Abstract
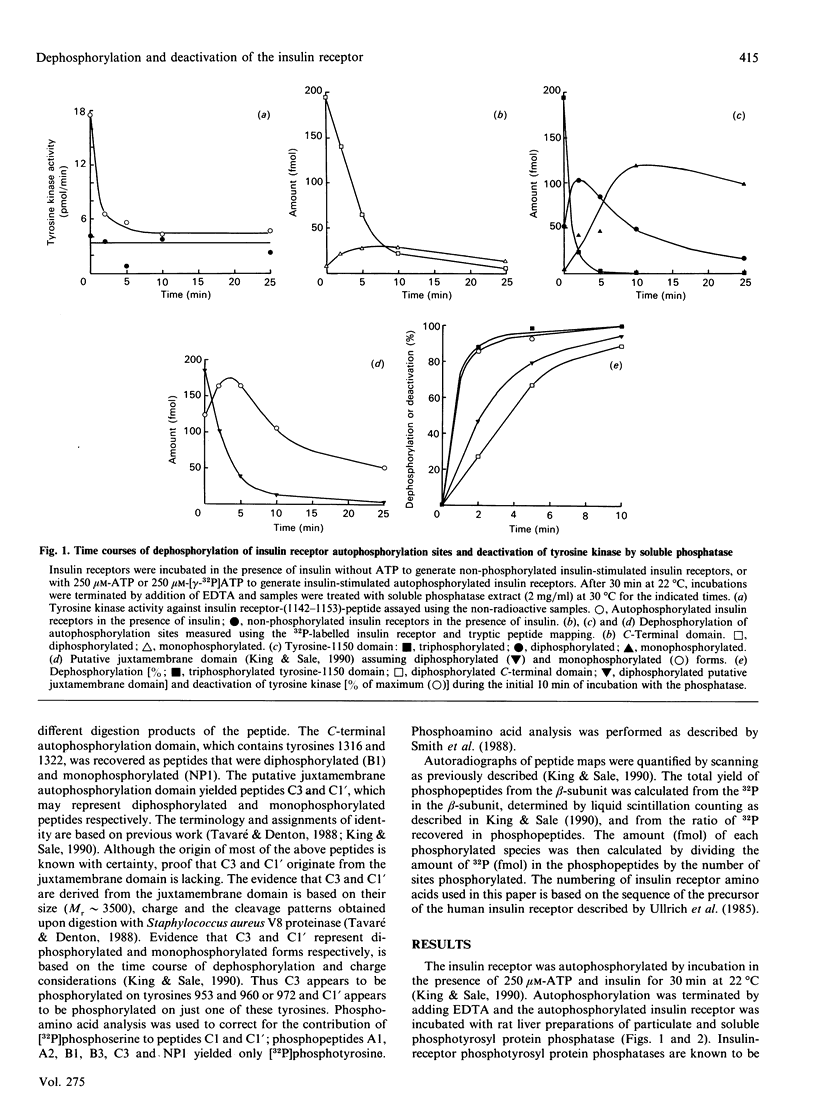
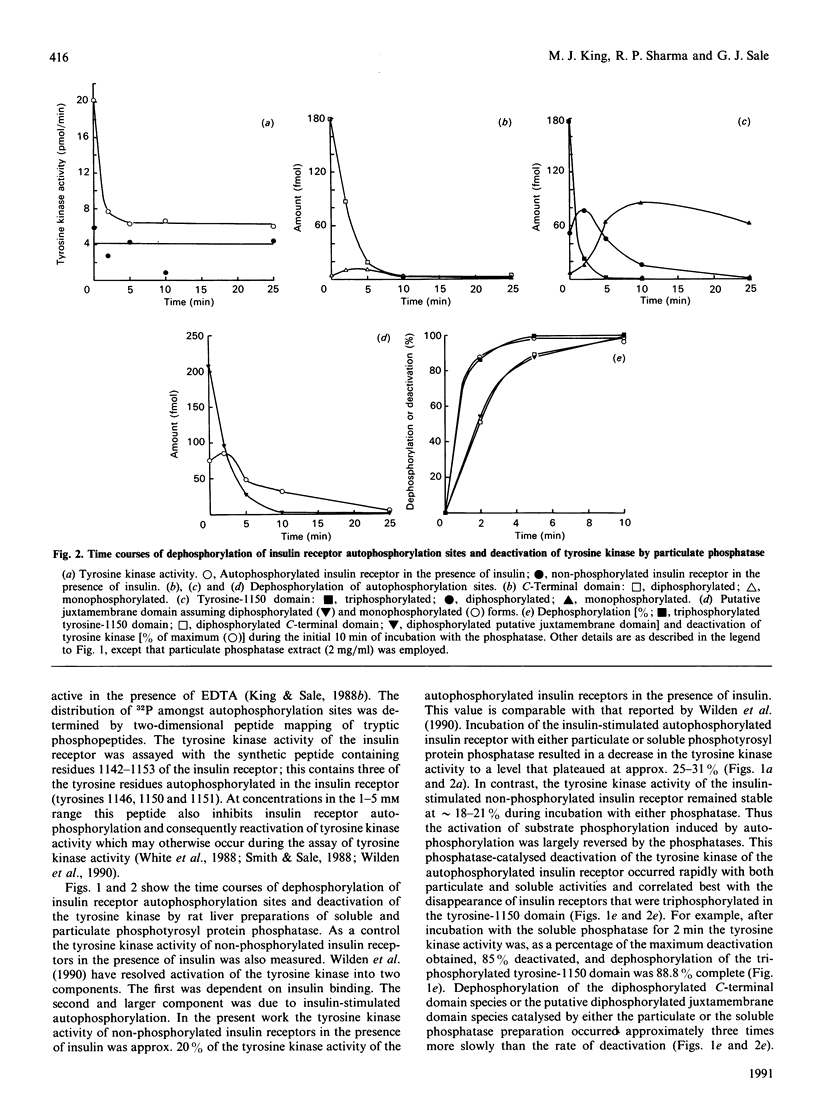
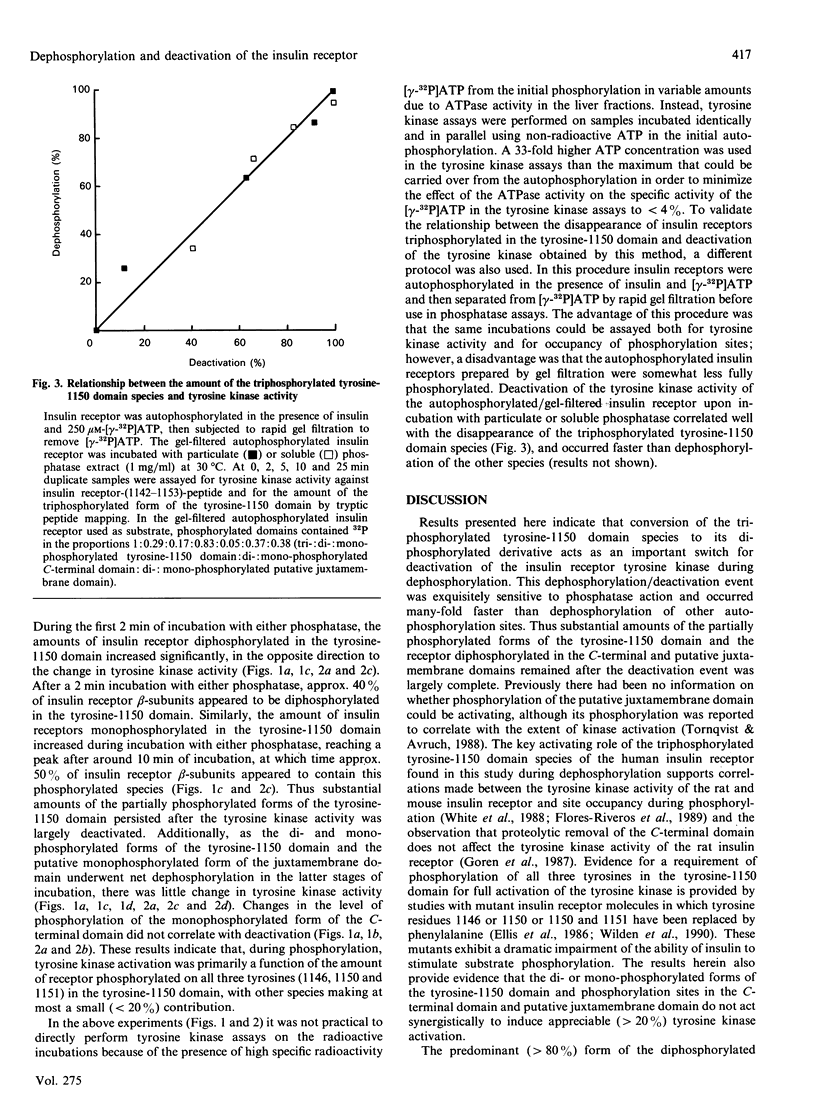
Insulin receptor tyrosine kinase activation, induced by insulin-stimulated autophosphorylation, was measured using a synthetic peptide containing residues 1142-1153 of the insulin receptor and shown to be reversed by both particulate and soluble phosphotyrosyl protein phosphatases from rat liver. Deactivation of the tyrosine kinase was highly sensitive to phosphatase action and was correlated best with disappearance of insulin receptors triphosphorylated in the tyrosine-1150 domain. Dephosphorylation of the di- and mono-phosphorylated forms of the tyrosine-1150 domain generated during dephosphorylation or of phosphorylation sites in the C-terminal or putative juxta-membrane domains occurred 3- greater than 10-fold more slowly than deactivation of the tyrosine kinase, and these phosphorylated species did not appear to appreciably (less than 20%) contribute to tyrosine kinase activation. These results indicate that the transition from the triply to the doubly phosphorylated form of the tyrosine-1150 domain acts as an important switch for deactivation of the insulin receptor tyrosine kinase during dephosphorylation. The exquisite sensitivity of this dephosphorylation/deactivation event to phosphotyrosyl protein phosphatase action, combined with the high affinities of this phosphatases for substrates and the high activities of the phosphatases in cells, suggests that the tyrosine kinase activity expressed by insulin-stimulated insulin receptors is likely to be stringently regulated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charbonneau H., Tonks N. K., Kumar S., Diltz C. D., Harrylock M., Cool D. E., Krebs E. G., Fischer E. H., Walsh K. A. Human placenta protein-tyrosine-phosphatase: amino acid sequence and relationship to a family of receptor-like proteins. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Flores-Riveros J. R., Sibley E., Kastelic T., Lane M. D. Substrate phosphorylation catalyzed by the insulin receptor tyrosine kinase. Kinetic correlation to autophosphorylation of specific sites in the beta subunit. J Biol Chem. 1989 Dec 25;264(36):21557–21572. [PubMed] [Google Scholar]
- Goren H. J., White M. F., Kahn C. R. Separate domains of the insulin receptor contain sites of autophosphorylation and tyrosine kinase activity. Biochemistry. 1987 Apr 21;26(8):2374–2382. doi: 10.1021/bi00382a044. [DOI] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Assay of phosphotyrosyl protein phosphatase using synthetic peptide 1142-1153 of the insulin receptor. FEBS Lett. 1988 Sep 12;237(1-2):137–140. doi: 10.1016/0014-5793(88)80187-x. [DOI] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Dephosphorylation of insulin-receptor autophosphorylation sites by particulate and soluble phosphotyrosyl-protein phosphatases. Biochem J. 1990 Feb 15;266(1):251–259. doi: 10.1042/bj2660251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Insulin-receptor phosphotyrosyl-protein phosphatases. Biochem J. 1988 Dec 15;256(3):893–902. doi: 10.1042/bj2560893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok Y. C., Nemenoff R. A., Powers A. C., Avruch J. Kinetic properties of the insulin receptor tyrosine protein kinase: activation through an insulin-stimulated tyrosine-specific, intramolecular autophosphorylation. Arch Biochem Biophys. 1986 Jan;244(1):102–113. doi: 10.1016/0003-9861(86)90098-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986 Apr 18;232(4748):341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Roome J., O'Hare T., Pilch P. F., Brautigan D. L. Protein phosphotyrosine phosphatase purified from the particulate fraction of human placenta dephosphorylates insulin and growth-factor receptors. Biochem J. 1988 Dec 1;256(2):493–500. doi: 10.1042/bj2560493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Fujita-Yamaguchi Y., Kahn C. R. Characterization of phosphatidylinositol kinase activity associated with the insulin receptor. Eur J Biochem. 1986 Mar 3;155(2):345–351. doi: 10.1111/j.1432-1033.1986.tb09497.x. [DOI] [PubMed] [Google Scholar]
- Sale G. J. Recent progress in our understanding of the mechanism of action of insulin. Int J Biochem. 1988;20(9):897–908. doi: 10.1016/0020-711x(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Smith D. M., King M. J., Sale G. J. Two systems in vitro that show insulin-stimulated serine kinase activity towards the insulin receptor. Biochem J. 1988 Mar 1;250(2):509–519. doi: 10.1042/bj2500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sale G. J. Evidence that a novel serine kinase catalyses phosphorylation of the insulin receptor in an insulin-dependent and tyrosine kinase-dependent manner. Biochem J. 1988 Dec 15;256(3):903–909. doi: 10.1042/bj2560903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G., Subrahmanyam G. Purification and characterization of a protein-phosphotyrosine phosphatase from rat spleen which dephosphorylates and inactivates a tyrosine-specific protein kinase. J Biol Chem. 1989 May 15;264(14):7801–7808. [PubMed] [Google Scholar]
- Tavaré J. M., Denton R. M. Studies on the autophosphorylation of the insulin receptor from human placenta. Analysis of the sites phosphorylated by two-dimensional peptide mapping. Biochem J. 1988 Jun 1;252(2):607–615. doi: 10.1042/bj2520607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré J. M., O'Brien R. M., Siddle K., Denton R. M. Analysis of insulin-receptor phosphorylation sites in intact cells by two-dimensional phosphopeptide mapping. Biochem J. 1988 Aug 1;253(3):783–788. doi: 10.1042/bj2530783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6731–6737. [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988 May 15;263(14):6722–6730. [PubMed] [Google Scholar]
- Tornqvist H. E., Avruch J. Relationship of site-specific beta subunit tyrosine autophosphorylation to insulin activation of the insulin receptor (tyrosine) protein kinase activity. J Biol Chem. 1988 Apr 5;263(10):4593–4601. [PubMed] [Google Scholar]
- Tornqvist H. E., Gunsalus J. R., Nemenoff R. A., Frackelton A. R., Pierce M. W., Avruch J. Identification of the insulin receptor tyrosine residues undergoing insulin-stimulated phosphorylation in intact rat hepatoma cells. J Biol Chem. 1988 Jan 5;263(1):350–359. [PubMed] [Google Scholar]
- Tornqvist H. E., Pierce M. W., Frackelton A. R., Nemenoff R. A., Avruch J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J Biol Chem. 1987 Jul 25;262(21):10212–10219. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Wilden P. A., Backer J. M., Kahn C. R., Cahill D. A., Schroeder G. J., White M. F. The insulin receptor with phenylalanine replacing tyrosine-1146 provides evidence for separate signals regulating cellular metabolism and growth. Proc Natl Acad Sci U S A. 1990 May;87(9):3358–3362. doi: 10.1073/pnas.87.9.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]


