effecting bacteroid cell cycle differentiation (Fig. 1).
RESULTS
ΔcbrA symbiosis suppressor mutations are in divL and cckA
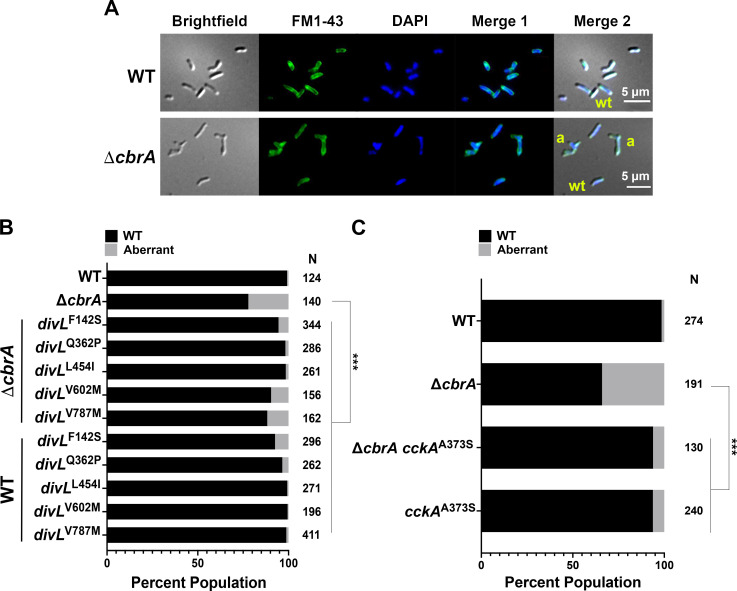
The ΔcbrA mutant has a severe symbiosis defect observed as underdeveloped white nodules unable to support M. sativa growth under nitrogen-limiting conditions due to the loss of nitrogen fixation (16). However, phenotypically wild-type nodules, elongated and pink, will rarely develop on these same plants. S. meliloti were isolated and cultured from each phenotypically wild-type nodule, and one colony per nodule was purified for further study. The absence of cbrA was confirmed using PCR for each isolate, and then its symbiotic phenotype was examined. Those found to provide a consistently improved outcome of pink nodule development and increased plant growth in comparison to the ΔcbrA parent strain were further studied (Fig. 2A).
Fig 2.

The ΔcbrA symbiosis defect can be suppressed by spontaneous second-site mutations. (A) Representative symbiosis results between M. sativa and one isolated symbiosis suppressor, later identified as ΔcbrA divLQ362P, in comparison to its ΔcbrA parent strain and wild type at 28 days post-inoculation. (B) The ΔcbrA mutant has pleiotropic free-living phenotypes that include succinoglycan overproduction and membrane instability. These phenotypes were assessed for the divL-linked (Tn5-divL) and cckA-linked (Tn5-cckA) transposons in an otherwise wild type or ΔcbrA background. Succinoglycan production was assayed using growth on calcofluor-supplemented media and visualized with UV light (top panel, 10° cell culture dilution), and membrane stability was assayed using growth on 10 mM DOC-supplemented media (bottom panel, 10−1 to 10−2 cell culture dilution). No detectable alteration of phenotype in either genetic background was observed for these and all other free-living phenotypes examined herein as well as symbiosis between S. meliloti and M. sativa (data not shown).
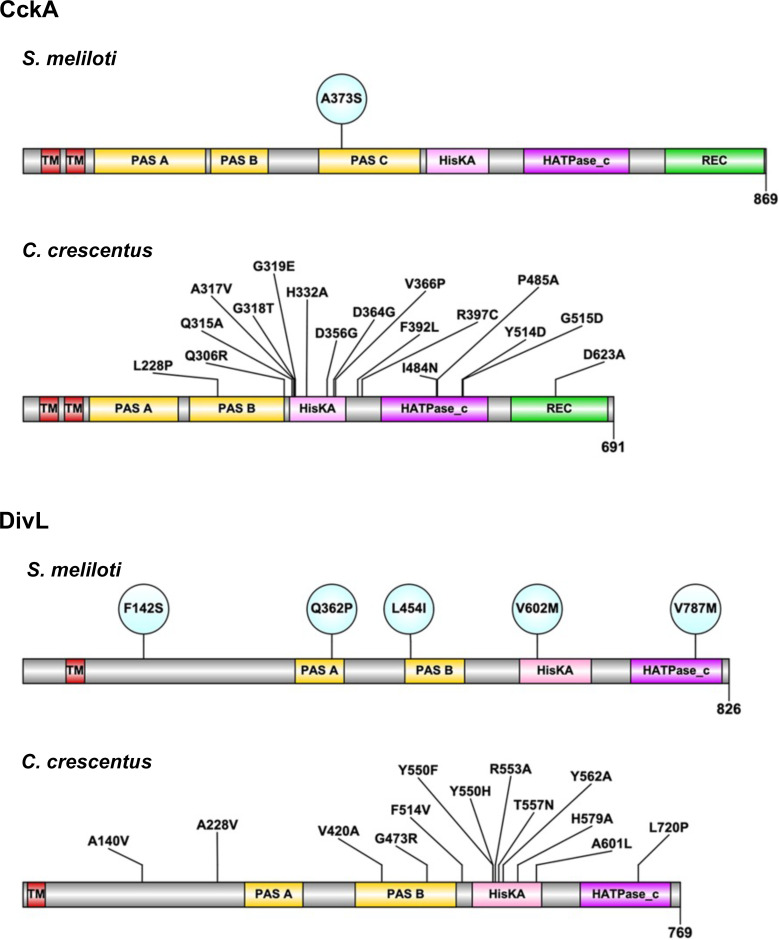
Whole-genome sequencing identified the precise genetic location of each ΔcbrA symbiosis suppressor mutation. For nine of these suppressors, a mutation is located within predicted components of the CtrA TCS: the atypical HK DivL and the hybrid histidine kinase CckA. Interestingly, three independently isolated suppressors contain the cckAA373S allele, and as the only mutation within cckA identified, this suggests the affected activity is particularly important to symbiosis (Fig. 3). The domain architecture of S. meliloti CckA is similar to its ortholog in C. crescentus except for the prediction of an additional PAS (PER ARNT SIM) domain, between the transmembrane and HisKA domains. The PAS-C domain contains the cckAA373S allele and aligns most closely with the PAS-B domain of C. crescentus CckA.
Fig 3.
The predicted domain architecture of S. meliloti DivL and CckA with each ΔcbrA symbiosis suppressor allele indicated. Each cckA and divL mutation isolated as a symbiosis suppressor is indicated as a circle within the CckA and DivL domain structure (InterProScan). Each C. crescentus ortholog and relevant mutations that have been previously studied is included for comparison.
Six suppressors are located within divL, four of which are unique, while divLV787M was independently isolated twice (Fig. 3). Mutations in divL are located throughout its ORF and each within a distinct domain, making it the most diverse target for suppressor mutations. S. meliloti DivL domain architecture is also similar to its C. crescentus ortholog with both having a transmembrane domain followed by two PAS domains, HisKA, and HATPase_C domains.
The allelic exchange was performed to confirm that each divL and cckA mutation is solely responsible for ΔcbrA suppression. To do this, a transposon linked to either divL or cckA was isolated. Free-living and symbiotic phenotypes were then assayed in both wild-type and ΔcbrA backgrounds, with and without each transposon. Since the presence of either transposon results in no observable phenotypic change compared to either parent strain (Fig. 2B), they were used to create divL and cckA single mutants as well as ΔcbrA divL and ΔcbrA cckA double mutants with clean genetic background. To simplify phenotypic descriptions below, rather than individually list each specific allele or mutant, we collectively refer to all divL and cckA mutants as “single mutants” and all ΔcbrA divL and ΔcbrA cckA mutants as “double mutants” when they share a phenotypic outcome, with any individual exceptions then identified.
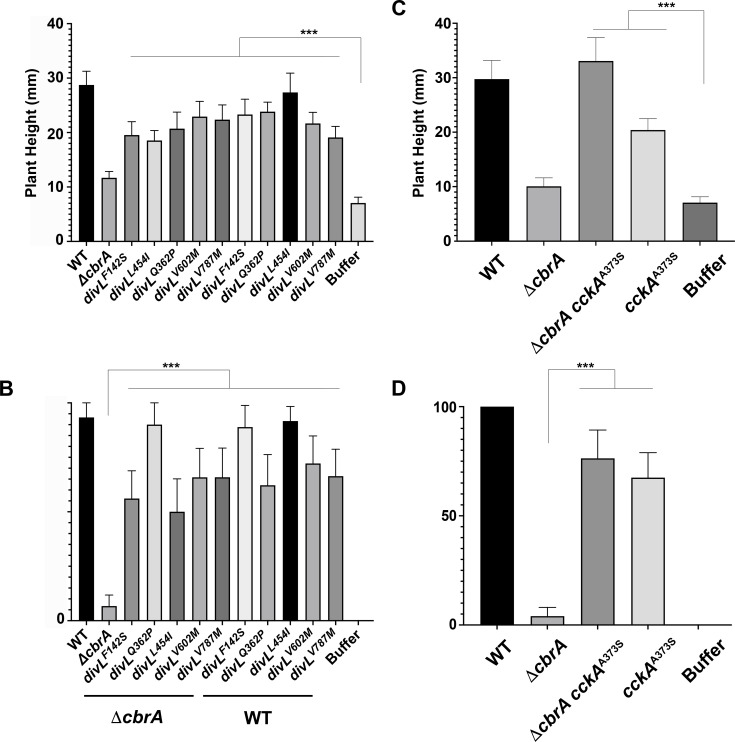
All divL and cckA suppressors rescue the ΔcbrA symbiosis for plant height and pink nodule formation as double mutants. Compared to uninoculated plants, ΔcbrA shows no significant difference in plant height (Fig. 4A and C). In contrast, ΔcbrA double mutants are significantly different from uninoculated plants and more closely resemble the wild type. Regarding nodule formation, ΔcbrA double mutants significantly increased the percentage of pink nodule development to ≥50% compared to ΔcbrA at 5%–10% (Fig. 4B and D). These observations are consistent with DivL and CckA functioning downstream of CbrA to regulate symbiosis. The symbiotic efficiency of each divL and cckA single mutant was also accessed and found not to be statistically different from the wild type (Fig. 4A and C).
Fig 4.
Suppressor alleles of divL and cckA improve the symbiotic outcome of ∆cbrA. M. sativa plants were inoculated with S. meliloti and at 28 days post-inoculation plant height, and the percentage of phenotypically wild-type pink nodules was quantified (N = 10 plants per strain). (A and C) While the average height of plants inoculated with wild type is approximately 29 mm, the average height of plants inoculated with ΔcbrA is not significantly different from uninoculated (buffer control) plants (P = 0.9749) at approximately 10 mm. In contrast, the plant height of those inoculated with either a ΔcbrA divL or ΔcbrA cckA double mutant is significantly different from uninoculated (buffer control) plants (P < 0.0001) and more closely resembles those inoculated with wild type. (B and D) Regarding nodule development, plants inoculated with either a ΔcbrA divL or ΔcbrA cckA double mutant have a significantly increased percentage of at least 50% pink nodules in contrast to the ΔcbrA mutant rate of between 5%–10% (P < 0.0001). There is no significant difference in either (A and C) plant height or (B and D) pink nodule development in the divL and cckA single mutants when compared to the wild type. Statistical significance and P values for all comparisons were determined using a two-way ANOVA and Tukey Kramer test.
Succinoglycan overproduction and detergent sensitivity do not cause ΔcbrA symbiosis defects
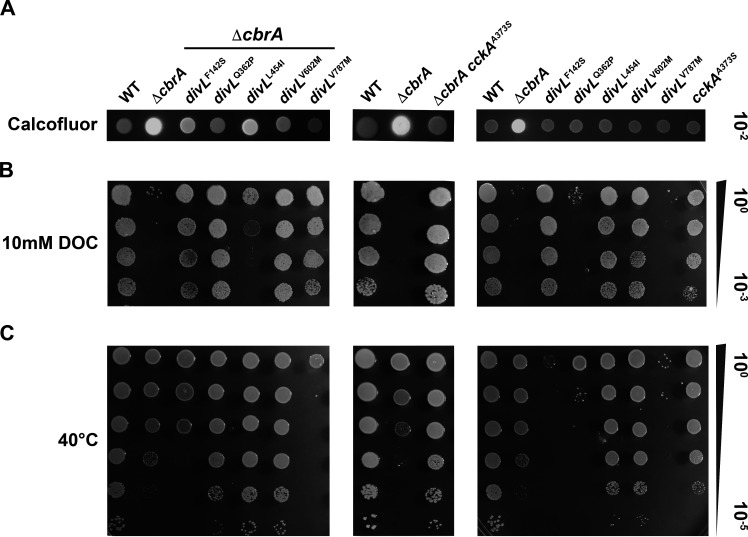
The exopolysaccharide succinoglycan is required for infection thread formation during early stages of host colonization, specifically the low molecular weight forms that are overproduced by ΔcbrA (18, 19). Thus, succinoglycan is not expected to be causative in ΔcbrA symbiosis defects, and therefore, suppressors specific to bacteroid differentiation may not impact this phenotype. To test this hypothesis, the relative production of succinoglycan was evaluated using media supplemented with calcofluor and visualized with UV-light. When succinoglycan production is assessed, wild type is calcofluor-dim, and ΔcbrA is calcofluor-bright (Fig. 5A). When compared to ΔcbrA, the divL and cckA double mutants display a decrease in calcofluor fluorescence relative to the parental strain, although to variable degrees, and therefore at least partially suppress ΔcbrA succinoglycan overproduction (Fig. 5A, left and middle panels).
Fig 5.
Free-living ΔcbrA succinoglycan overproduction and membrane instability phenotypes are suppressed by divL and cckA alleles. (A, left and middle panels) Succinoglycan production was assessed with calcofluor-supplemented media at 30°C using a serial-dilution spot assay and visualized by UV fluorescence. Wild type is calcofluor-dim, and ΔcbrA is calcofluor-bright. ΔcbrA double mutants display a decrease in calcofluor-fluorescence relative to the ΔcbrA parent strain to varying degrees with ΔcbrA divLL454I having an intermediate phenotype. (A, right pane) Each divL and cckA single mutant displays a calcofluor-dim phenotype indistinguishable from wild type. (B, left and middle panels) Membrane stability was assessed with 10 mM DOC-supplemented media at 30°C using a serial-dilution spot assay. As a control, a replicate succinoglycan production assay with the same samples was performed (A), and no growth defects are observed in the absence of DOC. Wild type is DOC-resistant, and ΔcbrA is DOC-sensitive as seen by its inability to grow. Each ΔcbrA double mutant has increased DOC-resistance phenotype with the exception again of divLL454I, which displays an intermediate phenotype. (B, right panel) Each divL and cckA single mutant displays a DOC-resistance phenotype like wild type except for divLQ362P and divLV787M, which exhibit a severe sensitivity to DOC nearly indistinguishable from ΔcbrA. (C) Growth was assessed at 40°C to determine whether any mutants exhibit a temperature-sensitive phenotype using a serial-dilution spot assay. As a control, a replicate succinoglycan production assay with the same samples was performed at 30°C (A), and no growth defects are observed at the standard temperature for S. meliloti. ΔcbrA grows nearly as well as wild type at 40°C, as do most ΔcbrA double mutants. Two exceptions are divLV787M and to a lesser extent divLF142S. (C, right panel) Most divL and cckA single mutants grow nearly as well as wild type. Two exceptions are divLV787M and divLF142S, which have a more severe temperature-sensitive phenotype in the presence of CbrA. In addition, cckAA373S displays a mild temperature-sensitive growth defect in an otherwise wild-type background.
However, the relatively weak suppression by divLL454I indicates that this phenotype can be genetically separated from symbiosis outcome. Thus, succinoglycan overproduction is unlikely to be a primary factor in the ΔcbrA symbiotic defect given that ΔcbrA divLL454I is symbiotically proficient (Fig. 4A and B). divL and cckA single mutants exhibit little to no observable effect on succinoglycan production (Fig. 5A, right panel).
The ΔcbrA mutant displays sensitivity to the detergent DOC, suggesting membrane instability for reasons that remain unclear (Fig. 5B) (18, 20). Each divL and cckA allele suppresses ΔcbrA DOC-sensitivity with wild-type growth with the notable exception of divLL454I, which displays an intermediate phenotype (Fig. 5B, left and middle panels). As with succinoglycan overproduction, this indicates that membrane instability is unlikely to be a primary factor in the ΔcbrA symbiotic defect given that ΔcbrA divLL454I is symbiotically proficient (Fig. 4A and B). Most cckA and divL single mutants display wild-type growth on DOC with the exceptions of divLQ362P and divLV787M, which exhibit DOC sensitivity similar to ΔcbrA (Fig. 5B, right panel). Unlike ΔcbrA, however, these mutants establish an effective symbiosis (Fig. 4A and B), further indicating that cell wall instability is unlikely to be a causative factor in the ΔcbrA symbiotic defect.
Growth at 40°C was used to assess mutants for temperature-sensitive (TS) growth as a potential outcome of cell cycle defects. Compared to wild type, ΔcbrA shows only a very mild growth defect (Fig. 5C). However, when combined with ΔcbrA, the divLF142S and divLV787M alleles cause noticeable TS growth defects (Fig. 5C, left panel), and these are even more severe in the wild-type background (Fig. 5C, right panel). Similarly, divLQ362P displays a TS phenotype as a single mutant, but with ΔcbrA, this phenotype is negligible (Fig. 5C, left compared to right panel). Thus, the activity of these mutant proteins remains responsive to CbrA in vivo and therefore do not represent complete null alleles. Instead, they likely weaken a critical interaction, either intra- or intermolecular, that limits their ability to promote the minimal level of CtrA activity, which is essential in S. meliloti, required for reproduction.
divL and cckA alleles identify core functions within the S. meliloti cell cycle pathway
As observed previously (16), ΔcbrA displays free-living cell cycle phenotypes of minicell production and filamentous morphology for approximately 30% of cells, whereas wild-type cells are bacilli with two cell poles measuring 1–2 µm in length (Fig. 6A).
Fig 6.
Free-living ΔcbrA cell morphology defects are suppressed by divL and cckA alleles. (A) Microscopy was performed on asynchronous logarithmic cultures to visualize cell morphology (brightfield DIC), cell membranes (FM1-43), and DNA (DAPI). The FM1-43 and DAPI images were combined (Merge 1), and all three images were combined (Merge 2) to highlight the filamentous cell morphologies observed. Example wild-type cells are labeled wt and filamentous cells are labeled a. (B and C) A population analysis was performed for cell morphology with N representing the number of cells examined for each strain. As a group, aberrant cells are those with either a length of >5 µm, those filamentous cells with three or more cell poles, and those that are minicell cocci with no observable cell pole. The wild type as well as all divL or cckA single and double mutants were found to be significantly different from ΔcbrA (P < 0.0001). Significance was determined using a two-way ANOVA and Tukey Kramer test.
Interestingly, minicells account for approximately 6% of the ΔcbrA cell population and are nearly 18% of all aberrant cells (data not shown), suggesting an underlying cell division defect. The divL and cckA suppressor alleles rescue ΔcbrA cell morphology defects to varying degrees with an aberrant cell population of ≤10% (Fig. 6B and C).
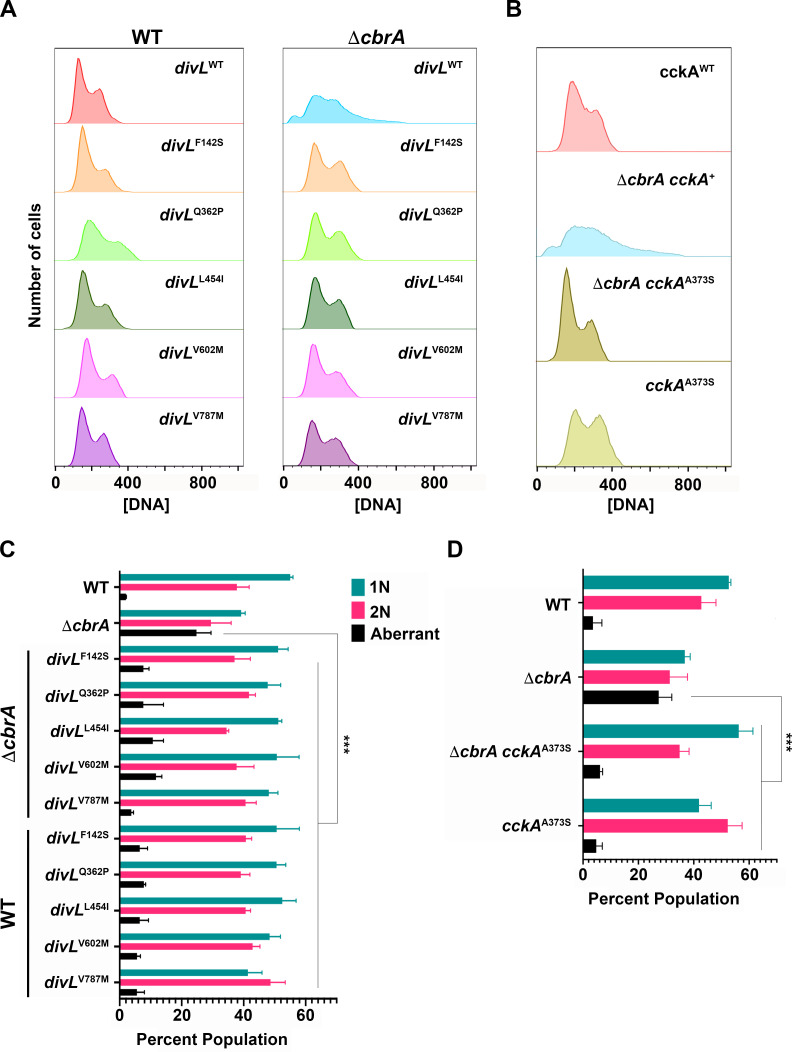
S. meliloti initiates DNA replication once-and-only-once per cell cycle such that asynchronous logarithmic cell populations display two peaks of DNA content (4): G1 cells with 1N and G2 cells with 2N genome content (Fig. 7A and B). This strict coordination between DNA replication initiation and cell division is uncoupled in ΔcbrA with a significant population of polyploid cells having >2N genome content. The divL and cckA alleles restore wild-type cell cycle progression to ΔcbrA with 1N and 2N cell populations that lack both minicells (<1N) and polyploid cells (>1N; Fig. 6A and B). The mutations in divL and cckA therefore affect core cell cycle functions required for reproduction that are not unique to bacteroid cell cycle differentiation during symbiosis.
Fig 7.
Free-living ΔcbrA polyploid defect requires wild-type DivL and CckA activity. Total cellular DNA was quantified by flow cytometry for cells growing asynchronously in the logarithmic phase with N = 3 independent biological replicates for all strains analyzed. One hundred thousand events were collected from each culture utilizing BD FACSAria II and subsequently analyzed using FlowJo. (A and B) Wild type displays a clear 1N and 2N peak of DNA, while ΔcbrA has in addition a <1N peak of minicells and >2N tail of polyploid cells. (A) Each divL and ΔcbrA divL double mutant is significantly different from ΔcbrA (P ≤ 0.0026) but not from the wild type. (B) The cckAA373S and ΔcbrA cckAA373S mutants are also significantly different from ΔcbrA (P = 0.0001) but not from the wild type. (C and D) The distribution of 1N, 2N, and aberrant (<1N and >2N) for cell populations growing asynchronously in the logarithmic phase was analyzed. In the ΔcbrA background, all divL and cckA alleles suppress the aberrant phenotypes observed in the ΔcbrA mutant (P < 0.0026). However, divLV787M and cckAA373S single mutants show a significant increase in the proportion of 2N cells (P = 0.0375 and 0.026, respectively). In addition, cckAA373S shows a significant decrease in the number of 1N cells compared to wild type (P = 0.0162). Significance was determined using a two-way ANOVA and Tukey Kramer test.
The distribution of cell populations falling into 1N, 2N, and aberrant genome content was quantified (Fig. 7C and D). Most divL single and ΔcbrA double mutants are not significantly different in DNA distribution compared to wild type (Fig. 7C). However, divLV787M has an altered distribution with significantly more 2N cells than wild type (Fig. 7C). The cckAA373S single mutant also has a significant reduction in the 1N cell population and a concurrent increase in the 2N cell population compared to wild type (Fig. 7D). These observations indicate that aberrant DivLV787M and CckAA373S mutant activity is associated with a mild G2 delay in cell division.
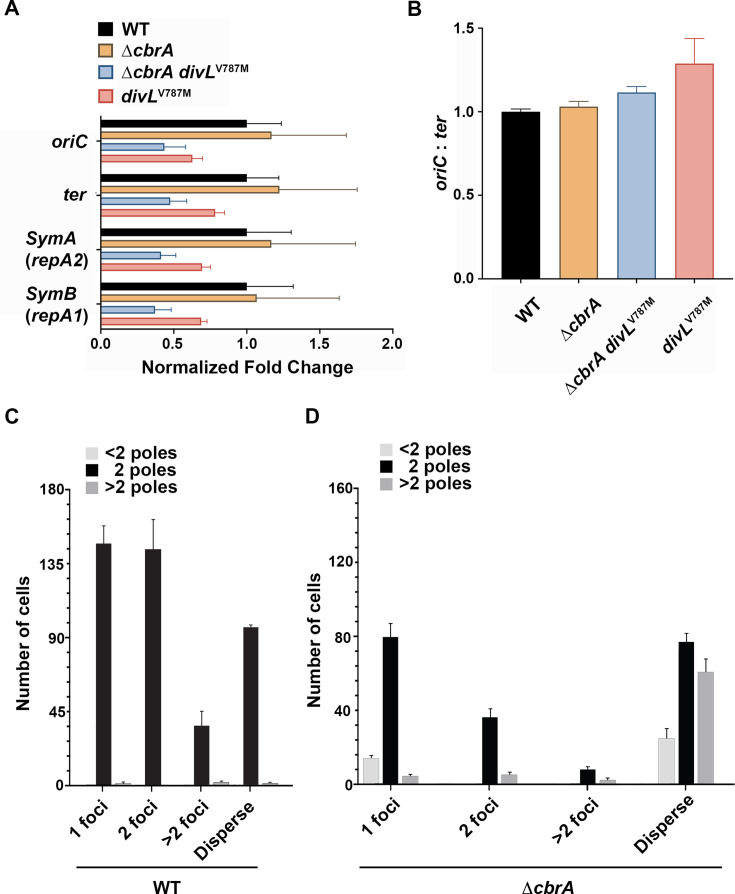
To test whether ΔcbrA polyploidy results from additional DNA replication initiation events prior to cell division, qPCR was performed to measure relative chromosome origin (oriC) and terminus (ter) copy number. If the primary ΔcbrA defect is related to dysregulation of DNA replication with multiple initiation events at the oriC prior to completing replication at the ter, then it will have an increased oriC copy number relative to wild type. In contrast, if the primary defect is related to a delay in cell division, then its oriC copy number would remain wild type as a new replication initiation event would not occur until a prior round of chromosome replication is completed. For wild type, oriC and ter products were each normalized to 1, and mutants were then normalized to wild type. Interestingly, the oriC and ter copy number for ΔcbrA is indistinguishable from wild type (Fig. 8A), as are copy numbers for megaplasmids SymA and SymB (Fig. 8A). It was therefore not surprising that ΔcbrA divLV787M and divLV787M show no significant deviation from wild type for any replicon (Fig. 8A). Similarly, assessment of DNA replication initiation events through quantification of the oriC:ter ratio using the 2−ΔCT method shows no statistically significant differences (Fig. 8B).
Fig 8.
The ΔcbrA polyploid phenotype is not due to aberrant over-initiation of DNA replication initiation events. (A) RT-qPCR analysis was performed on whole-genome extractions from cells growing asynchronously in the logarithmic phase (N = 3 independent biological replicates for each strain). The 2-ΔCT method was used for relative comparison between the four strains. The wild-type average value for each DNA target was normalized to 1. For each mutant strain, the average value for each DNA target was divided by the wild-type average value for that same target (ori, ter, repA2, and repA1). Similar results for pSymA and pSymB were obtained with repB and repC targets (data not shown). No comparisons among strains were found to be statistically significant using a two-way ANOVA. (B) The relative number of chromosome DNA replication initiation events was determined using the 2−ΔCT method and represented as the oriC:ter ratio. No comparisons among the four strains were found to be statistically significant using one-way ANOVA. (C and D) DnaN-mCherry localization was examined in wild type (N = 427) and ΔcbrA (N = 311) cells growing in the logarithmic phase. Each cell was categorized based on the number of DnaN-mCherry foci, with active DNA replication reflected in cells with 2 or >2 foci and those preparing to start or end replication having a single focus. Cells with a dispersed phenotype do not have an active replisome. DnaN-mCherry localization in wild type and ΔcbrA populations was significantly different using the chi-squared test (P < 0.0001). Cells were further divided based on their morphology with those displaying a wild-type bacillus morphology having 2 poles, aberrant filamentous cells having >2 poles, and minicells represented as those with <2 poles.
DnaN is a component of the replisome, so DnaN-mCherry was used as a proxy to visualize the number of replication events within individual cells growing in the logarithmic phase. S. meliloti cells in S phase will display a single focus that splits into two foci as one daughter chromosome is segregated to the opposite cell pole (12). Because of its tripartite genome, as cells transition through S phase, they display >2 foci as the symbiotic megaplasmids are replicated. For both wild type and ΔcbrA, cells were categorized based on their DnaN-mCherry phenotype: 1 focus, 2 foci, >2 foci, or a dispersed fluorescence phenotype (Fig. 8C and D). Active replication is represented by the presence of at least 1 focus, while cells with dispersed fluorescence are not actively replicating DNA as no replisomes are formed. The distribution of DnaN phenotypes is significantly different between wild type and ΔcbrA populations (P < 0.0001). Notably, ΔcbrA cells show an over-representation of cells that display a dispersed fluorescence pattern (23% ± 5% in wild type vs 51% ± 3% in ΔcbrA) and an under-representation of cells with foci (77% ± 5% in wild type vs 49% ± 3% in ΔcbrA). This observation further suggests that the ΔcbrA mutant experiences a significant delay in the timing of DNA replication initiation due either to an extended G1 or G2 phase, rather than excessive initiation during S phase.
Both wild type and ΔcbrA cells were additionally categorized based on the number of poles (Fig. 8C and D). As expected, the majority of wild type cells have 2 cell poles (99.7%) and display typical bacillus morphology regardless of DnaN phenotype. For ΔcbrA filamentous cells (>2 poles), the overwhelming majority (85%) exhibit a dispersed phenotype. These observations further suggest a significant delay in DNA replication initiation as cells with a dispersed phenotype lack an active replisome. This could result from either a G1 or a G2 arrest that delays re-entry into S phase and DNA replication initiation. Cumulatively, these observations demonstrate that the ΔcbrA polyploid phenotype is not caused by an aberrant increase in the number of DNA replication initiation events per cell cycle and instead likely reflects a role for CbrA in regulating cell division.
CbrA regulates gene expression through two distinct pathways
CtrA plays a role in regulating cell cycle progression with both direct and indirect gene targets, some of which overlap with CbrA-regulated genes. Specifically, Smc00888 and Smc00887 are CbrA-regulated genes (20) subsequently identified as being CtrA-regulated (14). The expression of an Smc00888::GUS transcription fusion was therefore examined in wild type, ΔcbrA, and each double mutant. While Smc00888::GUS expression is decreased in ΔcbrA, each ΔcbrA double mutant rescues Smc00888 expression to wild-type levels (Fig. 9A). This is consistent with each suppressor restoring cell cycle phenotypes to ΔcbrA by decreasing CtrA activity to wild-type levels.
Fig 9.
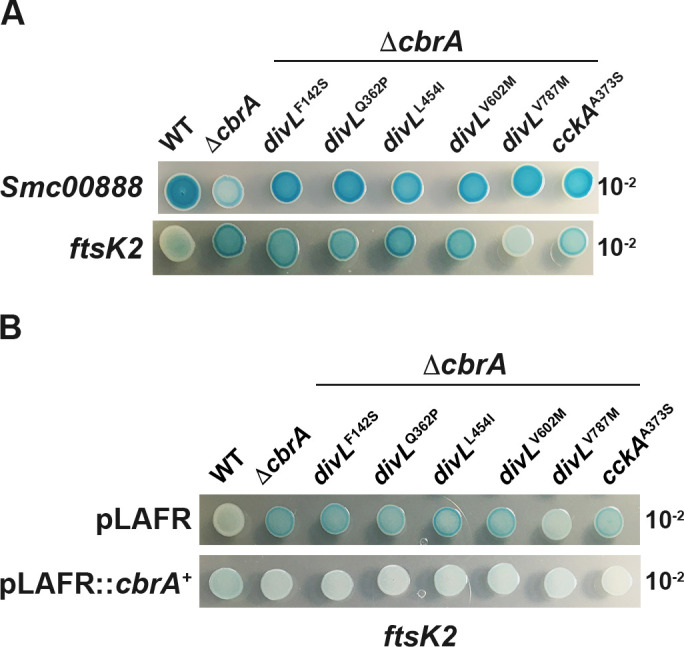
CbrA regulates cell cycle genes through two distinct pathways. Qualitative expression levels of an Smc00888::GUS and ftsK2::GUS transcription fusion were examined using a serial-dilution spot assay on LB/MC media supplemented with an X-GUS indicator dye. (A, top panel) ΔcbrA results in increased expression of Smc00888::GUS relative to wild type, and each divL and cckA allele restores wild-type expression levels. A similar pattern was observed for Smc00887::GUS expression; however, its overall level of activity is significantly decreased relative to Smc00888::GUS (data not shown). (A, bottom panel) The expression of ftsK2::GUS is increased in ΔcbrA; however, this phenotype is not restored to wild-type levels with the exception of divLV787M. (B) CbrA-dependence for ftsK2::GUS expression levels was examined through complementation analysis. (Top panel) With the pLAFR1 empty vector, high-level expression of ftsK2::GUS is observed in each mutant compared to wild type with the exception of ΔcbrA divLV787M. (Bottom panel) In contrast, the presence of pLAFR1::cbrAWT restores wild-type expression in each mutant background.
Another gene identified as a direct target of CtrA-regulation is minC (14), and its overexpression leads to filamentous cell growth (21). Thus, it was of interest to examine minC expression as a gene expected to influence cell division. Unfortunately, the introduction of the minC::GUS transcription fusion into ΔcbrA presents with highly unstable colony phenotypes, perhaps because the fusion does disrupt normal minCDE expression (21), and was therefore not able to be studied further. Of note however, minC::GUS is stable in the wild type and in each of the ΔcbrA double mutants with qualitatively similar expression levels across these strain backgrounds (data not shown). Thus, the genetic instability of this minC::GUS fusion in ΔcbrA suggests that there is an additive disruption in cell division activities that impairs reproduction.
ΔcbrA is polyploid with filamentous growth resulting from a cell cycle defect independent from the licensing of DNA replication initiation (Fig. 7). FtsK2 is predicted to coordinate chromosome resolution with cytokinesis and is CtrA-regulated although in an indirect manner (14). An ftsK2::GUS transcription fusion was constructed to maintain ftsK2 function and introduced into the wild type, ΔcbrA, and double mutant backgrounds. ftsK2::GUS expression increases in ΔcbrA compared to wild type; however, most divL and cckA alleles have little to no impact on this increased expression in ΔcbrA double mutants (Fig. 9A). An exception is ΔcbrA divLV787M, which decreases ftsK2::GUS expression relative to ΔcbrA (Fig. 9A). However, it appears that increased transcription of fstK2 in ΔcbrA and its double mutants does not correlate with either aberrant morphology (Fig. 6B) or genome content (Fig. 7C).
To test whether increased ftsK2 expression in ΔcbrA is due to the loss of cbrA or the loss of its adjacent genes, fbpB or SMc00777, complementation was performed.
The expression of ftsK2::GUS is unchanged with the pLAFR empty vector (Fig. 9A compared to B). In contrast, ftsK2::GUS expression is similar to wild type in each mutant background with pLAFR1::cbrAWT (Fig. 9B). Thus, CbrA is responsible for increased ftsK2::GUS expression in ΔcbrA with most divL and cckA alleles unable to rescue this phenotype. While ftsK2 was identified as an indirect transcriptional target of CtrA in a depletion experiment (14), these results suggest that the excess CtrA observed in ΔcbrA (13, 15, 16) is not the predominant transcription factor downstream of CbrA responsible for regulating ftsK2 expression.
Genetic characterization of divL and cckA suppressor alleles
In C. crescentus, DivL oscillates between direct interaction with either DivK~P, but not unphosphorylated DivK, or CckA (22, 23). This partner exchange regulates CckA localization to the new cell pole and its kinase/phosphatase switch within the CtrA TCS. Specifically, full CckA kinase activity requires DivL binding in vivo, and this interaction is blocked when DivK is phosphorylated and can titrate DivL away from CckA. Based on this C. crescentus model, CbrA as a DivK kinase will downregulate CckA kinase activity and thereby decrease CtrA activity (Fig. 1). In ΔcbrA, lower DivK~P levels would allow increased DivL binding to CckA, activating its kinase activity and thereby increasing CtrA activity (Fig. 1). Thus, DivL and CckA suppressor alleles of ΔcbrA are predicted to act through the downregulation of CckA kinase activity.
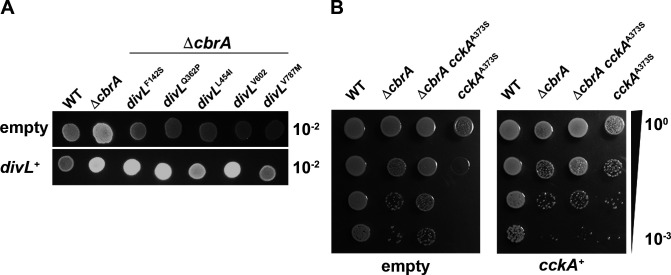
To genetically characterize each divL allele, pBB1RMCS-3::divLWT was introduced into ΔcbrA divL double mutants, and succinoglycan production was assayed as an output for complementation. Each ΔcbrA divL mutant displays a wild-type calcofluor-dim phenotype relative to ΔcbrA with the pBB1RMCS-3 empty vector (Fig. 10A, top row). Several ΔcbrA divL mutants with pBBR1MCS-3::divLWT display a calcofluor-bright phenotype similar to ΔcbrA (Fig. 10A, bottom row). Thus, these alleles are recessive to divLWT and may either reduce its CckA binding or increase its affinity for DivK/DivK~P (Fig. S1), resulting in an overall decrease in CtrA activity in the ΔcbrA background. In contrast, ΔcbrA divLL454I displays an intermediate calcofluor phenotype with pBBR1MCS-3::divLWT and has only partial complementation, while ΔcbrA divLV787M displays a calcofluor-dim phenotype like wild type and is therefore dominant (Fig. 10A, bottom row). Thus, DivLL454I and DivLV787M can likely bind CckA and thereby prevent full complementation by DivLWT. For both alleles, it appears they are either unable to effectively promote CckA kinase activity or aberrantly enhance its phosphatase activity upon binding (Fig. S1), thereby decreasing CtrA activity in ΔcbrA.
Fig 10.
Complementation assay performed with divL and cckA alleles tests genetic relationship to the wild type. The low copy number pBBR1MCS-3 plasmid (empty) was used to create a complementation plasmid with either divLWT or cckAWT ORF and each with its native promoter region included as predicted by Softberry BPROM. Phenotypes for each strain were accessed using a serial-dilution spot assay on either (A) calcofluor-supplemented LB media grown at 30°C or (B) on LB media grown at 40°C. (A, top row) In the presence of pBBR1MCS-3, the wild type has a calcofluor-dim phenotype, while ΔcbrA is calcofluor-bright. Each ΔcbrA divL double mutant has a calcofluor-dim phenotype relative to ΔcbrA in the presence of pBBR1MCS-3. (A, bottom row) In the presence of pBBR1MCS-3::divLWT, the wild type maintains a calcofluor-dim phenotype, while ΔcbrA remains calcofluor-bright. The divLF142S, divLQ362P, and divLV602M alleles are fully complemented by pBBR1MCS-3::divLWT to the ΔcbrA calcofluor-bright phenotype, which indicates these alleles are recessive. The intermediate calcofluor-bright phenotype of ΔcbrA divLL454I in the presence of pBBR1MCS-3 divLWT indicates this allele is semi-dominate, while the calcofluor-dim phenotype of divLV787M shows that it is dominant. (B, left) During growth at 40°C with pBBR1MCS-3, cckAA373S displays a significant TS growth defect in contrast to ΔcbrA and ΔcbrA cckAA373S mutants. (B, right) During growth at 40°C with pBBR1MCS-3::cckAWT, cckAA373S growth is now similar to other mutant backgrounds, ΔcbrA and ΔcbrA cckAA373S, which indicates this allele has been complemented and is recessive.
In an otherwise wild-type background, pBBR1MCS-3::cckAWT results in an aberrant calcofluor-bright phenotype (data not shown), and therefore, complementation could not be tested using this output. Instead, cckAA373S complementation was performed using TS growth of the cckAA373S single mutant as the pBBR1MCS-3 empty vector exacerbated this otherwise weak phenotype (Fig. 5C, middle and right panels; and Fig. 10B, left panel). Importantly, CckAA373S remains responsive to CbrA signaling at 40°C (Fig. 5C, middle and right panels; Fig. 10B, left panel), and both CckAWT and CckAA373S are equally expressed and soluble at both 30°C and 40°C (Fig. 11A). Thus, the TS phenotype used to evaluate complementation is not due to instability or unfolding of CckAA373S that would result in null activity.
Fig 11.
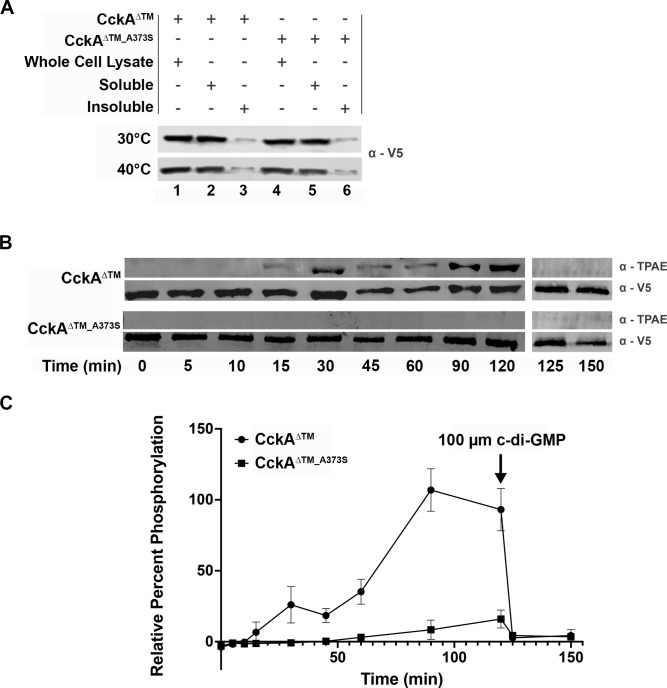
S. meliloti CckAΔTM displays kinase activity and responds to c-di-GMP with a switch to phosphatase activity. (A) V5 affinity-tagged CckAΔTM and CckAΔTM_A373S proteins were expressed in BL21 grown at 30°C and 40°C. Both protein variants are expressed (lanes 1 and 4) and soluble (lanes 2 and 5) with minimal insoluble protein (lanes 3 and 6) at both growth temperatures. (B) V5-CckAΔTM (row 1, α-TPAE phosphorylated protein; and row 2, α-V5 total protein) and V5-CckAΔTM_A373S (row 3, α-TPAE phosphorylated protein; and 4, α-V5 total protein) were incubated with ATPγS in a kinase assay for 120 min with samples taken at the indicated time points. V5-CckAΔTM autophosphorylation is detectable at 15 min (lane 4), while V5- CckAΔTM_A373S autophosphorylation is imperceptible until 60 min when a weak signal becomes detectable (lane 7). At 120 min, 100 µm c-di-GMP was added to each kinase reaction to test for phosphatase activity. For V5-CckAΔTM, complete dephosphorylation is observed within 5 min at the 125 min timepoint. (C) For each sample, the average amount detected with α-TPAE was normalized to the average total protein detected with α-V5 at each time point to quantify the percentage of phosphorylated protein. The V5-CckAΔTM value was maximal at 90 min and therefore normalized to 100%. V5-CckAΔTM_A373S autophosphorylation activity is significantly diminished compared to V5-CckAΔTM from 30 min onward (P < 0.01). N = 3 independent biological replicates for all enzymatic assays. Significance was determined using a two-way ANOVA and Tukey Kramer test.
Growth of each mutant strain is less robust than wild type either with the pBBR1MCS-3 empty vector or the pBBR1MCS-3::cckAWT complementation vector (Fig. 10B), indicating that each has a less efficient cell cycle supporting reproduction.
However, cckAA373S with pBBR1MCS-3 displays very limited growth at 40°C (Fig. 10B, left panel). Importantly, cckAA373S pBBR1MCS-3::cckAWT grows as well as the other mutant strains, ΔcbrA and ΔcbrA cckAA373S, when combined with pBBR1MCS-3::cckAWT (Fig. 10B, right panel). This complementation indicates that the cckAA373S allele is recessive and attenuating ΔcbrA phenotypes through either decreased binding to DivL or an inherently decreased kinase activity even when bound to DivL (Fig. S1).
CckAA373S is significantly impaired for kinase activity
The soluble cytoplasmic portion of CckA and CckAA373S proteins was V5-tagged for purification and enzymatic characterization. As the single mutant cckAA373S exhibits a weak TS phenotype when grown at 40°C, V5-CckAΔTM and V5-CckAΔTM_A373S were expressed at both 30°C and 40°C. Western blot analysis shows that both proteins are equally stable and located primarily within the soluble cell fraction at both temperatures (Fig. 11A). This, combined with no additive TS phenotype as a ΔcbrA cckAA373S double mutant compared to ΔcbrA (Fig. 5C, middle and right panels; Fig. 10B, left panel), strongly suggests that CckAA373S retains some functional activity and is not an unstable or unfolded protein in vivo. Thus, the V5-CckAΔTM_A373S protein can be assessed for in vitro enzymatic activities compared to V5-CckAΔTM.
CckAA373S is complemented by CckAWT (Fig. 10B), suggesting it has lost the ability to either bind DivL or perform kinase activity (Fig. S1). If CckAA373S kinase activity is decreased relative to CckAWT, then this would be reflected in an in vitro enzyme assay. The non-radioactive ATP analog ATP-γ-S was used in kinase assays, as one advantage is its increased stability to spontaneous hydrolyzation, and phosphorylated protein was quantified by western blot using antibodies to the thiophosphate ester produced by post-reaction alkylation. Proteins were purified using their V5 tag and then incubated with ATP-γ-S over the course of 2 hours. Samples at the indicated timepoints were probed by western blot for phosphorylated protein (α-TPAE) and total protein (α-V5; Fig. 11B and C). CckAΔTM exhibits autophosphorylation within 15 min and displays increasing phosphorylation over time. In contrast, CckAΔTM_A373S does not demonstrate detectable autophosphorylation activity, and at 2 hours, the total concentration of phosphorylated protein is significantly decreased relative to CckAΔTM. Together, these results show CckAΔTM_A373S is defective for autophosphorylation in vitro and is consistent with the complementation of cckAA373S in vivo reflecting its activity as a recessive allele (Fig. 10B).
In C. crescentus, CckA has been shown to bind c-di-GMP, and this enhances its switch to phosphatase activity (23–26). To test whether this regulation may also be present in S. meliloti, c-di-GMP was added to each kinase reaction at 120 min, and samples were subsequently taken at 125 and 150 min (Fig. 11B and C). Within 5 min of ci- di-GMP addition, minimal phosphorylated CckAΔTM is detectable, showing c-di-GMP can bind this protein and enhance its phosphatase activity. Since the level of phosphorylated CckAΔTM_A373S at 120 min is nearly undetectable, it is unclear whether the addition of c-di-GMP causes a further decrease. Overall, these data show c-di-GMP may be a regulator of the S. meliloti CtrA TCS pathway in vivo by binding CckA and enhancing its phosphatase activity.
DISCUSSION
CbrA functions as a DivK kinase to regulate CtrA activity during free-living growth (13, 15, 16), and this study represents a forward-genetic screen for mutations that suppress the severe symbiotic deficiency of ΔcbrA in order to gain insight into the molecular requirements underlying bacteroid cell cycle differentiation. CtrA levels are decreased in bacteroids compared to free-living cells, which indicates that this may be a requirement for symbiosis. Consistent with this, ΔcbrA and ΔdivJ mutants have increased CtrA during free-living growth and are unable to establish an effective symbiosis (15, 16). The identification of symbiosis suppressors in divL and cckA further supports the hypothesis that the ΔcbrA defect is due, at least in part, to the mis-regulation of CtrA. Significant TS growth defects are observed for several divL alleles, and in particular, the strong TS phenotype of divLV787M as both a single and double mutant indicates that divL is an essential gene in S. meliloti (Fig. 5C), as in C. crescentus, and possibly due to the requirement for minimal CtrA activity to support reproduction during free-living growth.
ΔcbrA has pleiotropic free-living phenotypes, including succinoglycan overproduction and membrane sensitivities, in addition to cell cycle phenotypes of filamentous growth and polyploidy (16, 18). While retaining moderate succinoglycan overexpression and DOC-sensitivity (Fig. 5A and B), divLL454I restores symbiosis to ΔcbrA (Fig. 4A and B). Importantly, it also restores free-living cell cycle progression to ΔcbrA (Fig. 6B, 7A and C). Moreover, divLQ362P and divLV787M single mutants display a strong DOC-sensitive phenotype (Fig. 5B) while maintaining free-living cell cycle progression and the ability to establish an effective symbiosis (Fig. 4A, B, 6C, 7B and D). These observations underscore the relative importance of the role CbrA plays in regulating cell cycle outcomes as the critical requirement for symbiosis .
For S. meliloti, DNA replication initiation is strictly limited to once-and-only-once per cell division (4); however, ΔcbrA has a >2N polyploid phenotype (Fig. 7). Our data demonstrate this phenotype is not caused by an increased rate of DNA replication initiation (Fig. 8) and instead points toward a cell division defect. Although many CtrA-regulated genes remain uncharacterized, this conclusion is consistent with the identification of minCDE, along with cell envelope functions, as transcription targets (14). While misexpression of either DnaA or its regulator, Hda, can alter the rate of chromosomal DNA replication initiation in S. meliloti (12), neither of these genes has been identified as CtrA-regulated (14). Consistent with this, while CtrA depletion and the hyperactivation of CtrA activity in ΔcbrA result in filamentous polyploid cells (Fig. 7) (16), these cells maintain a wild-type copy number for all replicons (Fig. 8). This contrasts with the C. crescentus divK null phenotype, which exhibits a strong G1 arrest with 1N cells due to the hyperactivation of CtrA, thereby blocking DnaA replication initiation at the origin (27, 28). Thus, it remains unclear whether, and if so how, CtrA regulates DNA replication initiation or the establishment of daughter cell replicative asymmetry in S. meliloti.
The only cckA symbiosis suppressor allele recovered was cckAA373S within its PAS-C domain (Fig. 3), although it was independently isolated three times and therefore reflects the importance of this residue for regulating CckA activity during bacteroid differentiation. Moreover, this allele is the most effective symbiosis suppressor as reflected in plant growth and pink nodule formation (Fig. 4C and D). It also restores wild-type phenotypes to the ΔcbrA mutant during free-living growth, including cell cycle progression (Fig. 6C, 7B and D) and CtrA-regulated gene expression (Fig. 9A).
Complementation analysis further reveals that cckAA373S is a recessive mutation (Fig. 10B), and consistent with this, the purified CckAΔTM_A373S protein displays significantly reduced autophosphorylation activity in vitro (Fig. 11B and C). These observations combined reinforce prior observations (13) and further indicate that decreasing the flow of phosphate toward CtrA likely plays a critical role in allowing bacteroid cell cycle differentiation.
For CckAA373S, the most closely aligned domain in C. crescentus is PAS-B (Fig. 3). This C. crescentus PAS-B domain is predicted to interact closely with its own DHp- CA catalytic core based on structural analyses and is also needed for responses to DivL and c-di-GMP (24, 26, 27, 29–31). Given the role that c-di-GMP plays in modulating CckA activity in C. crescentus, it was of interest to determine whether this signaling molecule could regulate S. meliloti CckA as well. When c-di-GMP is added to maximally phosphorylated CckAΔTM, a rapid rate of dephosphorylation is observed (Fig. 11B and C). Thus, S. meliloti CckA can bind this important signaling molecule, and binding enhances its phosphatase activity in vitro.
In C. crescentus, DivL is required to ensure the proper localization of CckA and enhance its kinase activity, which subsequently leads to downstream phosphorylation of CtrA (22). The PAS domains of DivL have been identified as regions required for CckA binding, while the HisKA and CA domains of DivL have been proposed to be docking surfaces for DivK~P (22, 23, 25, 29, 31). Two PAS domain mutations were isolated as suppressors of a C. crescentusΔdivJ mutant: divLV420A and divLG473R (32) (Fig. 3). They suppress the cell cycle defects of a ΔdivJ mutant while simultaneously conferring TS sensitivity, particularly in the wild-type background, by decreasing CtrA~P levels.
Notably, C. cresentus DivLA601L (Fig. 3) stimulates CckA kinase activity while abolishing its interaction with DivK~P (22, 29). It is therefore not surprising that four of the five isolated S. meliloti divL suppressors are located within these domains (Fig. 3), presumably modulating direct interaction with either DivK/DivK~P or CckA and thereby affecting CtrA activity.
Complete complementation of divLF142S, divLQ362P, and divLV602M suggests they suppress ΔcbrA phenotypes through a loss of interaction with CckA or an increased affinity for either DivK or DivK~P (Fig. 10A; Fig. S1). Conversely, divLL454I and divLV787M exhibit incomplete or no complementation (Fig. 10A), respectively, suggesting they are capable of binding CckA but have lost the ability to effectively promote CckA kinase activity or enhance phosphatase activity as a binding partner (Fig. S1). It will be of significant interest to further characterize each of these mutant proteins to test these hypotheses and gain insight into the molecular functions of DivL in S. meliloti that are required for cell cycle progression.
MATERIALS AND METHODS
Cell culture techniques
Escherichia coli strains were grown at 37°C in LB, and S. meliloti strains were grown at 30°C in LB containing 2.5 mM MgSO4 and 2.5 mM CaCl2 (LB/MC), unless otherwise stated. Serial dilution spot assays were performed to test phenotypes using overnight cultures grown in LB/MC that were first diluted to OD600 = 0.1, and then serial diluted from 100 to 10−5; only the relevant dilutions are presented. Succinoglycan production and DOC sensitivity were assayed as described previously (16, 18). Temperature sensitivity was assayed on LB/MC/calcofluor/HEPES at 30°C and 40°C. Antibiotics were added to the media as appropriate as previously described (13, 15, 18, 20). GUS expression was assayed on LB/MC with 1 µg/mL X-Gluc (5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid cyclohexylammonium salt).
Genetic techniques
All strains were created using either ΦN3 transduction or tri-parental mating as previously described (16). In order to move each divL and cckA suppressor into a clean CS6000 genetic background, a Tn5 linked to either divL or cckA was isolated by the introduction of pLAFR::Tn5 into HABsup9 and HABsup5, respectively, as previously described (33). Random Tn5 mutants were pooled, and each pool was used to create a ΦN3 lysate for CS6000 transduction. Calcofluor-dim colonies were isolated, and >60% linkage to the calcofluor-dim phenotype was confirmed via transduction into CS6000.
The presence of each suppressor allele in CS6000 background was confirmed by PCR and DNA sequencing. These linked transposons were then used to transduce each divL and cckA allele into the wild-type Sm1021 genetic background to create single mutants.
The presence of each suppressor allele in the Sm1021 background was confirmed by PCR and DNA sequencing (Table 1).
TABLE 1.
Strains, plasmids, and primers used in this study
| Bacterial strain, plasmid, or primer | Genetic characteristics | Antibiotic resistance | Source or reference |
|---|---|---|---|
| S. meliloti | |||
| Rm1021 | Wild-type strain SU47 | Sm | Ausubel |
| CS6000 | Rm1021 ΔcbrA | Sm | (16) |
| CS7001 | CS6000 divLQ362P | Sm | This study |
| HABsup1A | CS6000 divLV602M | Sm | This study |
| HABsup1B | CS6000 divLF142S | Sm | This study |
| HABsup2 | CS6000 divLL454I | Sm | This study |
| HABsup3 | CS6000 divLF142S | Sm | This study |
| HABsup5 | CS6000 cckAA373S | Sm | This study |
| HABsup6 | CS6000 cckAA373S | Sm | This study |
| HABsup7 | CS6000 cckAA373S | Sm | This study |
| HABsup9 | CS6000 divLV787M | Sm | This study |
| JP005 | CS6000 divLV602M Tn5-divL | Sm Nm | This study |
| JP006 | CS6000 divLL454I Tn5-divL | Sm Nm | This study |
| JP007 | CS6000 divLF142S Tn5-divL | Sm Nm | This study |
| JP008 | CS6000 divLV787M Tn5-divL | Sm Nm | This study |
| JP010 | CS6000 divLQ362P Tn5-divL | Sm Nm | This study |
| JP011 | Rm1021 Tn5-divL | Sm Nm | This study |
| JP012 | CS6000 Tn5-divL | Sm Nm | This study |
| JP013 | Rm1021 divLQ362P Tn5-divL | Sm Nm | This study |
| JP014 | Rm1021 divLF142S Tn5-divL | Sm Nm | This study |
| JP015 | Rm1021 divLL454I Tn5-divL | Sm Nm | This study |
| JP016 | Rm1021 divLV602M Tn5-divL | Sm Nm | This study |
| JP017 | Rm1021 divLV787M Tn5-divL | Sm Nm | This study |
| JP001 | CS6000 cckAA373S Tn5-cckA | Sm Nm | This study |
| JP002 | Rm1021 Tn5-cckA | Sm Nm | This study |
| JP003 | Rm1021 cckAA373S Tn5-cckA | Sm Nm | This study |
| JP004 | CS6000 Tn5-cckA | Sm Nm | This study |
| HAB2067 | JP017 pBBR1-MCS-3 | Sm Nm Tc | This study |
| HAB2069 | JP017 pHAB3059 | Sm Nm Tc | This study |
| HAB2031 | Rm1021 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2028 | Rm1021 pHAB3057 | Sm Tc | This study |
| HAB2050 | Rm1021 pHAB3059 | Sm Tc | This study |
| HAB2022 | CS6000 pHAB3059 | Sm Tc | This study |
| HAB2035 | CS6000 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2039 | CS6000 pHAB3057 | Sm Tc | This study |
| HAB2057 | HABsup3 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2052 | HABsup3 pHAB3059 | Sm Tc | This study |
| HAB2045 | CS7001 pHAB3059 | Sm Tc | This study |
| HAB2058 | CS7001 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2037 | HABsup2 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2048 | HABsup2 pHAB3059 | Sm Tc | This study |
| HAB2043 | HABsup1A pBBR1-MCS-3 | Sm Tc | This study |
| HAB2054 | HABsup1A pHAB3059 | Sm Tc | This study |
| HAB2055 | HABsup9 pHAB3059 | Sm Tc | This study |
| HAB2059 | HABsup9 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2056 | HABsup6 pHAB3057 | Sm Tc | This study |
| HAB2060 | HABsup6 pBBR1-MCS-3 | Sm Tc | This study |
| HAB2070 | JP003 pBBR1-MCS-3 | Sm Nm Tc | This study |
| HAB2074 | JP003 pHAB3057 | Sm Nm Tc | This study |
| MB672 | Rm1021 pMB697 | Sm Sp | (20) |
| HAB7010 | Rm1021 pMB697 | Sm Sp | This study |
| HAB7011 | HABsup9 pMB697 | Sm Sp | This study |
| HAB7012 | HABsup6 pMB697 | Sm Sp | This study |
| HAB7013 | HABsup1A pMB697 | Sm Sp | This study |
| HAB7024 | CS6000 pMB697 | Sm Sp | This study |
| HAB7025 | HABsup3 pMB697 | Sm Sp | This study |
| HAB7026 | JP009 pMB697 | Sm Sp | This study |
| HAB7027 | HABsup2 pMB697 | Sm Sp | This study |
| HAB8001 | Rm1021 pJP1001 | Sm Nm | This study |
| HAB8002 | CS6000 pJP1001 | Sm Nm | This study |
| HAB8003 | CS7001 pJP1001 | Sm Nm | This study |
| HAB8004 | HABsup1A pJP1001 | Sm Nm | This study |
| HAB8005 | HABsup1B pJP1001 | Sm Nm | This study |
| HAB8006 | HABsup2 pJP1001 | Sm Nm | This study |
| HAB8007 | HABsup3 pJP1001 | Sm Nm | This study |
| HAB8008 | HABsup5 pJP1001 | Sm Nm | This study |
| HAB8009 | HABsup6 pJP1001 | Sm Nm | This study |
| HAB8010 | HABsup7 pJP1001 | Sm Nm | This study |
| HAB8011 | HABsup9 pJP1001 | Sm Nm | This study |
| RH005 | Rm1021 pK19ms::dnaN-mCherry | Sm Gm | This study |
| RH006 | CS6000 pK19ms::dnaN-mCherry | Sm Gm | This study |
| E. coli | |||
| MT616 | MM294 pRK600 | Cm | Finan |
| DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1Δ(lacZYA-argG) | BRL Corp. | |
| BL21 | (DE3) pLysS ompT hsdSB (rB− mB−) gal dcm | Cm | Invitrogen |
| Plasmids | |||
| pBBR1-MCS-3 | Low copy vector | Tc | (34) |
| pHAB3057 | pBBR1-MCS-3::cckAWT | Tc | This study |
| pHAB3059 | pBBR1-MCS-3::divLWT | Tc | This study |
| pSF-OXB20-HN2-V5-TEV | High Copy Expression vector, N-terminal V5 | Kn | Oxford Genetics |
| pHAB4011 | pSF-OXB20-HN2-V5-TEV:: cckAΔTM | Kn | This study |
| pHAB4038 | pSF-OXB20-HN2-V5-TEV:: cckAΔTM_A373S | Kn | This study |
| pLAFR1 | Low copy vector | Tc | (35) |
| pLAFR2070 | cbrAWT complementation vector | Tc | (18) |
| pVO155 | Suicide vector with uidA cassette | Nm | (36) |
| pMB697 | pVO345::SMc00888-uidA | Sp | (18) |
| pJB1001 | pVO155::ftsk2-uidA | Nm | This study |
| pK19ms::dnaN-mCherry | pK19mobsacB with dnaN-mCherry translation fusion | Kn Gm | (12) |
| Primers | Sequence 5′–3′ | ||
| cat1 | AACTCACCCAGGGATTGGCT | (16) | |
| cat2 | ACCAGACCGTTCAGCTGGATA | (16) | |
| HAB0002 | GCAGGCATTCAGTCGCT | This study | |
| HAB0003 | GGTACGCTTGATTCATCG | This study | |
| HAB0004 | ATGTCCTGACGGCCATC | This study | |
| HAB0005 | ACGACCTTCCGCATCCT | This study | |
| HAB0007 | AGCGACTGAATGCCTGC | This study | |
| HAB0008 | CGATGAATCAAGCGTACC | This study | |
| HAB0009 | GATGGCCGTCAGGACAT | This study | |
| HAB0010 | AGGATGCGGAAGGTCGT | This study | |
| HAB0011 | CAAACCGGAAACGGATTGGG | This study | |
| HAB0012 | TGAATGCGGGGTTCGGTTG | This study | |
| HAB0015 | GACTAGTCTGAACCAGGGTGAAGGTTGG | This study | |
| HAB0016 | GGGGTACCCCCGAGCTCTCGGAGGAACAGG | This study | |
| HAB0017 | CGAGCTCGCGGATACTGCTCATGAAAGG | This study | |
| HAB0018 | GACTAGTCAAGGGAGAGGAGGGCTTGTT | This study | |
| HAB0021 | GAATTCGATCGAGGTGATGCCGCAATCG | This study | |
| HAB0022 | GGATCCTCAGCTGTCGAGCATCTCGCG | This study | |
| CTP050 | ATAAGGATCCTGAACCAGGGTGAAGGTTGG | This study | |
| CTP049 | ATTACATATGGGCAAACAGACGGAGACAGA | This study | |
| CTP019 | AGGCCAGCTCGAAAAGGCGAT | This study | |
| CTP020 | AATCAGCGGCTGCAGTTCTACAAT | This study | |
| CTP021 | ATCCTCGACTACGCGGTCATT | This study | |
| CTP022 | TTCAACCGCTTCGAAAGCTAC | This study | |
| CTP023 | TGTCGGGTGCATCGGTTCGAA | This study | |
| CTP024 | GATGCCCCAGATCGCCCGGAA | This study | |
| CTP025 | AAACCTTGTCGCGGAAAGGCGTCT | This study | |
| CTP026 | TGTTCTTGTCGGCAATGAGAG | This study | |
| HAB0023 | GCAAAGAGGCGGGAGAGATT | This study | |
| HAB0024 | TCATCCACAGGAACCGCAAA | This study | |
| HAB0025 | CAGATGTGAACAAGTCGCCG | This study | |
| HAB0026 | TCCGCGGTCTGTTGATAAGG | This study | |
| HAB0027 | GATTTCTGGAGTTCTTGGGCG | This study | |
| HAB0028 | AGGCTTTTCACGTCGGGAG | This study | |
| HAB0029 | TTCTGGAGTTCTTGGGCGGT | This study | |
| HAB0030 | GCGACAAGGCTTTTCACGTC | This study | |
| HAB0031 | GGAGCGCTCGCAATTCATAC | This study | |
| HAB0032 | AGACCCTGCGGATCATCTCT | This study | |
| HAB0033 | GCAGGAGACGATGGATGGAA | This study | |
| HAB0034 | GAAACGCTGATCGCTCTCCA | This study | |
| HAB0035 | CGAGCTCCTTCGTGACTCTG | This study | |
| HAB0036 | AGAGAGGCGCAGACAATTCC | This study | |
| HAB0037 | CAGCCTCGCTTTCATGGAGA | This study | |
| HAB0038 | TCGGTAGAGGTGAACTTGCG | This study | |
| HAB0039 | GACGTGATCTGCAAGTCCCT | This study | |
| HAB0040 | CGGACCAATCTGCATCAGGA | This study | |
| HAB0041 | GAGCGGCACAAACAGGAATC | This study | |
| HAB0042 | CCTTGTTGGCCGGTTCAATG | This study | |
| ftsK2-1 | ACTAGTCCACTTCTGAAACGACAGC | This study | |
| ftsK2-2 | CTCGAGCGACGAACGCTGTATCAC | This study | |
Molecular DNA techniques
The presence of ΔcbrA in suppressor mutants was confirmed by PCR using primers cat1 and cat 2 as previously described (16). Whole-genome sequencing was performed (Genewiz), and this identified cckA and divL mutations. The presence of these mutations was confirmed via PCR and sequencing (Eton Bioscience; CTP050/CTP049 and HAB0011/HAB0012, respectively). Native promoters for divL and cckA were predicted using Softberry BPROM (37), and complementation plasmids were designed to include these regions. divLWT and cckA WT were amplified using HAB0015/HAB0016 and HAB0017/HAB0018, respectively. PCR products and pBBR1-MCS-3 were digested using SacI/SpeI or KpnI/SpeI for cckA and divL, respectively, creating pHAB3057 and pHAB3059. Primers HAB0021/HAB0022 were used to create CckAΔTM and CckAΔTM_A373S by deleting residues 1–75. PCR products and pSF-OXB20-NH2-V5-TEV were digested (EcoRI/BamHI) to create N-terminal V5-tagged protein fusions (pHAB4011 and pHAB4038). The upstream regulatory region of ftsK2 was amplified using ftsK2-1 and ftsk2-2 primers. The resulting PCR fragment and pVO155 were digested using SpeI/XhoI and ligated together to create pJB1001.
Symbiosis techniques
M. sativa symbiosis was assayed on BNM agar (28) as previously described (38). To isolate bacteria, nodules were removed and sterilized with 70% ethanol and then 20% bleach, rinsed several times, and placed in LB/MC supplemented with 0.3M glucose. Nodules were crushed, and serial dilutions of the bacterial suspension were grown on LB/MC/glucose with streptomycin, with one colony isolated from each nodule.
Microscopy and flow cytometry
Cell morphology, DNA content, and DnaN-mCherry localization of cells in logarithmically growing batch culture were performed as previously described (16). Cell morphology was assayed as on a Zeiss Axioskop 2 Mot Plus microscope with a Hamamatsu Orca-ER camera using Openlab software. DNA content was quantified by FACS using cells stained with 0.5 µM SYTOX Green. One hundred thousand cells were acquired on a BD FACSAria II and subsequently analyzed using FlowJo.
qPCR
qPCR analysis was performed on 25 ng of a whole-genome extraction from logarithmic batch cultures (OD600 = 0.6–0.8). Primers (10 mM) amplified the origin of replication (HAB0023/HAB0024) and terminus (HAB0027/HAB0028) of the chromosome along with repABC regions on the SymA (repA2B2C2: HAB0036/HAB0037, HAB0039/HAB0040, and HAB0041/HAB0042) and SymB (repA1B1C1: HAB0031/HAB0032, HAB0033/HAB0034, and HAB0035/HAB0036). SYBR-green was used to quantify PCR products and QuantStudio 3 Real-Time PCR System for data analysis. The 2−ΔCT method was used for quantification and relative comparisons (39–41). The wild-type average value for each target was normalized to 1. For each mutant strain, the average value for each DNA target was normalized to wild type for the same target.
Protein techniques
BL21 expressing CckAΔTM or CckAΔTM_A373S was grown at 30°C and 40°C. Cells were pelleted at 5,000 g for 10 mins at 4°C and resuspended in pre-chilled lysis buffer (42).
Samples were sonicated 3× for 30 s at 10% amplitude to generate a whole cell lysate, and then centrifuged at 16,000 × g for 25 min at 4°C to separate soluble from insoluble fractions. With Laemmli loading buffer added, samples were boiled for 10 min and separated by 10% SDS-PAGE. V5-tagged CckAΔTM or CckAΔTM_A373S protein in each sample was assessed by western blot analysis via transfer onto an Immobilon-FL Membrane. Membranes were incubated with monoclonal anti-V5 mouse antibody (1:1,000) in LI-COR Odyssey Blocking Buffer overnight at 4°C and subsequently treated with donkey anti-mouse IRDye 800CW antibody (1:20,000) for 2 hours at 4°C. Protein was visualized using the LI-COR detection system, and images were acquired with Image Studio 3.1. For enzymatic assays, V5-tagged CckAΔTM or CckAΔTM_A373S was immunoprecipitated from soluble fractions with anti-V5 agarose beads following the manufacturer’s supplied protocol (Sigma Aldrich). Enzymatic analysis of V5-tagged CckAΔTM and CckAΔTM_A373S was performed with ATP-γ-S following the manufacturer-supplied protocol (abcam) and as described (43). Samples were analyzed by western blot to quantify phosphorylated protein levels. Rabbit monoclonal thiophosphate ester (1:5,000) in Odyssey Blocking buffer at 4°C overnight, followed with goat anti-rabbit IRDye 800CW ab216773 (1:10,000). Following 2-hour incubation with 1 mM of ATP-γ-S at 30°C, 100 µM c-di-GMP was added to test for CckA phosphatase activity at 30°C. Samples were taken post-induction and probed for alkylated ATP-γ-S substrate via western blot using the LI-COR detection system, and images were acquired with Image Studio 3.1.
ACKNOWLEDGMENTS
We thank colleagues in the Department of Biology, including Dr. Linda Huang for fluorescence microscope access; Dr. Catherine McCusker for BD FACSAria II training; Dr. Jens Rister and Deepshe Dewett for QuantStudio 3 Real-Time PCR System use and guidance in data analysis. We would also like to thank Dr. Linda Haung and Dr. Rachel Shvirsky for their support in the completion of this article following the passing of Dr. Katherine E. Gibson on 7 June 2024. The current and previous members of the Gibson Lab would like to thank Dr. Katherine E. Gibson for her dedication during our research training in her lab. We also greatly appreciate her support and guidance during the research process and the publication process of this article. This article will be published posthumously, but her research legacy will continue in all of us. Dr. Gibson will be greatly missed.
K.E.G. was supported by the NIH (1 R15 GM099052-01, P.I.), NSF (IOS 1119866, P.I.), and the University of Massachusetts Boston Proposal Development Grant Program (P20230000054709, P. I.). The Nancy Goranson Graduate Student Research Fund supported H.A.B. and K.B.S. K.B.S. was also supported by a Sanofi Genzyme Doctoral Research Fellowship and the NIH through a U56 grant to the University of Massachusetts, Boston (3U56CA1186305-05S1). NSF supported E.N.K. and J.B. through Research Experiences for Undergraduates (REU) grants to R. Skvirsky, University of Massachusetts Boston (P.I. for DBI-1359241 and DBI-1659347).
Contributor Information
Karla B. Schallies, Email: Karla.Schallies@umb.edu.
Elizabeth Anne Shank, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jb.00399-23.
Models for divL and cckA suppression of the cbrA mutant.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Liu A, Contador CA, Fan K, Lam HM. 2018. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front Plant Sci 9:1860. doi: 10.3389/fpls.2018.01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gibson KE, Kobayashi H, Walker GC. 2008. Molecular determinants of a symbiotic chronic infection. Annu Rev Genet 42:413–441. doi: 10.1146/annurev.genet.42.110807.091427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Den Herder G, Parniske M. 2009. The unbearable naivety of legumes in symbiosis. Curr Opin Plant Biol 12:491–499. doi: 10.1016/j.pbi.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 4. Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, Mausset AE, Barloy-Hubler F, Galibert F, Kondorosi A, Kondorosi E. 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium–legume symbiosis. Proc Natl Acad Sci USA 103:5230–5235. doi: 10.1073/pnas.0600912103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, Kevei Z, Farkas A, Mikulass K, Nagy A, Tiricz H, Satiat-Jeunemaître B, Alunni B, Bourge M, Kucho K, Abe M, Kereszt A, Maroti G, Uchiumi T, Kondorosi E, Mergaert P. 2010. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327:1122–1126. doi: 10.1126/science.1184057 [DOI] [PubMed] [Google Scholar]
- 6. Wang D, Griffitts J, Starker C, Fedorova E, Limpens E, Ivanov S, Bisseling T, Long S. 2010. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science 327:1126–1129. doi: 10.1126/science.1184096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Penterman J, Abo RP, De Nisco NJ, Arnold MFF, Longhi R, Zanda M, Walker GC. 2014. Host plant peptides elicit a transcriptional response to control the Sinorhizobium meliloti cell cycle during symbiosis. Proc Natl Acad Sci USA 111:3561–3566. doi: 10.1073/pnas.1400450111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farkas A, Maróti G, Dürgő H, Györgypál Z, Lima RM, Medzihradszky KF, Kereszt A, Mergaert P, Kondorosi É. 2014. Medicago truncatula symbiotic peptide NCR247 contributes to bacteroid differentiation through multiple mechanisms. Proc Natl Acad Sci USA 111:5183–5188. doi: 10.1073/pnas.1404169111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrows JM, Goley ED. 2023. Synchronized swarmers and sticky stalks: Caulobacter crescentus as a model for bacterial cell biology. J Bacteriol 205:e0038422. doi: 10.1128/jb.00384-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zik JJ, Ryan KR. 2022. Cell cycle signal transduction and proteolysis in Caulobacter. In Biondi E (ed), Cell cycle regulation and development in alphaproteobacteria. Springer, Cham. [Google Scholar]
- 11. Brilli M, Fondi M, Fani R, Mengoni A, Ferri L, Bazzicalupo M, Biondi EG. 2010. The diversity and evolution of cell cycle regulation in alpha-proteobacteria: a comparative genomic analysis. BMC Syst Biol 4:52. doi: 10.1186/1752-0509-4-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frage B, Döhlemann J, Robledo M, Lucena D, Sobetzko P, Graumann PL, Becker A. 2016. Spatiotemporal choreography of chromosome and megaplasmids in the Sinorhizobium meliloti cell cycle. Mol Microbiol 100:808–823. doi: 10.1111/mmi.13351 [DOI] [PubMed] [Google Scholar]
- 13. Schallies KB, Sadowski C, Meng J, Chien P, Gibson KE. 2015. Sinorhizobium meliloti CtrA stability is regulated in a CbrA-dependent manner that is influenced by CpdR1. J Bacteriol 197:2139–2149. doi: 10.1128/JB.02593-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pini F, De Nisco NJ, Ferri L, Penterman J, Fioravanti A, Brilli M, Mengoni A, Bazzicalupo M, Viollier PH, Walker GC, Biondi EG. 2015. Cell cycle control by the master regulator CtrA in Sinorhizobium meliloti. PLoS Genet 11:e1005232. doi: 10.1371/journal.pgen.1005232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pini F, Frage B, Ferri L, De Nisco NJ, Mohapatra SS, Taddei L, Fioravanti A, Dewitte F, Galardini M, Brilli M, Villeret V, Bazzicalupo M, Mengoni A, Walker GC, Becker A, Biondi EG. 2013. The DivJ, CbrA and PleC system controls DivK phosphorylation and symbiosis in Sinorhizobium meliloti. Mol Microbiol 90:54–71. doi: 10.1111/mmi.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadowski CS, Wilson D, Schallies KB, Walker G, Gibson KE. 2013. The Sinorhizobium meliloti sensor histidine kinase CbrA contributes to free-living cell cycle regulation. Microbiology (Reading) 159:1552–1563. doi: 10.1099/mic.0.067504-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi H, De Nisco NJ, Chien P, Simmons LA, Walker GC. 2009. Sinorhizobium meliloti CpdR1 is critical for co-ordinating cell cycle progression and the symbiotic chronic infection. Mol Microbiol 73:586–600. doi: 10.1111/j.1365-2958.2009.06794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibson K.E, Campbell GR, Lloret J, Walker GC. 2006. CbrA is a stationary-phase regulator of cell surface physiology and legume symbiosis in Sinorhizobium meliloti. J Bacteriol 188:4508–4521. doi: 10.1128/JB.01923-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gage DJ. 2004. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68:280–300. doi: 10.1128/MMBR.68.2.280-300.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibson KE, Barnett MJ, Toman CJ, Long SR, Walker GC. 2007. The symbiosis regulator CbrA modulates a complex regulatory network affecting the flagellar apparatus and cell envelope proteins. J Bacteriol 189:3591–3602. doi: 10.1128/JB.01834-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng Jiujun, Sibley CD, Zaheer Rahat, Finan TM. 2007. A Sinorhizobium meliloti minE mutant has an altered morphology and exhibits defects in legume symbiosis. Microbiology 153:375–387. doi: 10.1099/mic.0.2006/001362-0 [DOI] [PubMed] [Google Scholar]
- 22. Tsokos CG, Perchuk BS, Laub MT. 2011. A dynamic complex of signaling proteins uses polar localization to regulate cell-fate asymmetry in Caulobacter crescentus. Dev Cell 20:329–341. doi: 10.1016/j.devcel.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Childers WS, Xu Q, Mann TH, Mathews II, Blair JA, Deacon AM, Shapiro L. 2014. Cell fate regulation governed by a repurposed bacterial histidine kinase. PLoS Biol 12:e1001979. doi: 10.1371/journal.pbio.1001979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mann TH, Seth Childers W, Blair JA, Eckart MR, Shapiro L. 2016. A cell cycle kinase with tandem sensory PAS domains integrates cell fate cues. Nat Commun 7:11454. doi: 10.1038/ncomms11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iniesta AA, Hillson NJ, Shapiro L. 2010. Polar remodeling and histidine kinase activation, which is essential for Caulobacter cell cycle progression, are dependent on DNA replication initiation. J Bacteriol 192:3893–3902. doi: 10.1128/JB.00468-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narayanan S, Kumar L, Radhakrishnan SK. 2018. Sensory domain of the cell cycle kinase CckA regulates the differential DNA binding of the master regulator CtrA in Caulobacter crescentus. Biochim Biophys Acta Gene Regul Mech 1861:952–961. doi: 10.1016/j.bbagrm.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hung DY, Shapiro L. 2002. A signal transduction protein cues proteolytic events critical to Caulobacter cell cycle progression. Proc Natl Acad Sci USA 99:13160–13165. doi: 10.1073/pnas.202495099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lori C, Ozaki S, Steiner S, Böhm R, Abel S, Dubey BN, Schirmer T, Hiller S, Jenal U. 2015. Cyclic di-GMP acts as a cell cycle oscillator to drive chromosome replication. Nature New Biol 523:236–239. doi: 10.1038/nature14473 [DOI] [PubMed] [Google Scholar]
- 29. Mann T.H, Shapiro L. 2018. Integration of cell cycle signals by multi-PAS domain kinases. Proc Natl Acad Sci USA 115:E7166–E7173. doi: 10.1073/pnas.1808543115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dubey BN, Lori C, Ozaki S, Fucile G, Plaza-Menacho I, Jenal U, Schirmer T. 2016. Cyclic di-GMP mediates a histidine kinase/phosphatase switch by noncovalent domain cross-linking. Sci Adv 2:e1600823. doi: 10.1126/sciadv.1600823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Childers WS, Shapiro L. 2014. A pseudokinase couples signaling pathways to enable asymmetric cell division in a bacterium. Microb Cell 2:29–32. doi: 10.15698/mic2015.01.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pierce DL, O’Donnol DS, Allen RC, Javens JW, Quardokus EM, Brun YV. 2006. Mutations in DivL and CckA rescue a divJ null mutant of Caulobacter crescentus by reducing the activity of CtrA. J Bacteriol 188:2473–2482. doi: 10.1128/JB.188.7.2473-2482.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol 149:114–122. doi: 10.1128/jb.149.1.114-122.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- 35. Friedman AM, Long SR, Brown SE, Buikema WJ, Ausubel FM. 1982. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289–296. doi: 10.1016/0378-1119(82)90167-6 [DOI] [PubMed] [Google Scholar]
- 36. Oke V, Long SR. 1999. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol Microbiol 32:837–849. doi: 10.1046/j.1365-2958.1999.01402.x [DOI] [PubMed] [Google Scholar]
- 37. Solovyev V. 2011. Automatic annotation of microbial genomes and metagenomic sequences, p 61–78. In Li RW (ed), Metagenomics and its applications in agriculture, biomedicine and environmental studies [Google Scholar]
- 38. Ehrhardt DW, Atkinson EM, Long SR. 1992. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti nod factors. Science 256:998–1000. doi: 10.1126/science.10744524 [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 40. Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 41. Fernández-Coll L, Maciag-Dorszynska M, Tailor K, Vadia S, Levin PA, Szalewska-Palasz A, Cashel M. 2020. The absence of (p)ppGpp renders initiation of Escherichia coli chromosomal DNA synthesis independent of growth rates. MBio 11:e03223-19. doi: 10.1128/mBio.03223-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laub MT, Biondi EG, Skerker JM. 2007. Phosphotransfer profiling: systematic mapping of two-component signal transduction pathways and phosphorelays. Methods Enzymol 423:531–548. doi: 10.1016/S0076-6879(07)23026-5 [DOI] [PubMed] [Google Scholar]
- 43. Upton EC, Maciunas LJ, Loll PJ. 2019. Vancomycin does not affect the enzymatic activities of purified VanSA. PLoS One 14:e0210627. doi: 10.1371/journal.pone.0210627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Models for divL and cckA suppression of the cbrA mutant.