Abstract
Aims
Improving renal function (IRF) is paradoxically associated with worse outcomes in acute heart failure (AHF), but outcomes may differ based on response to decongestion. We explored if the relationship of IRF with mortality in hospitalized AHF patients differs based on successful decongestion.
Methods and results
We evaluated 760 AHF patients from AKINESIS for the relationship between IRF, change in B-type natriuretic peptide (BNP), and 1-year mortality. IRF was defined as a ≥20% increase in estimated glomerular filtration rate (eGFR) relative to admission. Adequate decongestion was defined as a ≥40% decrease in last measured BNP relative to admission. IRF occurred in 22% of patients who had a mean age of 69 years, 58% were men, 72% were white, and median admission eGFR was 49 mL/min/1.73 m2. IRF patients had more severe heart failure reflected by lower admission eGFR, higher blood urea nitrogen, lower systolic blood pressure, lower sodium, and higher use of inotropes. IRF patients had higher 1-year mortality (25%) than non-IRF patients (15%) (P < 0.01). However, this relationship differed by BNP trajectory (P-interaction = 0.03). When stratified by BNP change, non-IRF patients and IRF patients with decreasing BNP had lower 1-year mortality than either non-IRF and IRF patients without decreasing BNP. However, in multivariate analysis, IRF was not associated with mortality [adjusted hazard ratio (HR) 1.0, 95% confidence interval (CI) 0.7–1.5] while BNP was (adjusted HR 0.5, 95% CI 0.3–0.7). When IRF was evaluated as transiently occurring or persisting at discharge, again only BNP change was significantly associated with mortality.
Conclusion
Improving renal function is associated with mortality in AHF but not independent of other variables and congestion status. Achieving adequate decongestion, as reflected by lower BNP, in AHF is more strongly associated with mortality than IRF.
Keywords: Kidney function, Acute heart failure, Congestion, Prognosis, B-type natriuretic peptide
Graphical Abstract
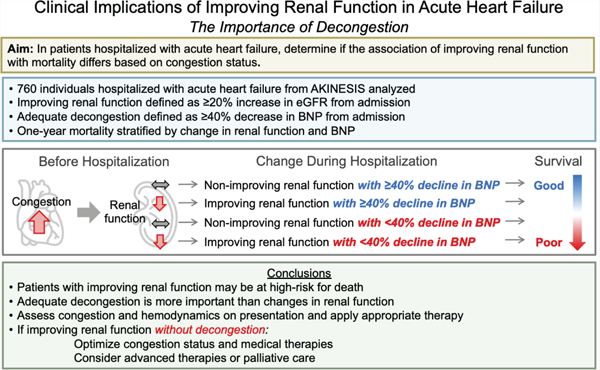
Improving renal function during a hospitalization for acute heart failure was found to identify patients at high-risk for one-year mortality; however, this risk was attenuated when patients were appropriately decongested, as defined by a ≥40% decrease in BNP, and when other high-risk clinical features were adjusted for.
Introduction
Kidney dysfunction is frequently observed in patients with heart failure (HF), especially in the setting of acute decompensated HF.1,2 Various pathophysiologic processes alter renal function in acute HF (AHF) with haemodynamic perturbations being a major driver.3 Prior studies have primarily focused on worsening renal function (WRF) during AHF hospitalization.2 WRF can be caused by both venous congestion and rapid decongestion with diuretics, and is associated with worse clinical outcomes.2,4,5 However, recent findings have demonstrated that WRF accompanied by decongestion is not associated with worse outcomes, whereas patients with WRF and residual congestion have a particularly poor prognosis.6–8
Improving renal function (IRF) is another frequently observed perturbation in AHF, occurring in approximately 10% to 30% of patients.9–11 IRF is thought to result from kidney decongestion during AHF and, theoretically, true improvements in renal function should portend a better rather than worse prognosis. Surprisingly, however, patients with IRF have been reported to have greater risk of mortality and HF readmissions.9–11 A possible explanation is that AHF patients with more severe HF and vulnerable kidneys are more likely to develop WRF as an outpatient prior to hospitalization that is subsequently identified as IRF during the AHF hospitalization.10,12 Despite IRF being a haemodynamically distinct event, its characteristics and prognostic implication have not been fully evaluated. Since change in venous congestion appears to modify the relationship of WRF with clinical outcomes, it seems plausible that the same may be true for IRF, if these two syndromes are linked.
We have previously shown that WRF during AHF hospitalization is associated with higher risk of mortality only when B-type natriuretic peptide (BNP) levels do not decrease with decongestive therapy.6 A declining BNP serves as a useful surrogate for the degree of change in venous congestion in the setting of AHF.13–16 In the current analysis, we aimed to determine if the relationship of IRF with mortality differs based on whether a hospitalized AHF patient is decongested based on changes in BNP.
Methods
Study population
This analysis utilized data from the Acute Kidney Injury Neutrophil gelatinase-associated lipocalin Evaluation of Symptomatic heart faIlure Study (AKINESIS). AKINESIS is a prospective, international, multicentre cohort study of AHF patients, which enrolled 927 patients at 16 sites in the United States and Europe. The methods have been described previously.17 In brief, patients were enrolled if they had findings consistent with AHF and had received or planned intravenous diuretic therapy. Exclusion criteria were (i) acute coronary syndrome, (ii) dialysis-dependence or planned initiation during the hospitalization, (iii) organ transplantation, (iv) enrolment in a drug treatment study within the past 30 days or prior to enrolment in this study, and (v) pregnant or vulnerable population determined by the institutional review board (IRB). The study was approved by IRBs at each study site and each patient signed informed consent. In the current analysis, we excluded 70 patients discharged from the emergency department, 54 patients without serial estimated glomerular filtration rate (eGFR) measurements, and 43 patients without serial BNP measurements, leaving 760 patients who formed the analytic study sample.
Definitions of changes in estimated glomerular filtration rate and B-type natriuretic peptide
Serum creatinine was measured every day of hospitalization. IRF was defined as an increase in eGFR ≥20% compared to admission, either occurring transiently during hospitalization or present at discharge. We subsequently examined outcomes in patients with transient IRF, defined as an increase in eGFR ≥20% during hospitalization compared to admission but not meeting criteria at discharge, and persistent IRF, defined as an increase in eGFR ≥20% compared to admission present at discharge. These cut-offs and definitions were based on prior studies to allow comparison of results across studies.9,10 Adequate decongestion was defined as a decrease of ≥40% in the last measured BNP compared to admission BNP. A cut-off of ≥40% decrease in BNP was chosen based on spline analysis (online supplementary Figure S1).
Outcomes
The primary outcome was all-cause mortality within 1 year of enrolment. The secondary outcome was HF readmission within 1 year. Outcomes were determined by chart review and phone follow-up. Follow-up was available in >98% of participants.
Statistical analysis
Continuous variables were described as means with standard deviations and categorical variables were described as percentages. Non-normally distributed data were described as medians and interquartile ranges (IQR). Patient characteristics were compared by ANOVA, Kruskal–Wallis or chi-square test as appropriate. The association of IRF, evaluated as the highest eGFR minus the admission eGFR, with 1-year mortality was examined using restricted cubic splines with three knots. Kaplan–Meier, log-rank and univariable and multivariable Cox regression analyses were used for the 1-year outcomes. Two multivariable Cox models were constructed. The first model included confounding factors that were significantly associated with 1-year mortality or HF readmission in univariable analysis (P < 0.05). Factors considered for the adjustments are listed in online supplementary Table S1. The second model included confounding factors selected from prior studies and included age, race, chronic obstructive pulmonary disease, oedema, systolic blood pressure (SBP), heart rate, sodium, haemoglobin, blood urea nitrogen (BUN), and high-sensitivity cardiac troponin I.18–25 As kidney function is prognostic for AHF outcomes, we performed a sensitivity analysis adding discharge eGFR to model 1 and 2 as previously done.10 All statistical analyses were performed using R version 3.6.1 for Windows and P-values of <0.05 were considered statistically significant for all analyses including interaction terms.
Results
Patient characteristics
Among the 760 patients, mean age was 69 ± 14 years, 63% were male, 80% had hypertension, 47% had coronary artery disease (CAD) and 45% had diabetes (Table 1). Median eGFR was 56 mL/min/1.73 m2 (IQR 39, 77 mL/min/1.73 m2) and median BNP was 575 pg/mL (IQR 235, 1108 pg/mL) on hospital admission.
Table 1.
Baseline characteristics of patients with and without improved renal function
| Non-IRF (n = 594, 78%) | IRF (n = 166, 22%) | P-value | |
|---|---|---|---|
| Age, years, mean (SD) | 69 (14) | 69 (14) | 0.43 |
| White, n (%) | 377 (64) | 119 (72) | 0.06 |
| Male sex, n (%) | 381 (64) | 97 (58) | 0.21 |
| BMI, kg/m2, mean (SD) | 31.7 (8.9) | 31.6 (9.0) | 0.94 |
| Coronary artery disease, n (%) | 288 (49) | 71 (43) | 0.22 |
| Hypertension, n (%) | 474 (80) | 134 (81) | 0.88 |
| Hyperlipidaemia, n (%) | 310 (52) | 77 (46) | 0.22 |
| Diabetes, n (%) | 271 (46) | 69 (42) | 0.40 |
| Atrial fibrillation, n (%) | 161 (27) | 54 (33) | 0.20 |
| COPD, n (%) | 159 (27) | 40 (24) | 0.55 |
| Smoking, n (%) | 96 (16) | 22 (13) | 0.43 |
| ACEI or ARB, n (%) | 185 (31) | 49 (30) | 0.76 |
| Beta-blockers, n (%) | 422 (71) | 113 (68) | 0.52 |
| Diuretic agents, n (%) | 417 (70) | 116 (70) | 1.00 |
| Oedema, n (%) | 449 (76) | 128 (77) | 0.76 |
| Jugular vein distension, n (%) | 154 (26) | 49 (30) | 0.41 |
| Rales, n (%) | 269 (45) | 76 (46) | 0.98 |
| Wheeze, n (%) | 91 (15) | 32 (19) | 0.27 |
| S3, n (%) | 33 (6) | 8 (5) | 0.86 |
| SBP, mmHg, mean (SD) | 142 (30) | 131 (27) | <0.01 |
| Heart rate, bpm, mean (SD) | 88 (23) | 88 (22) | 0.87 |
| Sodium, mg/dL, mean (SD) | 139 (7) | 137 (6) | <0.01 |
| Haemoglobin, g/dL, mean (SD) | 11.6 (2.5) | 11.8 (2.6) | 0.35 |
| BUN, mg/dL, median [IQR] | 23 [16, 34] | 31 [22, 53] | <0.01 |
| BUN/creatinine ratio, median [IQR] | 19 [15, 26] | 22 [17, 31] | <0.01 |
| Pre-hospital eGFR, mL/min/1.73 m2, median [IQR] (n = 329) | 61 [44, 83] | 68 [43, 90] | 0.17 |
| Admission eGFR, mL/min/1.73 m2, median [IQR] | 60 [43, 81] | 49 [31, 64] | <0.01 |
| Discharge eGFR, mL/min/1.73 m2, median [IQR] | 52 [38, 71] | 59 [41, 84] | <0.01 |
| Admission BNP, pg/mL, median [IQR] | 537 [241, 1077] | 680 [233, 1198] | 0.22 |
| Admission hs-cTnI, pg/mL, median [IQR] | 25.5 [12.6, 58.5] | 28.3 [13.8, 62.1] | 0.24 |
| Last BNP, pg/mL, median [IQR] | 314 [136, 726] | 365 [159, 850] | 0.23 |
| Median furosemide dose in first 3 days, mg/day, median [IQR] | 60 [40, 93] | 67 [40, 120] | 0.39 |
| Inotrope use in first 5 days, n (%) | 35 (6) | 21 (13) | <0.01 |
| Inotrope use in hospital, n (%) | 46 (8) | 32 (19) | <0.01 |
ACEI, angiotensin-converting enzyme inhibitor; ARB; angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; hs-cTnI, high-sensitivity cardiac troponin I; IRF, improved renal function; SBP, systolic blood pressure; SD, standard deviation.
Improving renal function occurred in 166 patients (22%). The median time to IRF was 4 days (IQR 3, 7 days). Compared to non-IRF patients, IRF patients had a lower, though not statistically significant, prevalence of chronic diseases including coronary artery disease, diabetes, hyperlipidaemia, and smoking (Table 1). IRF patients had features consistent with more severe AHF at admission including significantly higher BUN, higher BUN/creatinine ratio, lower SBP, and lower serum sodium. Additionally, IRF patients were more often treated with inotropes during hospitalization, though there was no difference in median loop diuretic dose during the first 3 days of hospitalization (Table 1). IRF patients had a lower admission eGFR than non-IRF patients (49 vs. 60 mL/min/1.73 m2, P < 0.01). Urine output and body weights were available in 480 (63%) and 531 (70%) patients, respectively. There was no difference in median daily urine output between IRF and non-IRF patients [1859 mL (IQR 1446, 2333 mL) vs. 1814 mL (IQR 1277, 2329 mL), P = 0.33] or median change in body weight between admission and discharge [−1.9 kg (IQR 0, −5.5 kg) vs. −1.4 kg (IQR 0, −4.0 kg), P = 0.38]. Among 329 patients (43%) with an eGFR available in the 3 months prior to hospitalization, pre-hospitalization eGFR was higher in IRF patients than non-IRF patients (68 vs. 61 mL/min/1.73 m2) consistent with IRF patients having a more substantial trajectory of loss of eGFR prior to hospital admission (Table 1).
Mortality and heart failure readmission in improving renal function (IRF) patients vs. non-IRF patients
By 1 year, 137 patients (18%) died and 154 patients (20%) had a HF readmission. Of deaths, 104 (76%), 13 (10%), 10 (7%), and 10 (7%) were from cardiovascular, infectious, pulmonary, and other causes, respectively. Spline analysis for 1-year mortality by ratio of highest measured eGFR to admission eGFR is shown in Figure 1. While any increase in eGFR appeared strongly related to 1-year mortality risk, this relationship was steepest for small improvements in eGFR and appeared to stabilize if eGFR improved by ≥40% from admission, although improvements of this degree or greater were uncommon (n = 68, 9%).
Figure 1.
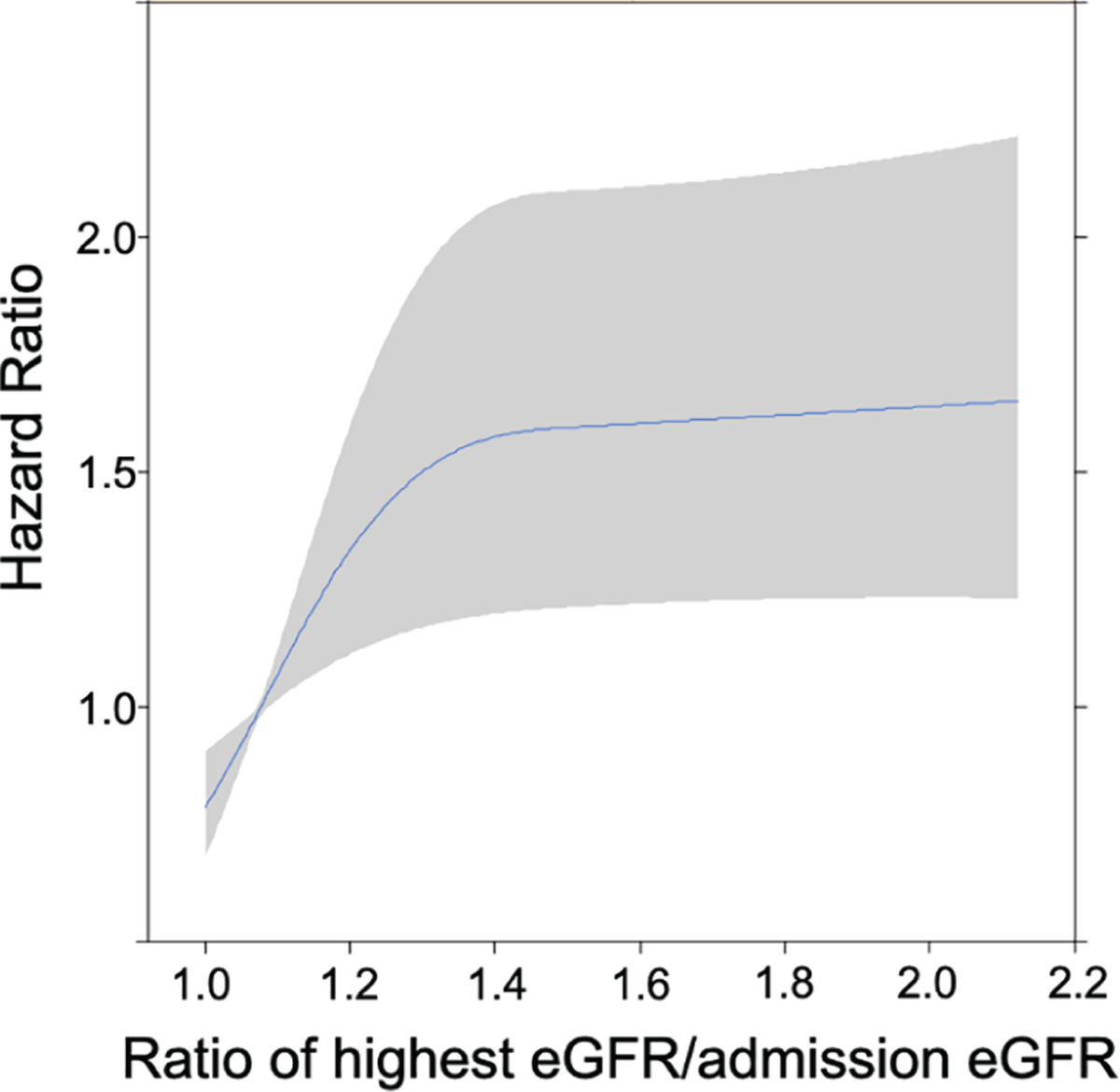
Unadjusted risk of 1-year mortality by the ratio of highest estimated glomerular filtration rate (eGFR) achieved during hospitalization to admission eGFR. Risk of mortality increased steeply with small improvements in eGFR; however, when eGFR improved ≥40% from admission eGFR, risk did not seem to increase more than the risk at 40% improvement. This is likely because few patients achieved an improvement in eGFR ≥40%.
Improving renal function patients had a significantly higher 1-year mortality than non-IRF patients (25% vs. 15%, P < 0.01 in unadjusted analysis; Figure 2A). The test for interaction between IRF (binary) and BNP (continuous) was statistically significant (P for interaction = 0.03), though the interaction of BNP on the risk estimate appeared more profound for non-IRF patients than IRF patients (online supplementary Figure S2). When patients were stratified by a trajectory of BNP decreasing ≥40% or <40%, those patients without IRF and with decreasing BNP had the lowest mortality (9%), followed by IRF patients with decreasing BNP (14%), then non-IRF patients without decreasing BNP (20%), and finally IRF patients without decreasing BNP had the highest mortality (31%, P < 0.01 in unadjusted analysis; Figure 2B). Compared to non-IRF patients with a decreasing BNP, unadjusted risk of mortality for IRF patients with decreasing BNP was not significantly different [hazard ratio (HR) 1.5, 95% confidence interval (CI) 0.7–3.3, P = 0.32], while non-IRF patients without decreasing BNP (HR 2.4, 95% CI 1.5–3.8, P < 0.01) and IRF patients without decreasing BNP (HR 3.8, 95% CI 2.3–6.4, P < 0.01) had significantly higher mortality risk. In univariate analysis, IRF was associated with a statistically significant 70% higher risk of 1-year mortality but was not associated with HF readmission (Table 2).
Figure 2.
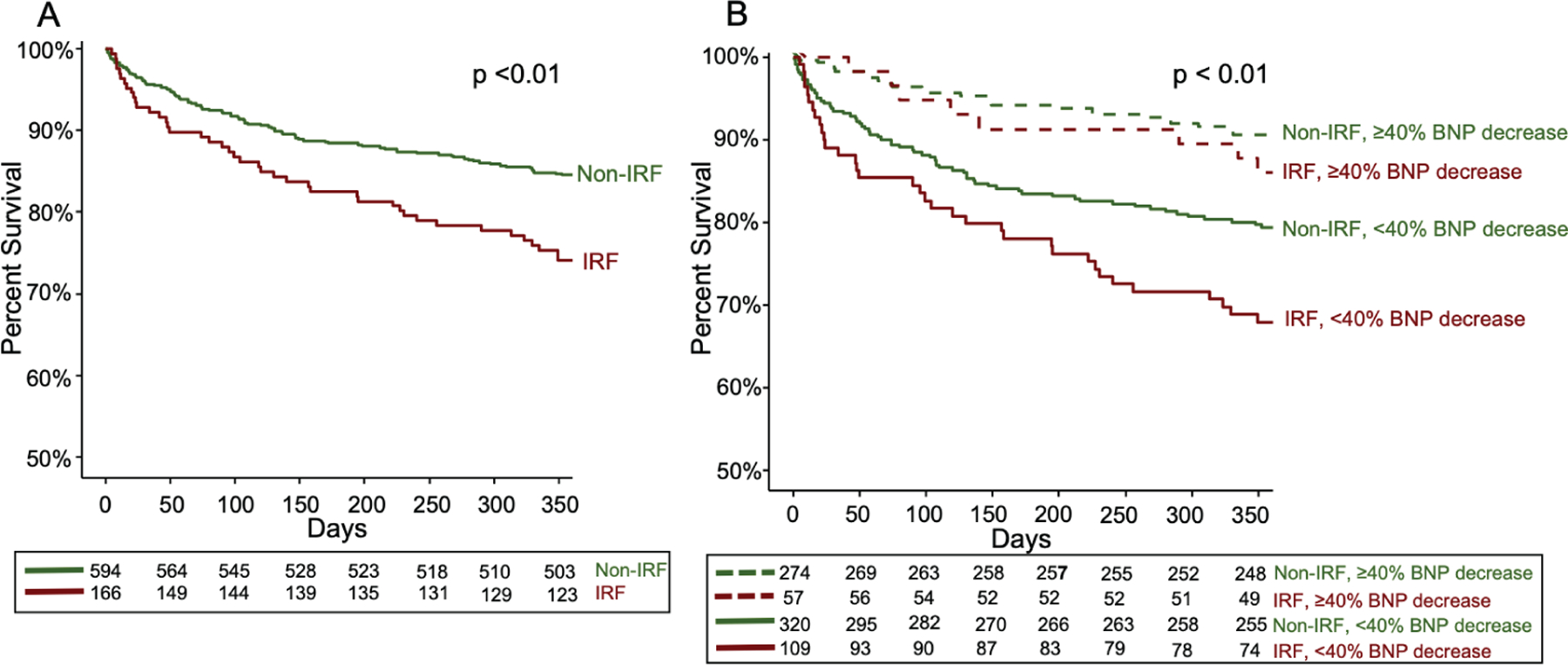
Survival curves for 1-year mortality for improving renal function (IRF) vs. non-IRF (A) and when IRF and non-IRF are further stratified by the presence or absence of a decreasing B-type natriuretic peptide (BNP) trajectory (B). Patients with IRF had worse 1-year survival than non-IRF patients (A). The presence or absence of a decreasing BNP trajectory further risk stratified IRF and non-IRF patients (B). Those patients achieving successful decongestion, as defined by a trajectory of decreasing BNP ≥40% from admission value, had improved survival compared to patients not achieving a decreasing BNP regardless of changes in renal function.
Table 2.
Univariable and multivariable Cox regression analysis for 1-year mortality and heart failure readmission by improved renal function and decreasing B-type natriuretic peptide
| HR (95% CI) |
|||
|---|---|---|---|
| Unadjusted | Model 1a | Model 2c | |
| Mortality | |||
| IRF | 1.7 (1.2–2.4) | 1.1 (0.8–1.7) | 1.0 (0.7–1.5) |
| Decreasing BNP | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) | 0.5 (0.3–0.7) |
| Unadjusted | Model 1b | Model 2c | |
|
| |||
| HF readmission | |||
| IRF | 1.1 (0.8–1.6) | 0.9 (0.6–1.4) | 0.9 (0.6–1.4) |
| Decreasing BNP | 0.9 (0.7–1.3) | 1.1 (0.8–1.5) | 1.0 (0.7–1.5) |
Bolded values have P-value <0.05.
BNP, B-type natriuretic peptide; CI, confidence interval; HF, heart failure; HR, hazard ratio; IRF, improved renal function.
Mortality model 1: age, white race, body mass index, chronic obstructive pulmonary disease, beta-blockers, rales, S3, systolic blood pressure, sodium, haemoglobin, blood urea nitrogen, and high-sensitivity cardiac troponin I.
HF readmission model 1: angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta-blockers, diuretics, systolic blood pressure, heart rate, and blood urea nitrogen.
Model 2: age, race, history of chronic obstructive pulmonary disease, oedema, systolic blood pressure, heart rate, sodium, haemoglobin, blood urea nitrogen, and high-sensitivity cardiac troponin I.
While IRF was associated with mortality in univariate models, in multivariable models IRF was not significantly associated with either mortality or HF readmission (Table 2, online supplementary Tables S1–S4). In contrast, a trajectory of BNP decreasing ≥40% was significantly associated with a lower risk for mortality, but not HF readmission (Table 2). Addition of discharge eGFR to these models did not change risk estimates for either outcomes (online supplementary Table S5). Spline analysis for risk of mortality by ratio of last BNP measured to admission BNP adjusted for multivariable models showed that the greater the trajectory of decreasing BNP, the lower the risk for mortality at 1 year (Figure 3).
Figure 3.
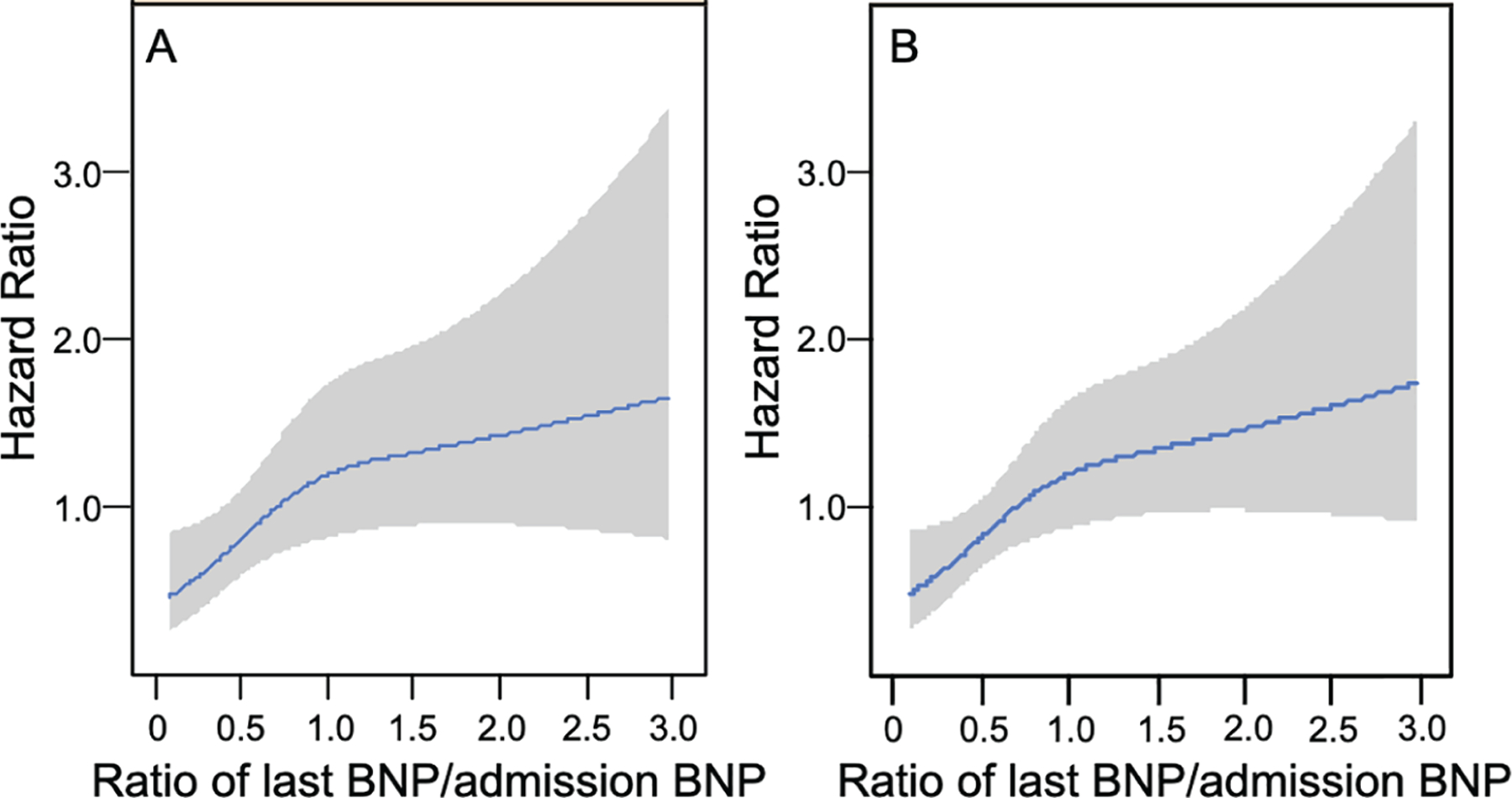
Adjusted risk of 1-year mortality by the ratio of last B-type natriuretic peptide (BNP) measured to admission BNP in multivariable model 1 (A) and model 2 (B). The greater the trajectory of decrease in BNP, the lower the risk of death.
To explore which factors attenuated the risk of IRF with mortality, we performed bivariate analysis with inclusion of each factor that was retained in our multivariable models. Lower admission SBP and higher admission BUN were each significantly associated with 1-year mortality and their inclusion each attenuated the association of IRF with mortality, rendering it no longer statistically significant. Other factors in the multivariate models minimally changed the risk estimate for IRF. Since BUN reflects cardiorenal interactions, we examined the correlation of admission eGFR and admission BUN and found them highly correlated (r = −0.69, P < 0.01). With a risk of collinearity, we examined the variance inflation factors in multivariate model 1 and 2 which were low at 1.14 and 1.23 for IRF and BUN, respectively, in model 1, and 1.10 and 1.19 for IRF and BUN, respectively, in model 2.
We also explored whether pre-admission kidney function influenced the relationship of IRF with mortality in the subgroup of 329 patients who had eGFR values available within 3 months prior to hospitalization. Given the reduced sample size, IRF was not significantly associated with 1-year mortality in univariable analysis. When evaluated in bivariate analysis, IRF was associated with a non-significant 53% higher risk of mortality before adjustment, which did not substantially change (non-significant 60% higher risk) with adjustment for pre-admission eGFR.
Outcomes with transient improvement of renal function (IRF) and persistent IRF vs. non-IRF
There were 60 patients (8%) with transient IRF and 106 patients (14%) with persistent IRF. Median times to transient IRF and persistent IRF were 5 days (IQR 3, 7 days) and 4 days (IQR 3, 7 days), respectively. Patients with non-IRF, transient IRF, and persistent IRF had significant differences for admission BUN, eGFR, SBP, and sodium (online supplementary Table S6).
Transient IRF patients had the highest 1-year mortality (30%) followed by persistent IRF (23%) then non-IRF (15%) (P < 0.01 in unadjusted analysis; online supplementary Figure S3A). When stratified by BNP decreasing ≥40% or <40%, non-IRF (7%), transient IRF (9%) and persistent IRF (17%) groups with decreasing BNP had lower 1-year mortality than non-IRF (20%), persistent IRF (27%) and transient IRF (38%) groups without decreasing BNP (P < 0.01 in unadjusted analysis; online supplementary Figure S3B). The test for interaction between persistent IRF (binary) and BNP (continuous) was significant (P for interaction = 0.04); however, between transient IRF (binary) and BNP (continuous) was not (P for interaction = 0.68).
In univariate analysis, transient IRF was associated with a 110% higher risk of 1-year mortality, while persistent IRF was not significantly associated with mortality, though there was broad overlap in CIs (Table 3). Neither was associated with HF readmission alone (Table 3). In multivariate analysis, transient IRF and persistent IRF were not associated with 1-year mortality, while BNP decreasing ≥40% was significantly associated with a lower risk for mortality (Table 3, online supplementary Tables S1 and S2). Addition of discharge eGFR did not change risk estimates (online supplementary Table S7).
Table 3.
Univariable and multivariable Cox regression analysis for 1-year mortality and heart failure readmission by transient improvement of renal function (IRF), persistent IRF and decreasing B-type natriuretic peptide
| HR (95% CI) |
|||
|---|---|---|---|
| Unadjusted | Model 1a | Model 2c | |
| Mortality | |||
| Transient IRF | 2.1 (1.3–3.4) | 1.1 (0.6–2.0) | 1.2 (0.7–2.0) |
| Persistent IRF | 1.5 (0.9–2.3) | 1.1 (0.7–1.9) | 0.9 (0.6–1.5) |
| Decreasing BNP | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) | 0.5 (0.3–0.7) |
| Unadjusted | Model 1b | Model 2c | |
|
| |||
| HF readmission | |||
| Transient IRF | 1.5 (0.9–2.4) | 1.5 (0.9–2.5) | 1.3 (0.8–2.3) |
| Persistent IRF | 0.9 (0.6–1.5) | 0.7 (0.4–1.2) | 0.7 (0.4–1.2) |
| Decreasing BNP | 0.9 (0.7–1.3) | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) |
Bolded values have P-value<0.05.
BNP, B-type natriuretic peptide; CI, confidence interval; HF, heart failure; HR, hazard ratio; IRF, improved renal function.
Mortality model 1: age, white race, body mass index, chronic obstructive pulmonary disease, beta-blockers, rales, S3, systolic blood pressure, sodium, haemoglobin, blood urea nitrogen, and high-sensitivity cardiac troponin I.
HF readmission model 1: angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta-blockers, diuretics, systolic blood pressure, heart rate, and blood urea nitrogen.
Model 2: age, race, history of chronic obstructive pulmonary disease, oedema, systolic blood pressure, heart rate, sodium, haemoglobin, blood urea nitrogen, and high-sensitivity cardiac troponin I.
Discussion
Among hospitalized AHF patients, we found patients with IRF had baseline characteristics consistent with more severe AHF. In the subgroup with eGFR measurements available prior to hospitalization, IRF patients had developed WRF prior to hospital admission with kidney function returning towards baseline levels during inpatient AHF treatment. IRF patients had higher 1-year mortality than non-IRF patients in unadjusted analysis. However, this association was attenuated after adjustment for admission BUN and SBP, known markers of more severe AHF.26 Importantly, we found mortality among IRF patients differed based on their BNP trajectories; those who had a ≥40% BNP decrease had lower mortality than patients who did not and the greater the trajectory of decrease in BNP, the lower the risk of mortality. When transient and persistent IRF were examined, findings were similar with respect to differential survival based on BNP trajectories. These findings give novel insights into the risk factors and clinical significance of IRF in AHF and emphasize the importance of achieving decongestion in AHF regardless of changes in kidney function.
Prior studies have reported that IRF in AHF is associated with an increased risk of mortality and rehospitalization.10,11 We found a strong association between IRF and mortality in univariate analysis but not after multivariate analysis. This risk attenuation was largely driven by higher admission BUN and lower admission SBP, both of which have been associated with mortality in hospitalized AHF patients.26 Indeed, IRF patients have features consistent with more severe HF at presentation including higher admission BUN, BUN/creatinine ratio, high-sensitivity cardiac troponin, and BNP, and lower SBP and serum sodium. This increased AHF severity is also reflected by the increased use of inotropes in IRF patients. Additionally, among the subset of patients with eGFR values available within 3 months of hospitalization, kidney function had worsened prior to admission in those who subsequently experienced IRF compared to the subset that did not. Supporting this, IRF patients had higher admission BUN, which was strongly correlated with admission eGFR. While BUN and IRF were found to be independent in multivariable models, BUN association with cardiorenal interactions cannot be fully discounted and a collinear relationship cannot be completely excluded.
When we examined transient and persistent IRF, we found only transient IRF was associated with worse outcomes in univariable analysis, though there was substantial overlap of the CIs for transient and persistent IRF. Again, the association of IRF with mortality was modified by the trajectory of change in BNP, where only BNP change and not IRF was associated with mortality in multivariable models. A prior study found only persistent IRF was associated with mortality, but this association was modified by congestion status with only persistent IRF patients who experienced a recurrence of WRF after discharge (recurrent congestion) having a higher risk of death.10 Thus, our findings are in line with this prior study, emphasizing the importance of achieving and maintaining decongestion in AHF regardless of trajectory of kidney function.
On its face, it would seem contradictory that IRF would be causally related to worse outcomes. An improvement in renal function would be expected to be a return to homeostasis and clinically associated with improved diuretic response and attempts at natriuresis. Consistent with this, we found other markers of severe AHF (BUN and SBP) attenuated the risk of IRF with mortality suggesting that IRF is not the cause of worse outcomes, but instead identifies high-risk AHF patients. Indeed, IRF may identify more severe AHF at presentation with susceptible kidneys that experience outpatient WRF prior to hospitalization and prompts more aggressive treatment with inotropes.12
Worsening renal function and IRF have been shown to have similar haemodynamic profiles and both are part of the haemodynamic continuum following dynamically changing kidney function in AHF.9–12 Pre-admission WRF that occurs in patients who experience IRF during AHF hospitalization likely results from increased central venous pressure (CVP) and renal congestion that improves with diuresis and reduction in CVP.3,10 Elevated venous pressure has been shown to increase neurohormonal activation and afferent arteriole resistance resulting in reduced eGFR and increased sodium reabsorption.27,28 Conversely, since other AHF patients develop WRF during diuresis where decongestion and reduced CVP lead to WRF, this would suggest the pathophysiologies of IRF and WRF during diuresis are distinct.12,29 In line with this, IRF patients had higher natriuretic peptide levels on admission suggesting greater congestion status. We and others have shown that change in BNP can discriminate WRF in AHF as clinically significant or not.6,16 Thus, we hypothesized that significant declines in BNP could further discriminate risk of IRF.
In this study, change in BNP was not associated with HF rehospitalization. While some prior studies have found an association between natriuretic peptides and risk of HF rehospitalization, prediction of HF rehospitalization remains difficult with prior models demonstrating poor predictive performance.30,31 BNP lack of association with readmission likely results from complexities beyond disease severity that BNP does not capture such as dietary choices, exercise habits, medication compliance and other social determinants. Additionally, recurrent congestion after discharge is a major driver of rehospitalization and outcomes, as shown in prior studies, but not measured in AKINESIS.10 Thus, it is not necessarily unexpected that both IRF and BNP changes were not associated with HF rehospitalization given these complexities.
Patients who experienced a ≥40% decline in BNP had lower 1-year mortality than patients who did not. Furthermore, the greater the trajectory of decrease in BNP, the lower the risk of mortality. Prior studies have reported an association of improved outcomes with a ≥30% decline in BNP while we found this association with a ≥40% decline. We hypothesize this difference in cut-offs arises because BNP levels decrease from both decongestion and increased clearance of BNP with IRF. These findings appear to modify the relationship of IRF with mortality and provide important information that may guide clinicians’ treatment decisions in AHF. When confronted with a patient with IRF, our findings and others show IRF identifies WRF prior to admission and more severe AHF at the time of admission. While IRF is not causally related to mortality, it identifies a high-risk patient population and potential modifiers of this mortality risk should be evaluated. This should be done by assessing congestion status as persons with IRF who have a trajectory of declining BNP during treatment have lower mortality risk. This finding emphasizes the importance of achieving adequate decongestion in AHF before discharge. Persons with IRF and without a reduction in BNP remain at high risk for mortality. We speculate that, for some patients with IRF, clinicians may be reticent to use diuretics or other medications that can decrease eGFR, reasoning that a trajectory of IRF reflects an improvement in end-organ perfusion and supports a less aggressive approach to fluid removal. Our data suggests the opposite, that instead IRF likely reflects WRF prior to admission and such patients should receive therapies to improve congestion. IRF without resolution of congestion, as assessed by changes in BNP, haematocrit, albumin or physical exam, indicates a need for further diuresis and optimization of disease-modifying therapies prior to discharge.32–34 An inability to achieve appropriate decongestion identifies a high-risk population where advanced therapies and advanced care planning should be considered (Graphical Abstract). Thus, clinicians should recognize that the AHF patient with IRF is a high-risk patient but should pursue therapies that promote decongestion and have less concern about short-term changes in kidney function.
Strengths and limitations
Our study has multiple strengths including its large sample size with patients recruited from 16 international sites. We had serial measures of BNP and eGFR allowing us to evaluate the prognostic information of BNP changes with eGFR changes concurrently. Availability of outpatient eGFR values within 3 months prior to admission in a sizable subset was also a key strength that provided valuable insights to the implications of IRF in this study.
Our study also has important limitations. As with any observational study, the influence of identified and unidentified confounders cannot be excluded. However, in this case, the associations of IRF were attenuated by available covariates that mark worse AHF, and therefore explained much of the association observed in unadjusted models. Thus, as the initial association was completely extinguished, remaining residual confounding seems unlikely. While BNP correlates with congestion and clinical outcomes, BNP levels can also be influenced by age, gender, race, obesity, kidney function and medical therapy. Since we examined BNP change on an individual level, many of these confounders are unchanged for an individual, but we cannot exclude confounders such as medical therapy or the change in kidney function influencing BNP levels. We lack adequate echocardiographic information on patients to include this information in our analyses. We also only have a limited number of physical exam findings recorded at study enrolment limiting our ability to explore change in congestion status by physical examination. Clinicians could have changed their treatment strategy in response to changes in kidney function; however, our data therefore also reflect real-world experiences in AHF management.
Conclusions
Patients with AHF who experience IRF have features consistent with more severe HF and often experience WRF as outpatients prior to hospital admission. IRF marks increased risk for 1-year mortality; however, this finding is explained by markers of worse AHF at presentation which rendered the association of IRF with mortality null in adjusted models. Both IRF and non-IRF patients who have a trajectory of decreasing BNP during their AHF treatment have better survival. Thus, clinicians should recognize IRF patients as a high-risk subset of AHF but should strive to achieve adequate decongestion regardless of short-term changes in kidney function.
Supplementary Material
Funding
The Acute Kidney Injury NGAL Evaluation of Symptomatic heart faIlure Study (AKINESIS) was funded by Abbott Laboratories (Chicago, IL, USA) and Alere, Inc (San Diego, CA, USA). They provided funding for study conduct at each investigational site during the conduct of the study. The sponsors assisted in the design of the study, data management, and study oversight. The sponsors did not participate in the analysis presented in this manuscript or preparation, review or approval of the manuscript.
N.W. and this work was supported (or supported in part) by Career Development Award Number IK2 CX002105 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service. The contents do not represent the view of the U.S. Department of Veterans Affairs or the United States Government. J.H.I. was supported by an award from the National Institutes of Diabetes and Digestive and Kidney Disease (K24 DK110427). S.D. received a Newman Fellowship in Acute Kidney Injury from the UCD Foundation, Dublin.
Footnotes
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest: J.H.I.: Investigator initiated research study from Baxter International, DSMB member for Sanifit Pharmaceuticals, Advisory Board member for AstraZeneca and Alpha Young. C.M. has received research support and speaker/consulting honoraria from several biomarkers companies but none directly related to this work. G.F. has no relevant disclosures directly related to the submitted manuscript; separate from submitted work, he is a trial member for Medtronic, Vifor, Boehringer Ingelheim, Bayer, Servier, Amgen and Novartis; he receives lecture fees from Servier, Novartis, and Boehringer Ingelheim. R.N. received grant funding from Abbott and Alere that ended in 2015. R.B. has received prior grant funding from Alere and current grant funding from Abbott; separate from this submitted work he always receives grant funding from Siemens and Lumos Diagnostics. P.T. has no disclosures directly related to the work submitted; she receives grant funding from the National Institutes of Health, Department of Homeland Security, and American Heart Association for unrelated research; she also receives consultant fees from Amgen, Novo-Nordisk, Esperion Therapeutics, Boehringer Ingelheim, and Sanofi. A.M. previously received grant funding from Abbott Laboratories and Alere Inc.; currently, he is a co-founder of Brainstorm Medical. P.T.M. previously received research funding from Abbott Laboratories and Alere Inc.; he currently received educational grant funding from Abbott; he also currently receives consulting fees from FAST biomedical. All other authors have nothing to disclose.
References
- 1.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P; Acute Dialysis Quality Initiative (ADQI) Consensus Group . Cardio-renal syndromes: report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J 2010;31:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 4.Aronson D, Abassi Z, Allon E, Burger AJ. Fluid loss, venous congestion, and worsening renal function in acute decompensated heart failure. Eur J Heart Fail 2013;15:637–643. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Mueller GA, Birkhahn R, Taub P, Vilke GM, Barnett O, McDonald K, Mahon N, Nunez J, Briguori C, Passino C, Murray PT, Maisel A. B-type natriuretic peptide trend predicts clinical significance of worsening renal function in acute heart failure. Eur J Heart Fail 2019;21:1553–1560. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012;5:54–62. [DOI] [PubMed] [Google Scholar]
- 8.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011;13:877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol 2010;106:1763–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail 2011;17:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail 2016;22:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, Tang WH, Skouri H, Verbrugge FH, Orso F, Hill L, Ural D, Lainscak M, Rossignol P, Metra M, Mebazaa A, Seferovic P, Ruschitzka F, Coats A. Evaluation of kidney function throughout the heart failure trajectory–a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:584–603. [DOI] [PubMed] [Google Scholar]
- 13.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–2368. [DOI] [PubMed] [Google Scholar]
- 14.Omar HR, Guglin M. Longitudinal BNP follow-up as a marker of treatment response in acute heart failure: relationship with objective markers of decongestion. Int J Cardiol 2016;221:167–170. [DOI] [PubMed] [Google Scholar]
- 15.Shah MR, Hasselblad V, Tasissa G, Christenson RH, Binanay C, O’Connor CM, Ohman EM, Stevenson LW, Califf RM. Rapid assay brain natriuretic peptide and troponin I in patients hospitalized with decompensated heart failure (from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Trial). Am J Cardiol 2007;100:1427–1433. [DOI] [PubMed] [Google Scholar]
- 16.Salah K, Kok WE, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, Verdiani V, Tijssen JG, Pinto YM. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail 2015;3:751 –761. [DOI] [PubMed] [Google Scholar]
- 17.Maisel AS, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Muller GA, Birkhahn R, Clopton P, Taub P, Vilke GM, McDonald K, Mahon N, Nunez J, Briguori C, Passino C, Murray PT. Neutrophil Gelatinase-Associated Lipocalin for Acute Kidney Injury During Acute Heart Failure Hospitalizations: The AKINESIS Study. J Am Coll Cardiol 2016;68:1420–1431. [DOI] [PubMed] [Google Scholar]
- 18.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. Jama 2003;290:2581–2587. [DOI] [PubMed] [Google Scholar]
- 19.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF Jr, Gheorghiade M, O’Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail 2004;10:460–466. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki H, Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Yamamoto Y, Yokoyama S, Asai K, Shimizu W. New scoring system (APACHE-HF) for predicting adverse outcomes in patients with acute heart failure: evaluation of the APACHE II and modified APACHE II scoring systems. J Cardiol 2014;64:441–449. [DOI] [PubMed] [Google Scholar]
- 22.Scrutinio D, Ammirati E, Guida P, Passantino A, Raimondo R, Guida V, Sarzi Braga S, Pedretti RF, Lagioia R, Frigerio M, Catanzaro R, Oliva F. Clinical utility of N-terminal pro-B-type natriuretic peptide for risk stratification of patients with acute decompensated heart failure. Derivation and validation of the ADHF/NT-proBNP risk score. Int J Cardiol 2013;168:2120–2126. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol 2010;55:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peacock WF 4th, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–2126. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor CM, Fiuzat M, Lombardi C, Fujita K, Jia G, Davison BA, Cleland J, Bloomfield D, Dittrich HC, Delucca P, Givertz MM, Mansoor G, Ponikowski P, Teerlink JR, Voors AA, Massie BM, Cotter G, Metra M. Impact of serial troponin release on outcomes in patients with acute heart failure: analysis from the PROTECT pilot study. Circ Heart Fail 2011;4:724–732. [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–580. [DOI] [PubMed] [Google Scholar]
- 27.Doty JM, Saggi BH, Sugerman HJ, Blocher CR, Pin R, Fakhry I, Gehr TW, Sica DA. Effect of increased renal venous pressure on renal function. J Trauma 1999;47:1000–1003. [DOI] [PubMed] [Google Scholar]
- 28.Abildgaard U, Amtorp O, Holstein-Rathlou NH, Agerskov K, SjØNtoft E, Christensen NJ, Leyssac PP. Effect of renal venous pressure elevation on tubular sodium and water reabsorption in the dog kidney. Acta Physiol Scand 1988;132:135–142. [DOI] [PubMed] [Google Scholar]
- 29.Duff S, Murray PT. Defining early recovery of acute kidney injury. Clin J Am Soc Nephrol 2020;15:1358–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, Kripalani S. Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frizzell JD, Liang L, Schulte PJ, Yancy CW, Heidenreich PA, Hernandez AF, Bhatt DL, Fonarow GC, Laskey WK. Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMA Cardiol 2017;2:204–209. [DOI] [PubMed] [Google Scholar]
- 32.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival. J Am Coll Cardiol 2013;62:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, Sarnak MJ. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail 2020;8:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M; EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013;34:835–843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


