Abstract
Objective:
The effects of sanitation and hygiene interventions on the gut microbiome and enteric pathogen burden are not well understood. We measured the association between free chlorine residue (FCR) levels in drinking water, microbiome composition, and stool enteric pathogens in infants and young children in Haiti.
Methods:
FCR levels were measured in household drinking water and enteric pathogen burden was evaluated using multiplex RT-PCR of stool among 131 children from one month to five years of age living in Mirebalais, Haiti. Microbiome profiling was performed using metagenomic sequencing.
Results:
Most individuals lived in households with undetectable FCR measured in the drinking water (112/131, 86%). Detection of enteric pathogen DNA in stool was common and did not correlate with household water FCR. The infant microbiome in households with detectable FCR demonstrated reduced richness (fewer total number of species, P = 0.04 Kruskall–Wallis test) and less diversity by Inverse Simpson measures (P = 0.05) than households with undetectable FCR. Infants in households with a detectable FCR were more likely to have abundant Bifidobacterium. Using in vitro susceptibility testing, we found that some Bifidobacterium species were resistant to chlorine.
Conclusions:
FCR in household drinking water did not correlate with enteric pathogen burden in our study.
Keywords: Gut microbiome, Chlorine, Infant microbiome, Free chlorine residual
Introduction
Repeated episodes of diarrhea cause stunting, and occur primarily in young children who lack access to safe water, adequate sanitation and healthcare [1,2]. Diarrheal disease remains a leading cause of death in children under 5 in Haiti [3], where the lack of safe water is manifested by an ongoing cholera outbreak that is associated with a high attack rate among young children [4].
While large scale public sanitation and water infrastructure has eliminated the transmission of waterborne pathogens, the impact of household-based water, sanitation, and hygiene (WASH) interventions on individual health is less clear. For example, point-of-use addition of chlorine to drinking water to maintain a level of ≥0.2 mg/L for pathogen inactivation, is considered a cornerstone of many WASH efforts [5,6], yet specific impacts on overall health remain unclear. A 2015 metanalysis of more than 30,000 participants estimated that home chlorination products resulted in a 23% reduction in the relative risk of diarrheal illness, but the evidence was considered low quality [7]. Since then, three rigorous clinical trials of household WASH interventions showed that combined point-of-use interventions, including household water chlorination, did not reduce stunting in young children [8–10].
Beyond a reduced likelihood of infection from specific pathogens [11], the other impacts of chlorinated water are not well understood. For example, an intact and diverse gut microbiota can prevent malnutrition and enteric infections [12], and specific gut microbial profiles are correlated with susceptibility to diarrheal pathogens [13,14]. Few studies have directly assessed the relationship between chlorinated household water and the gut microbiota. In one study, after one year of chlorinated water exposure, children had an increased abundance of microbes thought to be beneficial for gut health, and chlorine did not appear to alter overall diversity of the microbiome in treated children compared to untreated children [15]. The goal of our study was to gain a deeper understanding of the effect of chlorinated water on the gut bacteria, we measured the gut microbiome in a sample of children <1 to 59 months of age in the Centre Department of Haiti. Here, we identified specific gut microbial groups that may be impacted by chlorinated drinking water. Detectable FCR levels were not associated with enteric pathogen burden but were correlated with reduced microbial diversity and a higher abundance of Bifidobacterium in infants ≤ 12 months of age. To determine whether this association had a potential biological basis we evaluated chlorine resistance in Bifidobacterium strains and compared this with other commensal bacteria using isolates obtained from an independent cohort of healthy individuals from Bangladesh.
Methods
Recruitment and enrollment
Participants were recruited in Mirebalais, a community in the Central Plateau of Haiti, between May and July 2017, as part of a larger cross-sectional study conducted in the context of a public health campaign on the feasibility of a dual intervention combining oral cholera vaccine and household water treatment. During a census for this campaign, a subset of households was randomly invited to participate in a clinical and demographic survey, and a subsample of households with young children < 1–59 months of age were selected for bacteriologic assessment with consent of a parent/guardian.
Household questionnaire and interview procedures
Household questionnaires pertaining to household WASH practices, food security, nutrition, and vaccinations were administered, and direct observation was conducted by trained study staff. Diarrhea was defined as 3 or more watery stools in 24 hours. Middle upper arm circumference (MUAC) was measured by trained staff. Key WASH variables assessed by direct observation included access to hand washing facilities, type of toilet, and trash/garbage around the home environment.
Household water collection and chlorine measurement
Water was collected from the household’s main drinking water storage unit as identified by the head of household, and immediately tested in the field by trained staff. Levels of free chlorine were measured using the LaMatte Chlorine Kit by a trained laboratory technician. Briefly, a chlorine diethyl-p-phenylenediamine #1 tablet was added to 5 mL of the water sample and mixed until fully dissolved. The sample was then viewed using the OctaSlide Viewer using a color standard and the ppm (or mg/L) was recorded as free residual chlorine (FCR). Measures of <0.2 mg/L were at the limit of detection of the kit and referred to as undetected (und.). FCR values greater than 0.5 mg/L were defined as “high” in this analysis, based on the range of FCR observed in the household drinking water in our study.
DNA extraction from rectal swabs
Two sterile flocked rectal swabs were obtained in the field from each participating child between <1 and 59 months and stored frozen at −80°C until they were shipped to the Massachusetts General Hospital, Boston, USA, for microbial DNA extraction and additional analysis.
In Boston, USA genomic DNA from rectal swabs was isolated using the PowerLyzer PowerSoil kit (Qiagen) per the manufacturer’s instructions. Briefly, swabs were treated with a lysis buffer and incubated at 95°C for 10 minutes prior to bead beating for 10 minutes. DNA was eluted in 100 μL of molecular-grade water. Half of the eluate was submitted for metagenomic sequencing, and the remainder was retained for enteric pathogen qPCR. Microbiome sequencing and analysis and qPCR methods are in the Supplementary Methods. Whole-genome sequencing data can be found with NCBI Bioproject number PRJNA1067625 through the Sequence Read Archive.
Chlorine disc diffusion assay
We used bacteria representative of specific groups (see Results) to test gut microbe susceptibility to chlorine. Because bacterial isolates from our study participants were not available, we used bacterial isolates from the stool of study participants from Bangladesh because Bangladesh is also a cholera-endemic area. Isolates are described in the Supplementary Methods. Using the Kirby-Bauer Disk diffusion method, we applied variable concentrations of sodium hypochlorite (NaOCl) solution, a compound commonly used for water purification and surface cleaning; treatment of drinking water with <10 Nephelometric Turbidity units (NTU) with 1.88 mg/L NaOCl maintains an FCR ≥ 0.2 mg/L for 24 hours [16]. Bacterial strains were grown from frozen glycerol stocks streaked onto the specified agar at 37°C for 48 hours (Supplementary Table 1). To mimic the intestinal environment, liquid cultures for this experiment were incubated in anaerobic conditions at 37°C. Single colonies were inoculated into 5 mL of supplemented Brain-Heart-Infusion broth. After 48 hours, 100 μL was plated as a lawn onto a TSA (Tryptic soy agar) plate. Filter paper discs were treated with NaOCl solution, as described below, and placed on streaked agar plates and incubated anaerobically. After 48 hours, we measured the zone of inhibition, defined as the distance between the edge of the disc to the edge of bacterial growth ring. Filter paper antimicrobial susceptibility discs (Oxoid, Thermo Scientific) were aseptically inoculated with 20 μL of NaOCl solution (Fisher) at 6%, 3%, and 1% concentrations. The highest concentration was selected to approximate the concentration used in many household cleaning materials [16]. A disk treated with phosphate buffered saline (Corning) was used as a control.
Statistics
Statistical analyses and figures were prepared in Prism v9.2 (GraphPad Software, San Diego, CA). Comparisons of enteric qPCR results between age groups and FCR levels were tested using multiple T-tests (Mann-Whitney). One-way ANOVA was used to compare richness and Inverse Simpson measures between FCR levels, and analysis of molecular variance (AMOVA) (a non-parametric analysis of variance) was performed on microbiome data using Bray-Curtis distances at the specified bacterial ranks. Comparing relative abundance of select microbial families was tested using 2-way ANOVA. One-way ANOVA was used to test differences between the zones of inhibition in chlorine in vitro assays.
Results
Participant characteristics and WASH practices
We enrolled 131 participants <1 to 59 months of age from 108 households in the Centre Department of Haiti. Participants were grouped into two pre-determined age strata based on established observations of gut microbiota differences in young children [17]. Among participants, 41 were infants, defined as children ≤ 12 months of age (31%) and nearly half reported watery diarrhea in the previous two weeks (18/41, 44%, Table 1). Among young children, defined as >12 to 59 months), less than 30% reported watery diarrhea in the two weeks prior to the interview (26/90). Most households of infants and/or young children were found to have undetectable FCR at the time of the interview (32/41, 78% and 80/90, 88%, respectively, Table 1). We did not find a relationship between report of recent diarrhea and household FCR level (Odds of detectable FCR in drinking water in a household in which the child experienced diarrhea within two weeks, 2.46, 95% CI 0.89–6, P = 0.08, Fisher’s exact test).
Table 1.
Patient demographics, host factors, and WASH elements.
| Infants ≤ 12 months |
Young children > 12–59 months |
||||
|---|---|---|---|---|---|
| N = 41 | % | N = 90 | % | ||
|
| |||||
| Age | Average (mo), range | 7 (0–12) | 32 (14–57) | ||
| Sex | Female | 15 | 36·6 | 39 | 43·3 |
| Male | 26 | 63·4 | 51 | 56·7 | |
| Diarrhea during prior two weeksa | Watery | 18 | 43·9 | 26 | 28·8 |
| With blood | 4 | 9·8 | 10 | 11·1 | |
| FCR (mg/L) | Undetected | 32 | 78·0 | 80 | 88·9 |
| 0·2–0·3 | 3 | 7·3 | 2 | 2·2 | |
| >0·5 (range 0·9–3) | 6 | 14·6 | 8 | 8·9 | |
| MUAC measurement | Well-nourished | 35 | 85·4 | 72 | 80 0 |
| At risk for MAM | 1 | 2·4 | 2 | 2·2 | |
| MAM | 0 | 0 | 0 | 0 | |
| Missing | 5 | 12·2 | 16 | 17·8 | |
| Vitamin A supplementation within 6 monthsa | Yes | 27 | 65·9 | 58 | 64·4 |
| No | 13 | 31·7 | 27 | 30 0 | |
| Missing | 1 | 2·4 | 5 | 5·6 | |
| Zinc supplementation within 6 monthsa | Yes | 25 | 61·0 | 51 | 56·7 |
| No | 15 | 36·6 | 34 | 37·8 | |
| Missing | 1 | 2·4 | 5 | 5·6 | |
| Currently breastfeda | Yes | 34 | 82·9 | 16 | 17·8 |
| No | 6 | 14·6 | 33 | 36·7 | |
| Missing | 1 | 2·4 | 41 | 45·6 | |
| Toileta | Flush toilet | 0 | 0 | 3 | 3·3 |
| Ventilated, improved latrine | 11 | 26·8 | 19 | 21·1 | |
| Pit latrine with slab | 5 | 12·2 | 8 | 8·9 | |
| Pit latrine without slab | 10 | 24·4 | 17 | 18·9 | |
| Bucket | 0 | 0 | 1 | 1·1 | |
| No toilet | 6 | 14·6 | 17 | 18·9 | |
| Other | 0 | 0 | 4 | 4·4 | |
| Missing | 9 | 22·0 | 21 | 23·3 | |
| Soap use in householda | Almost never | 3 | 7.3 | 12 | 13·3 |
| Sometimes | 19 | 46·3 | 37 | 41·1 | |
| Often | 13 | 31·7 | 29 | 32·2 | |
| Almost always | 6 | 14·6 | 12 | 13·3 | |
| Trash/garbage observed in/around householdb | Yes | 11 | 26·8 | 23 | 25·6 |
| No | 30 | 73·2 | 67 | 74·4 | |
MUAC = mid-upper arm circumference. MAM = moderate acute malnutrition.
Data reported by parent/guardian report.
Wash elements measured by field staff observation (“Was trash seen in the yard or around the house?”).
Very few study participants had evidence of malnutrition as measured by MUAC, and most participants had received vitamin A and zinc supplements during the prior six months by report (Table 1). Among the infants enrolled, 82% were currently breastfeeding (34/41) and 18% of young children were currently breastfeeding (16/90); information on exclusive breastfeeding was not collected. Toileting infrastructure in the study households included flush toilets, pit latrines, and no toilet (Table 1). Soap use in the household was reported by the parent/guardian (Table 1).
Enteric pathogen burden was not associated with FCR
Presence of enteric pathogen DNA was measured using a multiplexed probe-based qPCR assay. In this study population, EAEC was highly prevalent among infants (36/41, 88%) and young children (64/90, 71%) (Figure 1). C. jejuni was the second most abundant detected pathogen among infants (20/41, 49%) while ETEC was second most abundant among young children (27/90, 30%). The DNA of other several other selected enteric bacterial pathogens was detected rarely, including C. coli, Salmonella, Shigella, V. cholerae, and Cryptosporidium. FCR levels did not correlate with the presence or quantity of enteric pathogen DNA in either group (Supplementary Figure 1). Individual pathogen-specific virulence genes were measured and did not differ between observed FCR levels (Supplementary Figure 2). We found no difference in pathogen DNA abundance in stool between high and low FCR households.
Figure 1.
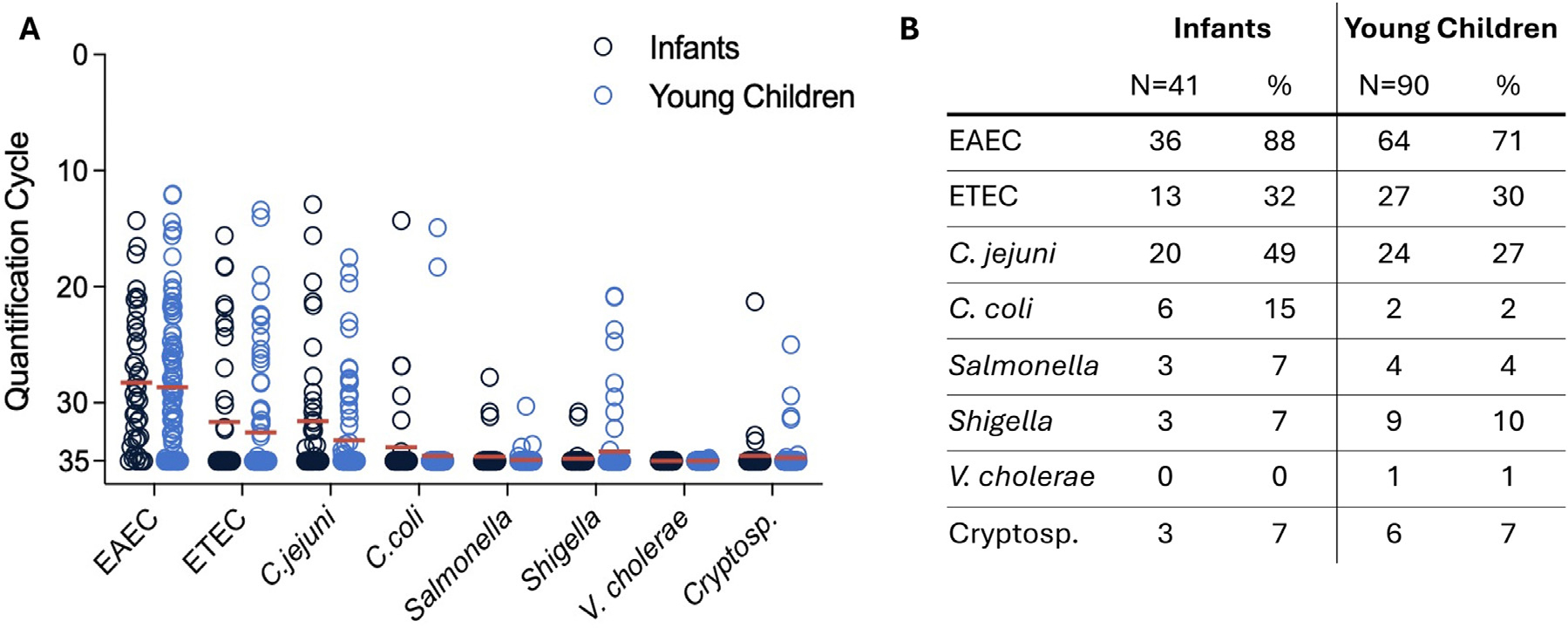
Semi-quantitative pathogen load in participant stool. Measured by multiplex qPCR, enteric pathogen burden is represented as quantification PCR cycles, with limit of detection at 35 cycles.34 (a) Quantification cycle of pathogen with each dot represents one participant and mean is shown with the horizontal red line. Multiple T-tests (Mann-Whitney), all q values >0·05 after multiple comparisons FDR. (b) Table of pathogen detection in percentage of participants. EAEC: Enteroaggregative E. coli, ETEC: Enterotoxigenic E. coli, C. jejuni: Campylobacter jejuni, C. coli: Campylobacter coli, V. cholerae: Vibrio cholerae, Cryptosp.: Cryptosporidium. Infants were defined as ≤12 months of age, and young children were defined as 12–59 months of age.
Gut microbial community structure corresponded with age, breastfeeding status, and household
As expected, the gut microbial community structure varied by age and by breastfeeding status (BF). Infants had higher levels of Actinobacteria and lower overall diversity by Inverse Simpson Index, a measure that quantifies average proportional abundances (Supplementary Figure 3a–c). The overall community structure between the infants and young children’s fecal microbiota differed (ANOVA, P < 0.001, Supplementary Figure 3d). Breastfeeding is known to be correlated with specific bacterial groups in the infant gut microbiota [18]. We found that in comparing BF status among all ages, there were differences in gut microbial composition in the BF group that were similar to the infant microbiome, while the non-BF group microbial composition of infants more closely resembled that of the young children (Supplementary Figure 4a). However, the magnitude of microbiome structure difference between age groups was more prominent than the differences attributable to breastfeeding (F test ratio of variance comparing group means of BF versus non-BF, relative to within-age group variability. Infant F test = 9.04; Young Children, F test = 11.06). We also examined gut microbial community clustering (“relatedness”) by household in order to account for household similarities in the microbiome. We found that participants from the same household did not demonstrate significant clustering (analysis of similarity, ANOSIM, P = 0.45, Supplementary Figure 4b). Compared to infants, young children overall had higher gut microbial diversity and increased Firmicutes, with less Actinobacteria and Bifidobacteriaceae (Supplementary Figures 3, 5).
Free chlorine residual levels in drinking water correlated with differences in infant microbiome
Infants in households with high FCR in household drinking water were found to have decreased species richness, indicating that fewer unique species were present within the microbiome (P = 0.04, Figure 2a). Overall species diversity, measured using the Inverse Simpson index to assess richness and evenness of the microbiome population, revealed decreased diversity in infants from high FCR households (mean ± SD= 5.9 ± 3.7) compared to infants in households with undetectable FCR (13.02 ± 8.9, Mann-Whitney t-test, P = 0.045, Figure 2b). The microbial composition of individuals from high FCR households was tightly clustered (composition was more similar to one another) compared to infants from households with undetectable FCR, and the overall compositions of these two groups were significantly different (measured by the distance from the centroid of the principal coordinates analysis (PCoA) ellipses, AMOVA, P = 0.049, Figure 2c). The gut microbiota of infants in this study population was dominated by Bifidobacteriaceae (Figure 2d). Additionally, visualization of microbial abundances in participants from households with undetectable FCR had a high stool abundance of Firmicutes families including Streptococcaceae, Lachnospiraceae, and Ruminococcaceae compared to individuals from households with high FCR (Figure 2d). To understand which bacterial families were responsible for the divergence of PCoA axes between these groups, we calculated the Pearson correlation coefficient of each bacterial family and found that the presence and/or abundance of Bifidobacteriaceae drove overall gut microbial variation in individuals leftward along the horizontal axis of the PCoA (position on axis1, −0.968, P < 0.001, length from center = 0.985, Figure 3a). Conversely, participants from households with a high FCR had significantly lower Streptococcaceae abundance (Figure 3b). Based on these results, we hypothesized that Bifidobacteriaceae may be more resistant to free chlorine than other abundant gut microbial species.
Figure 2.
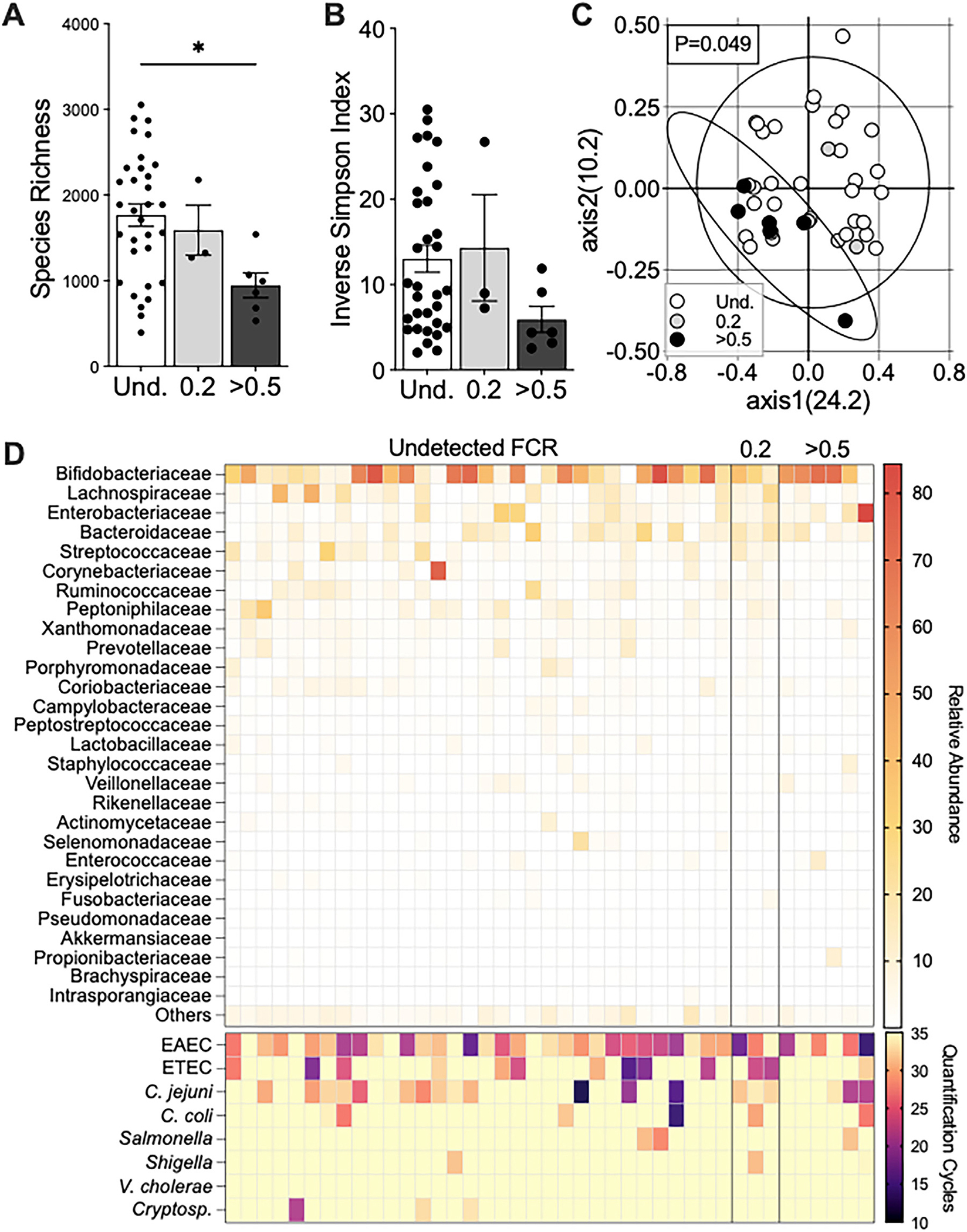
Infants in households with high FCR in the drinking water have a less diverse microbiome. Rectal swabs of participants were sequenced for bacteria using whole-genome sequencing. (a) Species richness with Kruskall–Wallis test and Dunn’s multiple comparisons test, P = 0.04 between undetected (Und.) and >0.5 FCR groups. (b) Alpha-diversity using Inverse Simpson by Kruskall–Wallis test (P > 0.05). Mann-Whitney test of Und. and >0·5 FCR, P = 0·045. Data shown as mean ± SEM; each dot resembles a participant. (c) Beta-diversity using Bray Curtis Dissimilarity index at the family level; values in parentheses are percent explained by axes and ellipses drawn on 95% CI; P = 0·049 for AMOVA comparison of ellipse centroid position. Three samples with FCR of 0·2 are removed because sample size is insufficient for the comparison. (d) Heatmap representation of family-level abundance (top) and enteric pathogen burden (bottom) per participant.
Figure 3.
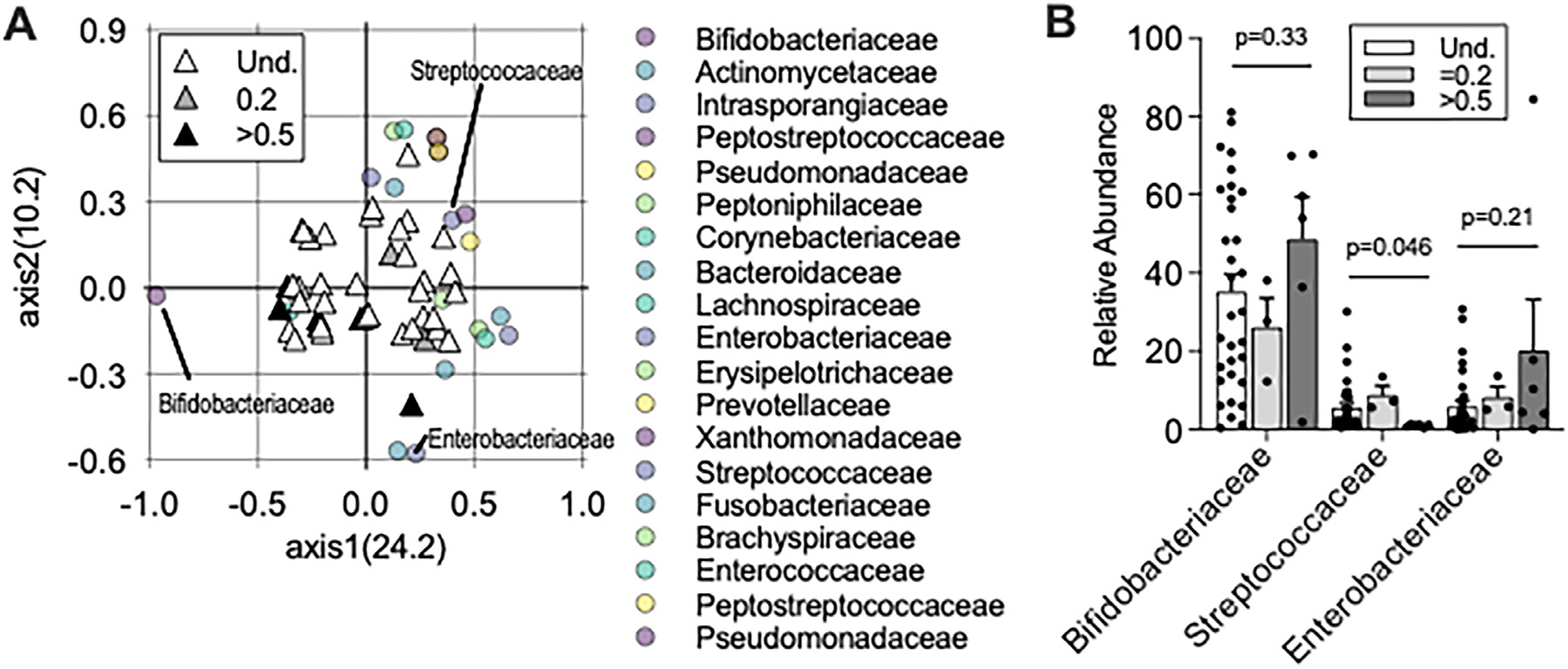
Infants in households with high FCR were predominant in Bifidobacteriaceae. (a) PCoA of Bray-Curtis Dissimilarity index using family-level identification of infant microbiota; values in parentheses are percent explained by axes. Correlation coefficients were calculated for each taxonomic family and plotted overlying the individual study participant PCoA. Triangles = Shading represents FCR levels in households, and one triangle is shown per individual. Circles = bacterial families. Only bacterial families with greater than 1% microbial abundance and a P value < 0·01 Pearson correlation coefficient are shown. (b) Relative abundance of 3 abundant microbial families shown by FCR levels. Mann-Whitney test of Undetected (Und.) and >0·5 FCR is shown; 2way ANOVA comparison among all three groups is P > 0·05.
The gut microbiota did not differ based on WASH practices
The gut microbiota community composition of young children did not differ by household water FCR levels (Supplementary Figure 5). Toilet type and staff observation of garbage around the household were not associated with differences in gut microbiome diversity or community structure in either group (Supplementary Figures 6, 7). In this study population with limited sample size, we also did not find microbiome variation between sexes, children reported to have diarrhea in the two weeks prior to interview, or those who reported taking Vitamin A or Zinc supplementation (Supplementary Figures 6, 7).
Bifidobacterium species were resistant to chlorine killing compared to other commensal gut microbes
To test our hypothesis of gut microbial resistance to chlorine in the infant gut microbiota, we measured bacterial susceptibility to killing by chlorine. In our analysis of common gut microbes identified in this population, species in the Streptococcaceae and Enterobacteriaceae families demonstrated dose-dependent growth inhibition by chlorine (Figure 4). Streptococcal species S. salivarius and S. agalactiae were associated with the highest axis1 values when we mapped species onto the infant level microbiota PCoA by FCR level (Supplementary Figure 8). Based on these observations, we suspected that the PCoA axis1 was inversely correlated with chlorine susceptibility. Consistent with this finding, Enterobacteriaceae species were also highly susceptible to chlorine and associated with high axis1 values.
Figure 4.
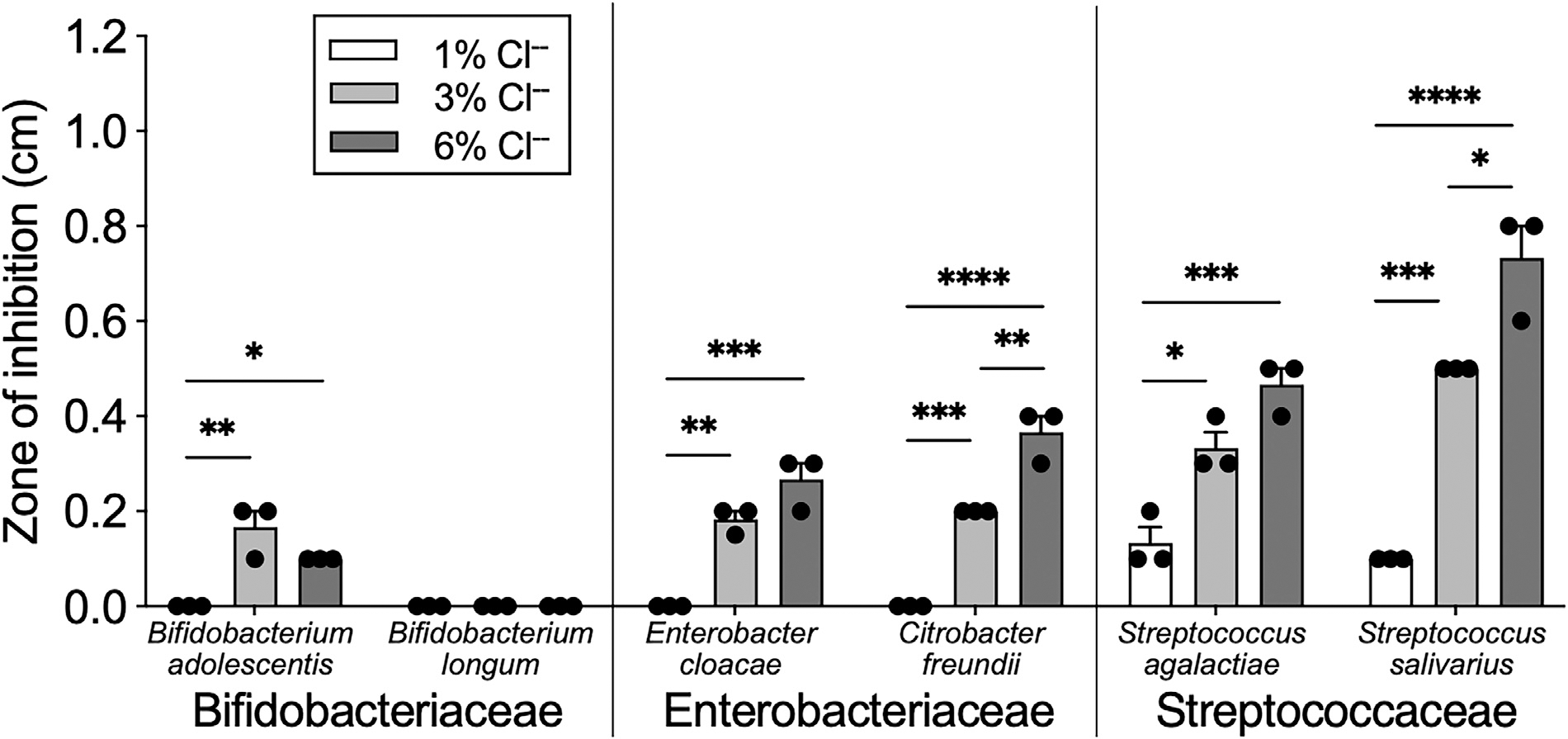
Bifidobacterium species are resistant to high levels of chlorine. Chlorine susceptibility was determined using Kirby-Bauer disk diffusion method.19 Zone of inhibition was measured as the distance between chlorine disk and edge of bacterial growth. All microbes were grown on TSA broth and cultured in anaerobic conditions for 48 hours at 37°C. Bars represent mean ± SEM with dots representing a biological replicate. One-way ANOVA with multiple comparisons for each species; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
We found that two Bifidobacterium strains, B. adolescentis and B. longum, exhibited little to no growth inhibition to chlorine (Figure 4). Additional strains were tested, and this pattern was consistent across both species (Supplementary Figure 9). When mapped onto the species-level infant gut microbiota by FCR, B. longum was found to have negative axis1 values, as expected, demonstrating a negative correlation between FCR and B. longum presence. Few other gut microbes in this cohort demonstrated the same presence/absence pattern according to FCR level (Supplementary Figure 9).
Discussion
Efforts to reduce the incidence of enteric infections through access to safe water and sanitation are critical for reducing childhood morbidity and mortality. Although treatment of water through chlorination is a widely utilized intervention for reduction of enteric pathogen burden [6,19,20], few studies address how chlorination affects the gut microbiome, a host factor that is critical for resistance to infection. Here we assessed the gut microbiome of infants and young children in central Haiti, in the context of WASH interventions including chlorination of household drinking water. Despite provision of chlorine tablets as part of a public health intervention, and an ongoing epidemic of cholera in the country, most households did not have detectable FCR in the household water. We did not find a relationship between FCR level in household water and report of recent diarrhea or of enteric pathogen genomic DNA in rectal swabs, and we observed that the gut microbiota of infants in high FCR households was skewed towards bacterial species typically associated with a healthy gut microbiota. Furthermore, the microbiome composition of infants in high FCR households included a higher abundance of Bifidobacterium, a bacterial group that was more resistant to killing by chlorine than other gut microbes.
We found that overall, young Haitian children had a Firmicutes-dominant microbiome with high species diversity, while infants had higher abundance of Bifidobacterium. Breastfed infants typically have a Bifidobacterium-dominant gut microbiota, distinct from non-breastfeeding infants and young children [17,21]. This relationship is thought to exist because Bifidobacteria assist in digestion of human milk oligosaccharides, and flourish in the setting of breastmilk [22]. As the infant grows and ingests other sources of nutrition, the gut microbiome typically increases in diversity and develops a Firmicutes-dominant phenotype [21]. Although the gut microbiome in breastfed compared to non-breastfed infants has not been previously studied in Haiti, our results are consistent with this pattern.
In a substudy of a randomized trial of chlorinated drinking water (mean FCR of 0.37 mg/L) or control (trace amounts of vitamin C) in urban Bangladesh, the gut microbiota in children six months to five years of age was minimally impacted one year after the intervention [15]. The parent trial found that chlorination of drinking water reduced diarrhea prevalence [20], but surprisingly, the substudy investigators found no change in qualitative enteric pathogen nucleic acid detection in children in the intervention arm. Investigators found that microbiota clustering and decreased richness within the water chlorination group was observed only among children 15–30 months of age, and not in younger children in the study [15]. Our study detected a similar trend of microbiota clustering and decreased richness and diversity but only in the youngest age group (infants ≤ 12 months) in households with detectable FCR in drinking water.
Our results indicate that the gut microbiota of infants in detectable FCR households, compared to those in undetectable FCR households, support an increased Bifidobacteriaceae abundance with less Streptococcaceae and Enterobacteriaceae. The latter two bacterial families are associated with a maturing childhood microbiota [21,23]. Thus, the high FCR household infants were more likely to have tightly clustered and Bifidobacteriaceae-dominant gut microbes that typically represent a healthier early-stage microbiome [21,24]. The clinical significance of our findings includes that Firmicutes families including Lachnospiraceae and Ruminococcaceae are known to be markers of a “transitioning” microbiome, and this change occurs as breastfeeding is reduced and solid foods are introduced. Our susceptibility testing demonstrated that Bifidobacteria spp. are indeed resistant to high concentrations of chlorine [25], and this finding supports our hypothesis that Bifidobacteriaceae were responsible for the FCR-dependent differences we observed in the gut microbiota of infants. In infants exposed to water with detectable FCR, we suspect that non-Bifidobacteria bacterial groups are suppressed, allowing for maintenance and dominance of Bifidobacterium for a longer time period compared to infants from households with undetectable FCR. This may be beneficial, because premature transition of the stages of development of the infant microbiome are known to have long-term detrimental effects [23,26].
We found no significant differences between cross-sectional FCR and the participant’s report of recent diarrhea or in enteric pathogen burden. Small sample size and our method of detecting enteric pathogens using molecular DNA that does not reflect a disease state[27] may have prevented us from accurately assessing these relationships. Diarrhea is common in this population, likely due to exposures outside of drinking water, inconsistency of water chlorination over time (and not accurately reflected in a single household measurement), or other non-measured intrinsic characteristics of the household water that impact the effectiveness of chlorination, such as high turbidity [16], temperature, pH, and duration of storage [6,28]. We did not collect data about exclusive breastfeeding, or the manner in which breastfeeding infants may have been exposed to household water. Future studies would with more detailed questionnaires regarding breastfeeding and food consumption with household water FCR measurements would better inform potential microbiome confounding factors. Nonetheless, our findings suggest that household drinking water FCR did impact infant microbiomes, and this may have occurred via formula or prepared food exposure, bathing, primary caregiver hygiene, and use of utensils. Additional limitations include the self-reported nature of questionnaire variables that could include recall bias. The strains tested in our in vitro assay are a proxy for the species of abundant commensal microbes found in the gut in this population, and further testing of different strains within the species tested is needed to draw conclusions about chlorine susceptibility in a specific species or genus of gut microbes.
The resurgence of cholera in Haiti as of October 2022 is primarily impacting children 1 to 4-years old who lack previous exposure [29]. Increasing chlorination of public water supplies and provision of chlorine tablets is a key objective in the National Plan for the Elimination of Cholera in Haiti [30], and access to safe water is essential to contain these outbreaks and reduce transmission of other diarrheal diseases. While this study demonstrates that a detectable FCR in household drinking water does not negatively affect the childhood or infant gut microbiome composition and may prevent early maturation of the infant microbiome, a detectable FCR was not associated with a reduction in enteropathogen burden. This finding underscores the need to understand how point-of-care interventions and household water chlorination impacts human health in all areas where safe water access is unreliable, and to identify and intervene upon other sources of enteropathogen transmission.
Supplementary Material
Acknowledgments
We thank the study participants and staff. The International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) gratefully acknowledges the government of the People’s Republic of Bangladesh, Global Affairs Canada, the Swedish International Development Cooperation Agency, and the Department for International Development.
Funding source
This work was supported by the National Institutes of Health (AI099243 to L.C.I. and J.B.H., and AI123494 to A.A.W).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Written informed consent was obtained from a parent or guardian for all study participants. The study protocol was approved by both the Partners Institutional Review Board in the US Protocol #2016P002781 and the Haitian National Bioethics Committee (Port-au-Prince, Haiti). The University of Washington investigators had access to only de-identified data and the study was deemed not human subjects by the institutional review board at this site.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2024.107165.
References
- [1].Chen L, Jiao J, Liu S, Liu L, Liu P. Mapping the global, regional, and national burden of diarrheal diseases attributable to unsafe water. Front Public Health 2023;11:1302748.0020. doi: 10.3389/fpubh.2023.1302748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brander RL, Pavlinac PB, Walson JL, John-Stewart GC, Weaver MR, Faruque ASG, et al. Determinants of linear growth faltering among children with moderate-to-severe diarrhea in the Global Enteric Multicenter Study. BMC Med 2019;17:214. doi: 10.1186/s12916-019-1441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vinekar K, Schaad N, Ber Lucien MA, Leshem E, Oboho IK, Joseph G, et al. Hospitalizations and deaths because of respiratory and diarrheal diseases among Haitian children under five years of age, 2011–2013. Pediatr Infect Dis J 2015;34:e238–43. doi: 10.1097/INF.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Severe K, Alcenat N, Rouzier V. Resurgence of cholera in Haiti amidst humanitarian crises. N Engl J Med 2022;387:2389–91. doi: 10.1056/NEJMc2213782. [DOI] [PubMed] [Google Scholar]
- [5].Mintz ED, Reiff FM, Tauxe RV. Safe water treatment and storage in the home. A practical new strategy to prevent waterborne disease. JAMA 1995;273:948–53. [PubMed] [Google Scholar]
- [6].World Health Organization Guidelines for drinking-water quality: fourth edition incorporating first addendum. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]
- [7].Clasen TF, Alexander KT, Sinclair D, Boisson S, Peletz R, Chang HH, et al. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev 2015;2015:CD004794. doi: 10.1002/14651858.CD004794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 2018;6:e302–15. doi: 10.1016/S2214-109X(17)30490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 2018;6:e316–29. doi: 10.1016/S2214-109X(18)30005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Humphrey JH, Mbuya MNN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health 2019;7:e132–47. doi: 10.1016/S2214-109X(18)30374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grembi JA, Lin A, Karim MA, Islam MO, Miah R, Arnold BF, et al. Effect of water, sanitation, handwashing and nutrition interventions on enteropathogens in children 14 months old: a cluster-randomized controlled trial in rural Bangladesh. J Infect Dis 2020;227:434–47. doi: 10.1093/infdis/jiaa549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanidad KZ, Zeng MY. Neonatal gut microbiome and immunity. Curr Opin Microbiol 2020;56:30–7. doi: 10.1016/j.mib.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferreira RBR, Gill N, Willing BP, Antunes LCM, Russell SL, Croxen MA, et al. The intestinal microbiota plays a role in Salmonella-induced colitis independent of pathogen colonization. PLoS One 2011;6:e20338. doi: 10.1371/journal.pone.0020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levade I, Saber MM, Midani F, Chowdhury F, Khan AI, Begum YA, et al. Predicting Vibrio cholerae infection and disease severity using metagenomics in a prospective cohort study. J Infect Dis 2021;223:342–51. doi: 10.1093/infdis/jiaa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nadimpalli ML, Lanza VF, Montealegre MC, Sultana S, Fuhrmeister ER, Worby CJ, et al. Drinking water chlorination has minor effects on the intestinal flora and resistomes of Bangladeshi children. Nat Microbiol 2022;7:620–9. doi: 10.1038/s41564-022-01101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilhelm N, Kaufmann A, Blanton E, Lantagne D. Sodium hypochlorite dosage for household and emergency water treatment: updated recommendations. J Water Health 2018;16:112–25. doi: 10.2166/wh.2017.012. [DOI] [PubMed] [Google Scholar]
- [17].Olm MR, Dahan D, Carter MM, Merrill BD, Yu FB, Jain S, et al. Robust variation in infant gut microbiome assembly across a spectrum of lifestyles. Science 2022;376:1220–3. doi: 10.1126/science.abj2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep 2020;10:15792. doi: 10.1038/s41598-020-72635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arnold BF, Colford JM. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg 2007;76:354–64. [PubMed] [Google Scholar]
- [20].Pickering AJ, Crider Y, Sultana S, Swarthout J, Goddard FG, Anjerul Islam S, et al. Effect of in-line drinking water chlorination at the point of collection on child diarrhoea in urban Bangladesh: a double-blind, cluster-randomised controlled trial. Lancet Glob Health 2019;7:e1247–56. doi: 10.1016/S2214-109X(19)30315-8. [DOI] [PubMed] [Google Scholar]
- [21].Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–8. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Turroni F, Milani C, Duranti S, Mahony J, van Sinderen D, Ventura M. Glycan utilization and cross-feeding activities by bifidobacteria. Trends Microbiol 2018;26:339–50. doi: 10.1016/j.tim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [23].Matsuyama M, Morrison M, Cao K-AL, Pruilh S, Davies PSW, Wall C, et al. Dietary intake influences gut microbiota development of healthy Australian children from the age of one to two years. Sci Rep 2019;9:12476. doi: 10.1038/s41598-019-48658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davis EC, Wang M, Donovan SM. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes 2017;8:143–71. doi: 10.1080/19490976.2016.1278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Elli M, Arioli S, Guglielmetti S, Mora D. Biocide susceptibility in bifidobacteria of human origin. J Global Antimicrob Resist 2013;1:97–101. doi: 10.1016/j.jgar.2013.03.007. [DOI] [PubMed] [Google Scholar]
- [26].Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- [27].Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016;388:1291–301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marois-Fiset J-T, Carabin A, Lavoie A, Dorea CC. Effects of temperature and pH on reduction of bacteria in a point-of-use drinking water treatment product for emergency relief. Appl Environ Microbiol 2013;79:2107–9. doi: 10.1128/AEM.03696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].World Health Organization Disease outbreak news. Cholera - Haiti: WHO; 2022. [Google Scholar]
- [30].Republic of Haiti Ministry of Public Health and Population, National Directorate for Water Supply and Sanitation. National plan for the elimination of cholera in Haiti, 2013–2022. Port-Au-Prince, Haiti: Ministry of Public Health and Population; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


