Abstract
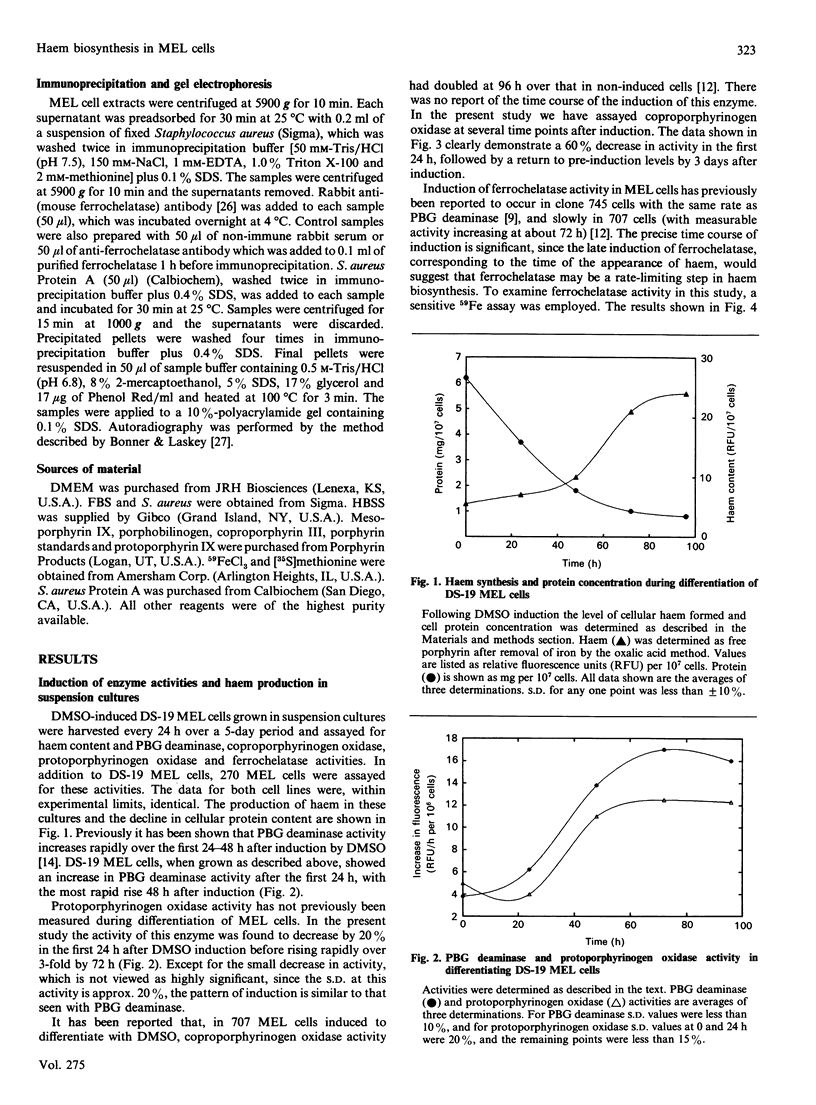
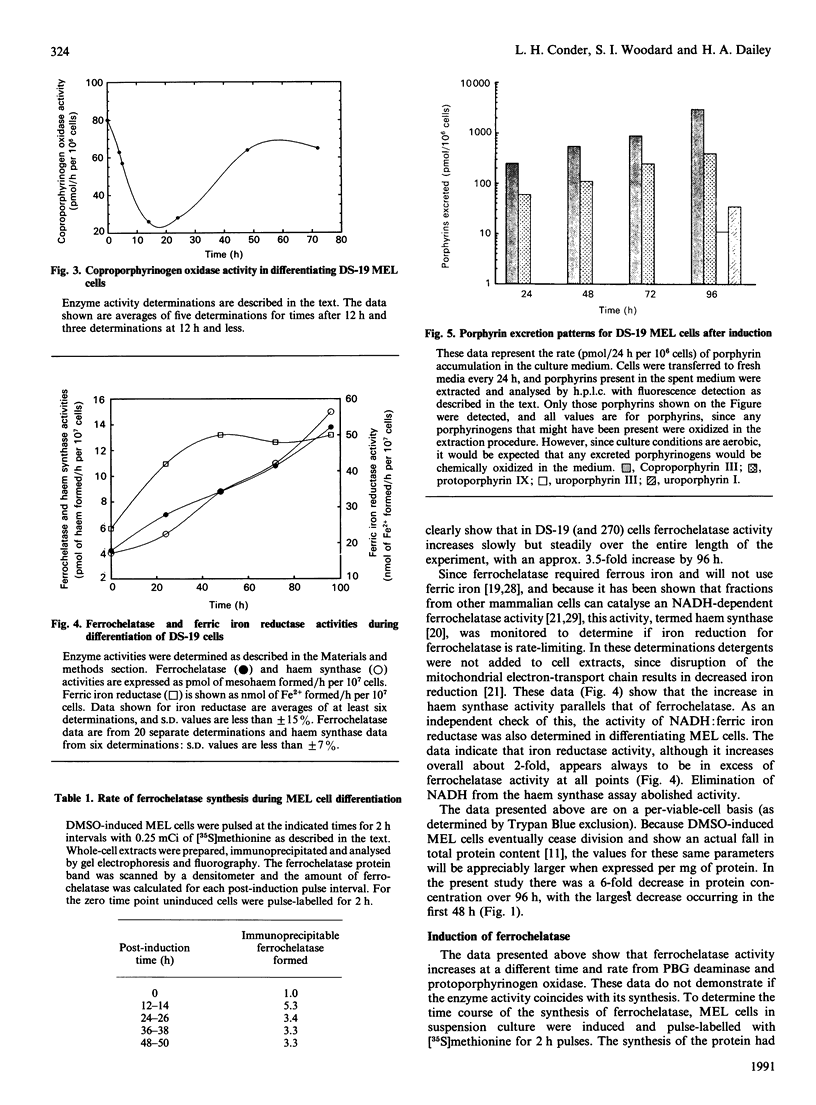
Murine erythroleukaemia (MEL) cells are virus-transformed erythroid precursor cells that, when induced to differentiate by dimethyl sulphoxide (DMSO), will initiate haem biosynthesis by the induction and synthesis de novo of all of the enzymes of the haem-biosynthetic pathway. The activities of porphobilinogen (PBG) deaminase (EC 4.3.1.8), coproporphyrinogen oxidase (EC 1.3.3.3), protoporphyrinogen oxidase (EC 1.3.3.4), ferrochelatase (EC 4.99.1.1) and NADH:ferric iron reductase, as well as the synthesis of the enzyme ferrochelatase and the levels of excreted porphyrins, were monitored during DMSO-induced differentiation of MEL cells in culture. The data demonstrate that PBG deaminase and protoporphyrinogen oxidase activities rise rapidly and early, in comparison with ferrochelatase activity, which rises more slowly, and coproporphyrinogen oxidase activity, which decreases by 60% within 24 h of induction before returning to initial levels by 72 h. NADH:ferric iron reductase activity increases slightly, but is always present at levels higher than needed for haem synthesis. Total immunoprecipitable ferrochelatase also rises slowly and parallels the increase in its activity, suggesting that it is not synthesized early in a slowly processed precursor form. Examination of culture media demonstrated that, whereas excretion of protoporphyrin and coproporphyrin occurs within 24 h of induction, coproporphyrin is excreted in amounts 4-15 times greater than protoporphyrin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Porphobilinogen deaminase: methods and principles of the enzymatic assay. Enzyme. 1982;28(2-3):146–157. doi: 10.1159/000459098. [DOI] [PubMed] [Google Scholar]
- Barnes R., Jones O. T. The availability of iron for haem synthesis in red blood cells. Biochim Biophys Acta. 1973 Apr 28;304(2):304–308. doi: 10.1016/0304-4165(73)90248-1. [DOI] [PubMed] [Google Scholar]
- Beaumont C., Deybach J. C., Grandchamp B., da Silva V., de Verneuil H., Nordmann Y. Effects of succinylacetone on dimethylsulfoxide-mediated induction of heme pathway enzymes in mouse friend virus-transformed erythroleukemia cells. Exp Cell Res. 1984 Oct;154(2):474–484. doi: 10.1016/0014-4827(84)90171-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E., Harbin B. M. Purification and characterization of mammalian and chicken ferrochelatase. Methods Enzymol. 1986;123:401–408. doi: 10.1016/s0076-6879(86)23049-9. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Karr S. W. Purification and characterization of murine protoporphyrinogen oxidase. Biochemistry. 1987 May 19;26(10):2697–2701. doi: 10.1021/bi00384a007. [DOI] [PubMed] [Google Scholar]
- Fadigan A., Dailey H. A. Inhibition of ferrochelatase during differentiation of murine erythroleukaemia cells. Biochem J. 1987 Apr 15;243(2):419–424. doi: 10.1042/bj2430419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C., Andrew T. L., Karr S. W., Dailey H. A. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem. 1988 Mar 15;263(8):3835–3839. [PubMed] [Google Scholar]
- Grandchamp B., Beaumont C., de Verneuil H., Nordmann Y. Accumulation of porphobilinogen deaminase, uroporphyrinogen decarboxylase, and alpha- and beta-globin mRNAs during differentiation of mouse erythroleukemic cells. Effects of succinylacetone. J Biol Chem. 1985 Aug 15;260(17):9630–9635. [PubMed] [Google Scholar]
- Grandchamp B., Phung N., Nordmann Y. The mitochondrial localization of coproporphyrinogen III oxidase. Biochem J. 1978 Oct 15;176(1):97–102. doi: 10.1042/bj1760097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F. C. Rapid isolation techniques for mitochondria: technique for rat liver mitochondria. Methods Enzymol. 1974;31:299–305. doi: 10.1016/0076-6879(74)31031-2. [DOI] [PubMed] [Google Scholar]
- Harbin B. M., Dailey H. A. Orientation of ferrochelatase in bovine liver mitochondria. Biochemistry. 1985 Jan 15;24(2):366–370. doi: 10.1021/bi00323a019. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Terasawa M., Yamauchi K., Kikuchi G. Effects of hemin on the synthesis and intracellular translocation of delta-aminolevulinate synthase in the liver of rats treated with 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biochem. 1980 Nov;88(5):1537–1543. doi: 10.1093/oxfordjournals.jbchem.a133124. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Watanabe N., Kikuchi G. Inhibition by hemin of in vitro translocation of chicken liver delta-aminolevulinate synthase into mitochondria. Biochem Biophys Res Commun. 1983 Sep 15;115(2):700–706. doi: 10.1016/s0006-291x(83)80201-0. [DOI] [PubMed] [Google Scholar]
- Laskey J. D., Ponka P., Schulman H. M. Control of heme synthesis during Friend cell differentiation: role of iron and transferrin. J Cell Physiol. 1986 Nov;129(2):185–192. doi: 10.1002/jcp.1041290209. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Aerobic ferrisiderophore reductase assay and activity stain for native polyacrylamide gels. Anal Biochem. 1983 Oct 1;134(1):235–239. doi: 10.1016/0003-2697(83)90290-7. [DOI] [PubMed] [Google Scholar]
- Parker D., Housman D. Regulation of protein synthesis and accumulation during murine erythroleukemia cell differentiation. J Biol Chem. 1985 Jan 10;260(1):604–609. [PubMed] [Google Scholar]
- Ponka P., Schulman H. M. Acquisition of iron from transferrin regulates reticulocyte heme synthesis. J Biol Chem. 1985 Nov 25;260(27):14717–14721. [PubMed] [Google Scholar]
- Rutherford T., Thompson G. G., Moore M. R. Heme biosynthesis in Friend erythroleukemia cells: control by ferrochelatase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):833–836. doi: 10.1073/pnas.76.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Srivastava G., Borthwick I. A., Brooker J. D., May B. K., Elliott W. H. Purification of rat liver mitochondrial delta-aminolaevulinate synthase. Biochem Biophys Res Commun. 1982 Nov 30;109(2):305–312. doi: 10.1016/0006-291x(82)91721-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Hayashi N., Kikuchi G. Evidence for the transcriptional inhibition by heme of the synthesis of delta-aminolevulinate synthase in rat liver. Biochem Biophys Res Commun. 1982 Apr 14;105(3):985–990. doi: 10.1016/0006-291x(82)91067-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem. 1980 Feb 25;255(4):1746–1751. [PubMed] [Google Scholar]


