Abstract
Background and Objectives
Previous systemic reviews, predominantly including observational studies, have shown that participation in social activities is a protective factor against cognitive decline. However, this association is subject to potential reverse causality, creating a knowledge gap in our understanding of the effect of social interaction interventions on cognitive function. Therefore, this study aims to conduct a systematic review and meta-analysis of randomized controlled trials to examine the effects of social interaction interventions on cognitive decline among older adults without dementia.
Research Design and Methods
This systematic review, registered in PROSPERO (CRD42022367828), systematically searched 6 databases from inception to May 6, 2022, to identify relevant articles on the effects of activities with social interaction components on cognitive function in community-dwelling older adults without dementia aged above 60. Two independent reviewers conducted study selection, data extraction, and bias assessment, with RevMan5.3 used for meta-analysis. Subgroup analysis was conducted to assess variation in intervention effects among subgroups.
Results
We included 11 studies for qualitative analysis and 8 studies for the meta-analysis. The results showed that social interaction intervention had a significant effect on executive function (standardized mean difference [SMD] = 1.60; 95% CI, 0.50 to 2.70; p = .004), but not attention and memory. The subgroup analysis showed a greater cognitive benefit for healthy older adults, but not those with mild cognitive impairment. Moreover, in-person social interaction positively affected global cognition, whereas online interaction did not.
Discussion and Implications
Social interaction interventions have a limited impact on cognitive function in older adults without dementia but showed potential effects on executive function. This finding offers insights for implementing social intervention in the community.
Keywords: Aging Population, Behavioral intervention, Cognitive decline, Executive function
Translational Significance: This study addresses the challenge of dementia prevention in aging populations through community-based interventions. Our systematic review and meta-analysis of randomized controlled trials showed that social interaction interventions significantly improve executive function in older adults without dementia. The findings suggest that enhancing social participation can promote cognitive health and healthy aging, particularly among those with normal cognitive functioning. These insights are vital for developing accessible strategies to mitigate the risk of cognitive decline in community settings.
The population is aging globally and according to the World Health Organization, the number of people aged 60 years and above is expected to double by 2050 (WHO, 2020), highlighting the increasing relevance of age-related issues. Among these concerns, dementia stands out as a common challenge for older adults, with its prevalence increasing with age and doubles every 5 years after age 65 (van der Flier & Scheltens, 2005). Currently, around 55 million people worldwide have dementia, with nearly 10 million new cases yearly. The WHO estimates that by 2050, the number of people with dementia will reach 139 million, accompanied by a staggering projected cost of over 2.8 trillion dollars by 2030, placing a significant burden on healthcare systems globally (Shin, 2022). Given that neurodegeneration is an irreversible without process and current cure for dementia, the quest for approaches to prevent onset or slow disease progression has become paramount in aging societies.
Recently, numerous studies have investigated lifestyle factors as potential interventions to prevent and slow down the decline of cognitive function (Eubank et al., 2022; Kivipelto et al., 2018). Targeting modifiable risk factors, such as smoking cessation, reduced alcohol consumption, increasing physical activity, and social engagement, may prove effective in slowing cognitive decline and reducing the risk of dementia (Livingston et al., 2020). For older adult, social activities present a relatively simple yet critical avenue for intervention, contributing to successful aging—an aspiration cherished by many older adults (Douglas et al., 2017). Observational studies in recent years have consistently shown the protective role of social activities against cognitive decline. However, this association is susceptible to potential reverse causality and other confounding factors inherent to observational studies. Furthermore, existing systematic reviews have predominantly focused on observational studies, leaving a gap in our understanding of the effect of social interaction interventions on cognitive function.
Therefore, this study aims to conduct a systematic review and meta-analysis of randomized controlled trial (RCT) studies to examine the effects of social interaction interventions on cognitive decline among older adults without dementia.
Method
Registration
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis reporting guidelines (Page et al., 2021). The study was registered in PROSPERO (CRD42022367828), and the PRISMA checklist is shown in Supplementary Table 1.
Eligibility Criteria
Participants
The study focused on community-dwelling older adults aged 60 years and above without any significant medical, psychiatric, or neurological conditions.
Intervention
Social interaction is the process by which we act and react to those around us, encompassing the acts people perform toward each other and the responses they give in return. Our study includes interventions that incorporate social interaction components involving elements of communication or interaction with others. However, we will exclude studies unable to independently assess the impact of social interaction on cognitive function, such as research involving multidomain interventions.
Comparator
We had no restrictions for the controlled group. All studies evaluating the effect of social interaction interventions on cognitive function in comparison with various control groups were considered eligible, including active (i.e., alternative interventions designed not to affect the targeted aspect) or passive controls (e.g., no intervention, standard care, or waiting list control).
Outcome
The primary outcome of interest is global cognitive function, whereas the secondary outcomes include specific cognitive domains such as memory, attention, executive function, and language. The included article should include the changes in neuropsychological test scores from baseline to postintervention for these outcomes.
Study design
We included randomized controlled trials aimed to assess the effects of social intervention on cognitive function among older adults.
Search Strategy
The selection was conducted with six electronic databases, including PubMed, MEDLINE, PsycINFO, Embase, CINAHL, and Cochrane Library, from the database inception to May 6, 2022. The search was restricted to publications written in English-language. The searched terms included “social activity,” “social engagement,” “social participation,” “social interaction,” “social intervention,” “leisure intervention,” “recreation groups,” “volunteering” combined with “cognitive function,” “cognitive performance,” “cognition,” “social cognition,” and “aged,” “older adults,” “elderly,” and “aging” (Supplementary Table 2). Furthermore, we searched the reference lists of included studies and published systematic reviews to increase the retrieval of studies.
Study Selection and Data Extraction
Duplicate studies in six databases were removed using EndNote version 20. Two reviewers (C.-C. Wei and M.-J. Hsieh) independently screened the titles and abstracts of the rest studies and retrieved those with potential eligibility for a full-text review. The two reviewers performed data extraction independently, and if they could not reach a consensus, a majority rule was made by adding the third reviewer’s (Y.-F. Chuang) opinion. The following data were extracted from each included study: publication, participants, intervention, comparison type, outcome measurement tools, and neuropsychological test scores. As there was missing data, we contacted the authors via email in an attempt to obtain any relevant information.
Quality Assessment
Two reviewers (C.-C. Wei and M.-J. Hsieh) independently evaluated the quality of the studies using the Cochrane risk of bias tool, which categorizes the risk of various biases into three grades: low risk (green mark), unclear risk (yellow mark), and high risk (red mark). The unclear risk of bias, often due to missing information, can make it difficult to fully assess the limitations and potential problems in the studies. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach categorized the quality of evidence in the results of the meta-analysis into four levels: very low, low, moderate, and high certainty.
Statistical Analysis
We conducted a meta-analysis using Review Manager version 5.4 and employed random-effects models to address inherent heterogeneity among the studies (Borenstein et al., 2010). The standardized mean difference (SMD) was utilized as the effect size measure, considering that neuropsychological test scores were continuous and measured on different scales, and the data from the last time point of each study were used to estimate the effect sizes in the presence of multiple measurement time points.
When the mean difference was unavailable, we calculated from baseline and the postintervention scores for both groups. Additionally, we calculated the change in SD from baseline and the postintervention SD using the following formula, where the pre–post correlation was 0.50 (Cumpston et al., 2019). Chi-square test and I² statistic were used to assess the existence and degree of heterogeneity.
We conducted subgroup analyses to explore potential sources of heterogeneity and to assess whether the effects varied across subgroups. We assessed the heterogeneity of social interaction on cognitive function between cognitive status (normal cognition vs MCI), mode of social interaction intervention (in-person vs online), and intervention dose (high vs low).
To assess whether various assessment tools in different cognitive domains would influence the results, we selected the most widely used assessment tool within each cognitive domain for primary analysis. Additionally, we conducted sensitivity analyses by replacing the assessment tools with alternatives. In cases of high heterogeneity, defined as I2 higher than 75%, a leave-one-out sensitivity analysis using R studio version 4.2.2 assessed that individual studies did not significantly influence our findings.
We used two-sided p ≤ .05 as a guide for statistical significance in the main analysis and p ≤ .1 for the subgroup analyses, considering potential low statistical power (Richardson et al., 2019).
Results
Study Selection
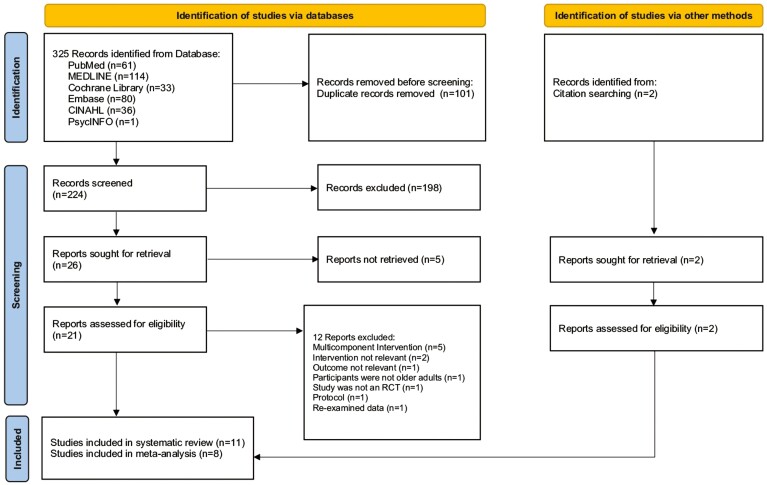
The study selection process was based on the PRISMA flow diagram as in Figure 1. Initially, 325 articles were retrieved from 6 databases and 2 from citation searching and 21 were potentially eligible for further assessment after removing duplicates and reviewing titles and abstracts. After assessing the full-text articles, 11 studies (Brydges et al., 2021; Carlson et al., 2008; Dodge et al., 2015; Iizuka et al., 2019; Kawakami et al., 2019; Lam et al., 2015; Mortimer et al., 2012; Morton et al., 2018; Myhre et al., 2017; Park et al., 2014; Pitkala et al., 2011) were included in the systematic review. Finally, as three studies (Carlson et al., 2008; Myhre et al., 2017; Park et al., 2014) had insufficient information for the effect sizes and eight studies were included in the meta-analysis.
Figure 1.
PRISMA flowchart of study selection.
Study Characteristics
The detailed characteristics of the included studies are described in Table 1.
Table 1.
Characteristics of Included Studies
| Study/country | Participants | Intervention | Control type | Outcomes measurement tools | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Cognitive status | Age (mean ± SD) | Setting | Type | Frequency and time | Duration | Total dose | Adherence | |||
|
Park et al., 2014
USA |
Photo group (n = 29) Quilt group (n = 35) Dual group (n = 42) Social activity (n = 36) Placebo (n = 39) |
Healthy | 60–90 y/o (71.7 ± 7.3) | Community | Recreation group | 15.9 hr per week | 3 months | 207.4 hr | 85% | No intervention | Attention: DSF |
|
Lam et al., 2015
China |
Social group (n = 131)
Cognitive group (n = 145) Physical group (n = 147) Cognitive–physical group (n = 132) |
MCI | ≥60 y/o (75.4 ± 6.5) | Social center | Recreation group | Three times a week; 1 hr | 12 months | 156.5 hr | 77% | Active control (physical activity) | Global function: ADAS-Cog, CMMSE, CDR-SOB Attention: DSF Executive function: VF |
|
Iizuka et al., 2019
Japan |
Face‐to‐face group (n = 25)
Non‐face‐to‐face group (n = 25) Control group (n = 22) |
Healthy | ≥65 y/o (76.8) | Community | Recreation group | Once a week; 1 hr | 12 weeks | 12 hr | 83% | Active control (non‐face‐to‐face Go classes) | Memory: LM II Attention: DSF, LM I, TMT‐A Executive function: VF, TMT‐B |
|
Carlson et al., 2008
USA |
Intervention group (n = 70)
Control group (n = 58) |
Healthy | ≥60 y/o (69) | Community | Volunteer (BECT) | 15 hr per week | 8 months | 521.8 hr | 89% | Waiting list control | Memory: Word List Recall, Rey-CFT Attention: TMT‐A Executive function: TMT‐B |
|
Brydges et al., 2021
USA |
Experience Corps group (n = 181)
Control group (n = 155) |
Healthy | ≥60 y/o (66.4 ± 5.6) | Community | Volunteer (BECT) | 15 hr per week | 24 months | 1565.3 hr | Not provided | Waiting list control | Attention and executive function: Stroop |
|
Pitkala et al., 2011
Finland |
Social activity plus therapeutic writing/group exercise/art experience (n = 117) Normal community care (n = 118) |
Healthy | ≥75 y/o (80 ± 3.6) | Day care centers | Group discussion | Once a week; 6 hr | 3 months | 78.3 hr | 94% | No intervention | Global function: ADAS-Cog |
|
Mortimer et al., 2012
China |
Tai Chi (n = 30) Walking (n = 30) Social Interaction (n = 30) No intervention (n = 30) |
Healthy | 60–79 y/o (67.8) | Community | Group discussion | Three times a week; 1 hr | 40 weeks | 120 hr | 90% | No intervention | Global function: MDRS Memory: RAVLT, Rey-CFT Attention: DSF, TMT-A Executive function: Stroop, VF, TMT‐B Language: BNT |
|
Dodge et al., 2015
USA |
CDR = 0: Intervention group (n = 24) Control group (n = 25) CDR = 0.5: Intervention group (n = 17) Control group (n = 17) |
Healthy and MCI | ≥70 y/o (80.5 ± 6.8) | Retirement community and senior centers | Online social | Five times a week; 0.5 hr | 6 weeks | 15 hr | 100% | No intervention | Global function: MMSE Memory: Word list recall Attention: Stroop, TMT‐A Executive function: Stroop, VF, TMT‐B |
|
Myhre et al., 2017
USA |
Facebook group (n = 14)
Online diary group (n = 13) Control group (n = 14) |
Healthy | 75–86 y/o (78.5) | Retirement community | Online social | Three times a week; 2 hr | 8 weeks | 48 hr | 95% | Active control (online diary) | Memory: RAVLT, Rey-CFT Attention: TMT‐A Executive function: TMT‐B |
|
Morton et al., 2018
United Kingdom |
Training group (n = 44)
Control group (n = 32) |
Healthy | 60–95 y/o (80.7 ± 8.8) | Care organization | Online social | Three times a week; 1.5 hr | 3 months | 58.7 hr | 83% | Care-as-usual | Global function: ACE-R |
|
Kawakami et al., 2019
Japan |
Spectator group (n = 29)
Control group (n = 29) |
Healthy | 65–85 y/o (73.7 ± 5.3) | Community | Visit sport events | Two games a month; 3 hr | 2 months | 12 hr | 100% | Waiting list control | Attention and executive function: Stroop |
Notes: ACE-R = Addenbrooke’s Cognitive Examination-Revised; ADAS-Cog = Alzheimer’s Disease Assessment Scale—Cognitive; BNT = Boston Naming Test; CMMSE = Chinese version of Mini-Mental State Examination; CDR-SOB = clinical dementia rating—sum of boxes; DSF = digit span forward; LM I = Logical Memory 1; LM II = Logical Memory 2; MDRS = Mattis Dementia Rating Scale; RAVLT = Rey Auditory Verbal Learning Test; Rey-CFT = Rey Complex Figure Test; TMT‐A = Trail Making Test Part A; TMT‐B = Trail Making Test Part B; VF = verbal fluency.
Participants
Among the 11 studies included in the systemic review, 9 examined the effect of social interaction on cognition in healthy older adults. In contrast, one study (Lam et al., 2015) included participants with mild cognitive impairment (MCI), as defined by subjective cognitive complaints and objective impairments in cognitive function. In another study (Dodge et al., 2015), the cognitive status of both normal subjects and those with MCI was defined by a global clinical dementia rating (CDR) score of 0 and 0.5, respectively. Participants were recruited from various settings, including the community (n = 8), senior social centers (n = 2), daycare centers (n = 1), and care organizations (n = 1), with one study (Dodge et al., 2015) recruiting from both the community and senior centers. Among the 1,430 older adults included in the systemic review, the mean age was from 66.4 to 80.7.
Interventions
Social interactions can be broadly categorized into group recreational activities (Iizuka et al., 2019; Lam et al., 2015; Park et al., 2014), group discussions (Mortimer et al., 2012; Pitkala et al., 2011), online social interactions (Dodge et al., 2015; Morton et al., 2018; Myhre et al., 2017), watching sporting events (Kawakami et al., 2019), and volunteering (Brydges et al., 2021; Carlson et al., 2008). The duration and frequency of interventions varied, ranging from 30 min to 6 hr per session, conducted five times per week or at least twice a month. The overall duration of intervention ranged from 6 weeks to 2 years, with a total dose ranging from 12 to 1565.3 hr. Most studies had high adherence rates, with the majority exceeding 80%, except for 1 (Lam et al., 2015) reporting 77%, and 1 (Brydges et al., 2021) not reporting adherence rates.
Comparators
The control conditions used in the study primarily consisted of a waitlist (n = 8) and no intervention (n = 4). Other comparisons included physical activity, health education, or care-as-usual. On the other hand, two of the studies (Iizuka et al., 2019; Myhre et al., 2017) included multiple control groups consisting of both active and passive control. When conducting the meta-analysis, we chose the active control group as our control group to eliminate intervention components that were not of interest to us.
Cognitive function
We referred to the classification of different cognitive domains and corresponding neuropsychological tests used by Smith et al. (2010), Engeroff et al. (2018), and Reger et al. (2004). We summarized the tests used in these domains across the 11 studies included in our analysis and presented the results in Supplementary Table 3.
Among the included studies, five studies (Dodge et al., 2015; Lam et al., 2015; Mortimer et al., 2012; Morton et al., 2018; Pitkala et al., 2011) reported outcomes for global cognitive function, three (Dodge et al., 2015; Iizuka et al., 2019; Mortimer et al., 2012) for memory, five (Brydges et al., 2021; Dodge et al., 2015; Iizuka et al., 2019; Kawakami et al., 2019; Mortimer et al., 2012) for attention, six (Brydges et al., 2021; Dodge et al., 2015; Iizuka et al., 2019; Kawakami et al., 2019; Lam et al., 2015; Mortimer et al., 2012) for executive function, and only one (Mortimer et al., 2012) for language. The corresponding extracted neuropsychological test scores for conducting the meta-analysis are shown in Supplementary Table 4.
Quality Assessment
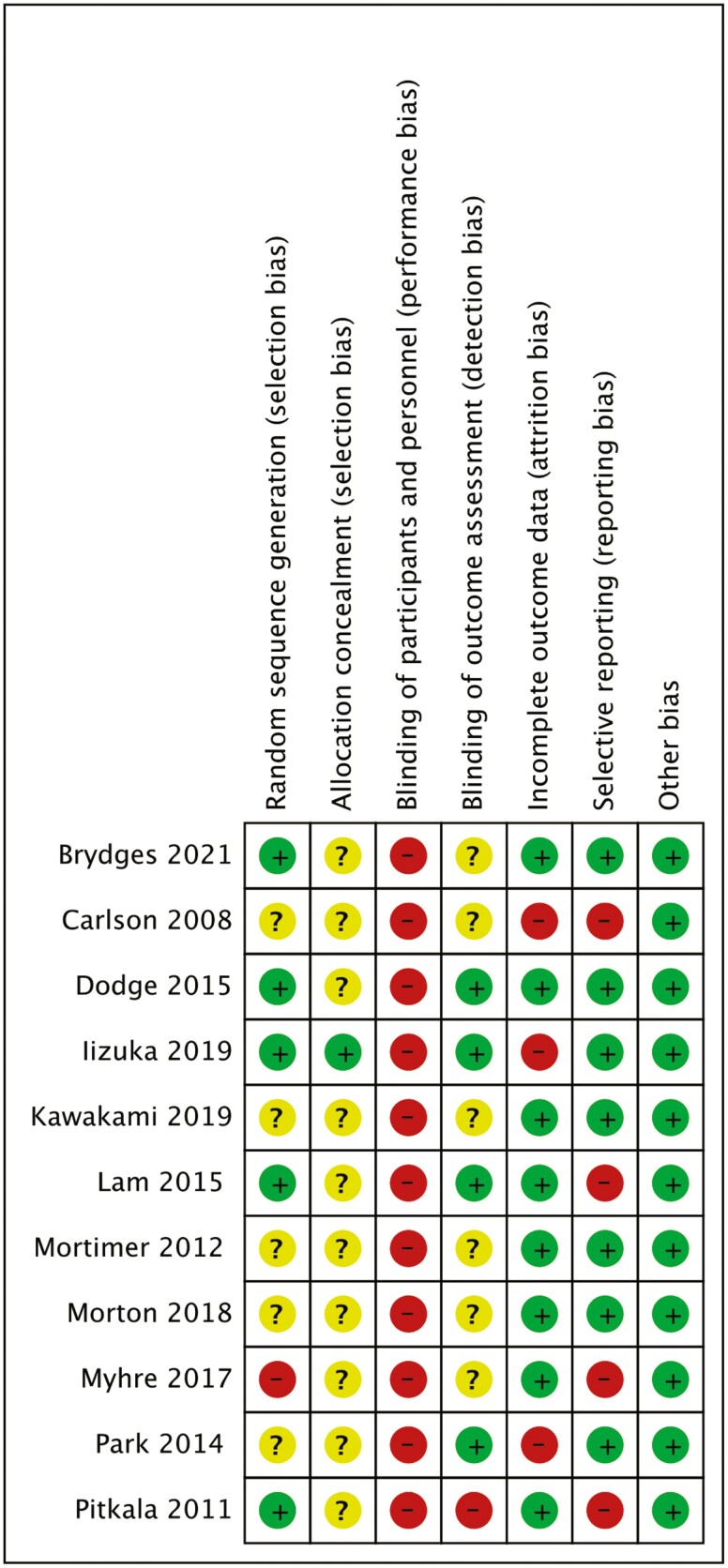
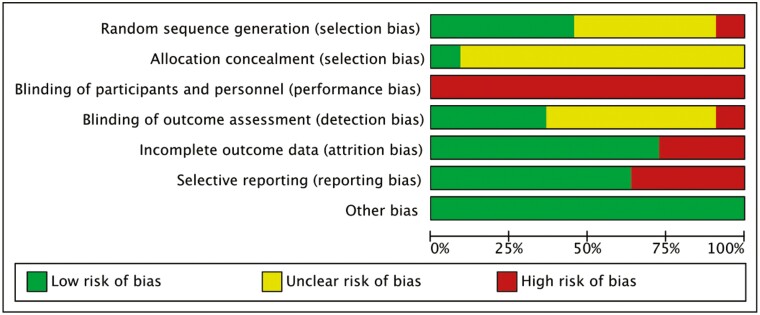
As shown in Figures 2 and 3, all articles were rated as high risk for participant blinding due to the inherent limitations of behavioral interventions. Additionally, most of the studies did not provide specific information on the methods of allocation concealment and random sequence generation, whereas one study (Myhre et al., 2017) was not randomized. As for the “other bias” category, we have assessed these studies as having a low risk of bias, meaning they appear to be free from other potential sources of bias, such as potential biases related to the specific study design used. The support for the judgment was detailed in Supplementary Table 5.
Figure 2.
Risk of bias summary. This figure summarizes each risk of bias item in all included studies. The risk levels are indicated by the following marks: low risk (green mark), unclear risk (yellow mark), and high risk (red mark). The unclear risk of bias, often due to missing information, can make it difficult to fully assess the limitations and potential problems in the studies.
Figure 3.
Risk of bias graph. Each risk of bias item is presented as a percentage across all included studies.
Based on the GRADE rating of evidence, global cognition has low credit quality due to the heterogeneity across studies. For specific cognitive domains, memory had moderate quality that might be related to a small sample size, whereas attention and executive function had high quality as indicated in Supplementary Table 6.
Meta-Analysis of Social Interaction and Cognitive Function Domains
Global cognitive function
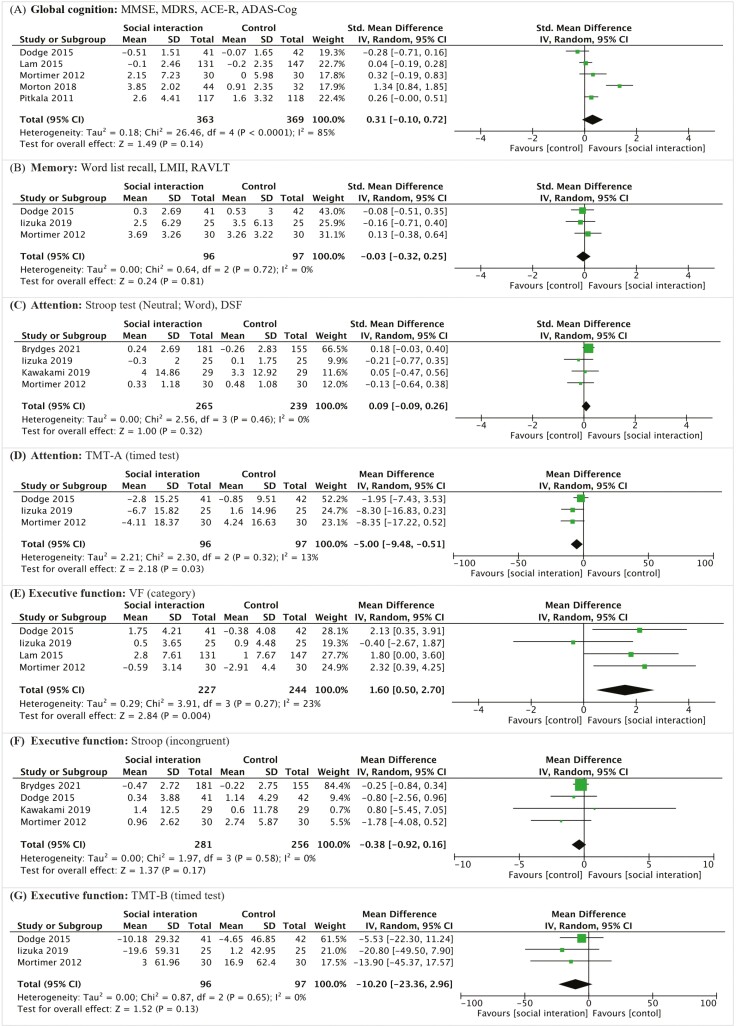
Global cognitive function was assessed in five studies (Dodge et al., 2015; Lam et al., 2015; Mortimer et al., 2012; Morton et al., 2018; Pitkala et al., 2011), using the Mini-Mental State Examination (MMSE; 2/5 studies), Mattis Dementia Rating Scale (MDRS; 1/5 studies), Addenbrooke’s Cognitive Examination—Revised (ACE-R; 1/5 studies), and Alzheimer’s Disease Assessment Scale—Cognitive Subscale (ADAS-Cog; 1/5 studies). The pooled SMD was 0.31 (95% CI, −0.10 to 0.72; p = .14, Figure 4A), for which high heterogeneity was detected (Q = 26.46; I2 = 85%; p < .00001).
Figure 4.
Forest plots of the effect of social interaction intervention on cognitive function. ACE-R = Addenbrooke’s Cognitive Examination Revised; ADAS-Cog = Alzheimer’s Disease Assessment Scale—Cognitive Subscale; CI = Confidence Interval; DSF = Digit Span Forward; IV = Inverse Variance; LMII = Logical Memory test II; MDRS = Mattis Dementia Rating Scale; MMSE = Mini-Mental State Examination; RAVLT = Rey Auditory Verbal Learning Test; SD = Standard Deviation; TMT‐A & TMT-B = Trail Making Test Parts A and B; VF test = Verbal Fluency test.
Memory
Memory was assessed in three studies (Dodge et al., 2015; Iizuka et al., 2019; Mortimer et al., 2012), using the word list recall (1/3 studies), Logical Memory test II (LMII; 1/3 studies) and Rey Auditory Verbal Learning Test (RAVLT; 1/3 studies). The pooled SMD was −0.03 (95% CI, −0.32 to 0.25; p = .81, Figure 4B), without significant heterogeneity (Q = 0.64; I2 = 0%; p = .72).
Attention
Attention was included in four studies (Brydges et al., 2021; Iizuka et al., 2019; Kawakami et al., 2019; Mortimer et al., 2012), assessed with the Stroop test neutral (2/4 studies) and Digit Span Forward (DSF; 2/4 studies). The pooled SMD was 0.09 (95% CI, −0.09 to 0.26; p = .32, Figure 4C), without significant heterogeneity (Q = 2.56; I2 = 0%; p = .46). Additionally, three studies (Dodge et al., 2015; Iizuka et al., 2019; Mortimer et al., 2012) used the Timed Test—Trail Making Test Part A (TMT-A) for the assessment. The pooled MD was 5.00 (95% CI, −9.48 to −0.51; p = .03, Figure 4D).
Executive function
Three assessment tools were utilized to evaluate executive function. Each test was discussed individually due to sufficient articles. Verbal fluency (VF) evaluation was included in four trials (Dodge et al., 2015; Iizuka et al., 2019; Lam et al., 2015; Mortimer et al., 2012), showing an SMD of 1.60 (95% CI, 0.50 to 2.70; p = .004, Figure 4E). However, the four studies (Brydges et al., 2021; Dodge et al., 2015; Kawakami et al., 2019; Mortimer et al., 2012) using the Stroop test (incongruent) had varied outcomes, with a pooled SMD of −0.38 (95% CI, −0.92 to 0.16; p = .17, Figure 4F). Three studies (Dodge et al., 2015; Iizuka et al., 2019; Mortimer et al., 2012) employed Trail Making Test Part B (TMT-B) for evaluation, yielding a pooled MD of −10.20 (95% CI, −23.36 to 2.96; p = .13, Figure 4G).
Language
We did not conduct a meta-analysis for language function as it was only included in one study (with the Boston Naming Test; Mortimer et al., 2012).
Sensitivity Analysis
There were no substantial changes in the overall pulled effect size after changing the assessment tool of both global cognition and cognitive domains (Supplementary Figure 1). The leave-one-out sensitivity analysis was conducted to assess the influence of individual studies on the overall findings in light of the high heterogeneity observed in global cognition (Supplementary Figure 2). A removal of Morton’s study (Morton et al., 2018) resulted in a significant decrease in heterogeneity. Consequently, it was excluded from the subgroup analysis to ensure higher reliability.
Subgroup Analysis
Statistical analysis of the subgroup differences revealed significant effects in cognitive status and mode of intervention (p = .08; Supplementary Figure 3A and B), which showed a greater cognitive benefit for healthy older adults (SMD = 0.27; p = .02) but not those with MCI (SMD = −0.06; p = .67). Moreover, in-person social interaction (SMD = 0.27; p = .02) positively affected, whereas online interaction (SMD = −0.06; p = .67) did not. However, when considering intervention dose as divided by the mean total dose of the included studies (≥92.5 hr and <92.5 hr), no statistically significant subgroup effect was found (p = .80; Supplementary Figure 3C).
Discussion
To our knowledge, this is the first systematic review and meta-analysis of the randomized controlled trials examining the effect of social interaction interventions on cognitive functions among older adults without dementia. The findings suggest that these interventions demonstrate a discernible effect on cognitive function, particularly in executive function, but the overall impact appears modest.
In contrast to the stronger relationships identified in previous systematic reviews and meta-analyses (Evans et al., 2019; Kelly et al., 2017; Kuiper et al., 2016; Piolatto et al., 2022), we found a smaller effect of social interaction on cognitive function for several reasons. First, previous SRMAs primarily included observational studies, which may face the challenge of reverse causation, that is, older adults with better cognitive function are more likely to maintain social engagement, and the inherent confounding effects such as healthier lifestyles, greater social support, and higher socioeconomic status among more socially active individuals might also lead to overestimating the association. Furthermore, the shorter follow-up periods in RCTs make it challenging to observe changes in cognitive function, as these changes typically require longer periods to become detectable. Consequently, RCTs often focus on observing changes in cognitive functions rather than incidences of dementia. Another difference is generalizability, as observational studies often include larger cohorts, making their results more representative. In contrast, the results of RCTs are more susceptible to the characteristics of the selected groups in the trial due to their smaller sample size, which leads to heterogeneity in the results across RCTs and a weaker overall association, as shown in this meta-analysis. These factors (Breitner et al., 2022; Peters et al., 2022) highlight the differences between observational studies and RCTs and explain why our findings are more conservative.
In response to substantial heterogeneity in global cognition, we performed a leave-one-out analysis and found that Morton et al.’s (2018) study contributed to the high heterogeneity of global cognition, which may be related to its differences in intervention when compared to other studies. Morton’s study used online social interactions, involving basic computer skills and operating specific social apps, which may have enhanced cognitive function. Dodge et al.’s (2015) study, on the other hand, focuses on simpler platform conversations that require no prior computer skills or application-specific operational learning. This may explain why Morton’s study found a greater improvement in global cognition.
In the subgroup analysis, the results showed a greater cognitive benefit among normal older adults but not the older adults with MCI. This could be related to the poor baseline cognitive function in older adults with MCI, making it more challenging for them to experience changes through social interaction. Additionally, most studies done in MCI participants involved intervention with online social interaction, which was known to have a weaker effect on cognitive improvement.
We found that social interaction intervention had a stronger effect on executive function than attention and memory. Executive function is a higher mental function involving self-regulation, planning, and inhibition. Participation in social interaction may facilitate the individuals’ ability to adopt different perspectives, mentally engage with others, and actively construct a nuanced understanding of each other’s minds. This process involves alternating between self and other perspectives, emphasizing the importance of effective communication and contributing to important components of executive function (Vygotsky, 1978; Ybarra et al., 2011).
This study is the first to explore the impact of social interaction interventions on cognitive function in older adults, covering various cognitive domains. We provided a more precise definition of social interaction and included a more comprehensive range of terms in our search strategy to cover multiple types of social interaction.
However, due to the limited number of articles, the study did not extensively investigate intervention types for specific cognitive domains and did not present a funnel plot to assess the presence of publication bias, considering insufficient statistical power (Egger et al., 1997). Despite the fact our study included a limited number of articles in the meta-analysis and may have had insufficient statistical power, we effectively tackled the challenges associated with observational studies and reached different conclusions.
Conclusion
The present study showed that social interaction interventions significantly improved executive function but had limited effects on global cognition, memory, and attention. Some of the included studies lacked rigorous research designs and clear reporting in terms of literature quality. For example, there was a need for explicit mention of random assignment methods or specific explanations of how allocation concealment was conducted. Thorough explanations of subject attrition rates and the use of intention-to-treat analysis to avoid bias were also lacking in several reviewed studies. Therefore, it is recommended that future research focus more strictly on these aspects and emphasize them in their reporting.
Future research is expected to examine and investigate the impact of different types of social interaction interventions on specific cognitive domains. As our meta-analysis is limited to studies up to May 2022, it is important to recognize that as more evidence from RCTs in this area accumulates, the overall conclusions could change. Periodic updates of the meta-analyses are encouraged. This ongoing research would enable public health and long-term care providers to offer more targeted interventions based on the specific needs or weaknesses in the cognitive domains of older adults, thereby slowing the cognitive decline.
Supplementary Material
Contributor Information
Chi-Chuan Wei, Institute of Public Health, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Min-Jia Hsieh, Institute of Public Health, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan; Department of Family Medicine, Taipei Veterans General Hospital, Taipei, Taiwan.
Yi-Fang Chuang, Institute of Public Health, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan; International Health Program, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Funding
None.
Conflict of Interest
None.
Data Availability
All data relevant to the study are included in the article or uploaded as Supplementary Information. Our review was preregistered in PROSPERO and can be accessed by searching the PROSPERO database for registration number CRD42022367828.
Author Contributions
Y.-F. Chuang and C.-C. Wei conceived and designed the study. C.-C. Wei and M.-J. Hsieh acquired the data. C.-C. Wei analyzed the data. C.-C. Wei, M.-J. Hsieh, and Y.-F. Chuang interpreted the data and drafted the manuscript. All authors contributed to the manuscript revision and approved the final submitted version.
References
- Borenstein, M., Hedges, L. V., Higgins, J. P., & Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods, 1(2), 97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- Breitner, J. C. S., Dodge, H. H., Khachaturian, Z. S., & Khachaturian, A. S. (2022). “Exceptions that prove the rule”—Why have clinical trials failed to show efficacy of risk factor interventions suggested by observational studies of the dementia-Alzheimer’s disease syndrome? Alzheimer’s and Dementia, 18(3), 389–392. 10.1002/alz.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges, C. R., Carlson, M. C., Andrews, R. M., Rebok, G. W., & Bielak, A. A. M. (2021). Using cognitive intraindividual variability to measure intervention effectiveness: Results from the Baltimore experience corps trial. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(4), 661–670. 10.1093/geronb/gbaa009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M. C., Saczynski, J. S., Rebok, G. W., Seeman, T., Glass, T. A., McGill, S., Tielsch, J., Frick, K. D., Hill, J., & Fried, L. P. (2008). Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist, 48(6), 793–801. 10.1093/geront/48.6.793 [DOI] [PubMed] [Google Scholar]
- Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., & Thomas, J. (2019). Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database of Systematic Reviews, 10, ED000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge, H. H., Zhu, J., Mattek, N., Bowman, M., Ybarra, O., Wild, K., Loewenstein, D. A., & Kaye, J. A. (2015). Web-enabled conversational interactions as a means to improve cognitive functions: Results of a 6-week Randomized Controlled Trial. Alzheimer’s & Dementia, 1(1), 1–12. 10.1016/j.trci.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, H., Georgiou, A., & Westbrook, J. (2017). Social participation as an indicator of successful aging: An overview of concepts and their associations with health. Australian Health Review, 41(4), 455–462. 10.1071/AH16038 [DOI] [PubMed] [Google Scholar]
- Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeroff, T., Ingmann, T., & Banzer, W. (2018). Physical activity throughout the adult life span and domain-specific cognitive function in old age: A systematic review of cross-sectional and longitudinal data. Sports Medicine, 48(6), 1405–1436. 10.1007/s40279-018-0920-6 [DOI] [PubMed] [Google Scholar]
- Eubank, J. M., Oberlin, D. J., Alto, A., Sahyoun, N. R., Asongwed, E., Monroe-Lord, L., & Harrison, E. A. (2022). Effects of lifestyle factors on cognition in minority population of older adults: A review. Frontiers in Nutrition, 9, 841070. 10.3389/fnut.2022.841070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, I. E., Martyr, A., Collins, R., Brayne, C., Clare, L., Anstey, K., & Peters, R. (2019). Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 70(s1), S119–S144. 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka, A., Suzuki, H., Ogawa, S., Kobayashi-Cuya, K. E., Kobayashi, M., Inagaki, H., Sugiyama, M., Awata, S., Takebayashi, T., & Fujiwara, Y. (2019). Does social interaction influence the effect of cognitive intervention program? A randomized controlled trial using Go game. International Journal of Geriatric Psychiatry, 34(2), 324–332. 10.1002/gps.5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, R., Sawada, S. S., Ito, T., Gando, Y., Fukushi, T., Yoshino, A., Kurita, S., Oka, K., Sakamoto, S., & Higuchi, M. (2019). Effect of watching professional baseball at a stadium on health-related outcomes among Japanese older adults: A randomized controlled trial. Geriatrics & Gerontology International, 19(8), 717–722. 10.1111/ggi.13687 [DOI] [PubMed] [Google Scholar]
- Kelly, M. E., Duff, H., Kelly, S., McHugh Power, J. E., Brennan, S., Lawlor, B. A., & Loughrey, D. G. (2017). The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259. 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto, M., Mangialasche, F., & Ngandu, T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Reviews Neurology, 14(11), 653–666. 10.1038/s41582-018-0070-3 [DOI] [PubMed] [Google Scholar]
- Kuiper, J. S., Zuidersma, M., Zuidema, S. U., Burgerhof, J. G. M., Stolk, R. P., Oude Voshaar, R. C., & Smidt, N. (2016). Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. International Journal of Epidemiology, 45(4), 1169–1206. 10.1093/ije/dyw089 [DOI] [PubMed] [Google Scholar]
- Lam, L. C., Chan, W. C., Leung, T., Fung, A. W., & Leung, E. M. (2015). Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: A cluster randomized controlled trial. PloS One, 10(3), e0118173. 10.1371/journal.pone.0118173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., ... Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396(10248), 413–446. 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer, J. A., Ding, D., Borenstein, A. R., DeCarli, C., Guo, Q., Wu, Y., Zhao, Q., & Chu, S. (2012). Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. Journal of Alzheimer’s disease, 30(4), 757–766. 10.3233/JAD-2012-120079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, T. A., Wilson, N., Haslam, C., Birney, M., Kingston, R., & McCloskey, L. G. (2018). Activating and guiding the engagement of seniors with online social networking: Experimental findings from the AGES 2.0 Project. Journal of Aging and Health, 30(1), 27–51. 10.1177/0898264316664440 [DOI] [PubMed] [Google Scholar]
- Myhre, J. W., Mehl, M. R., & Glisky, E. L. (2017). Cognitive benefits of online social networking for healthy older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 72(5), 752–760. 10.1093/geronb/gbw025 [DOI] [PubMed] [Google Scholar]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., ... Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. C., Lodi-Smith, J., Drew, L., Haber, S., Hebrank, A., Bischof, G. N., & Aamodt, W. (2014). The impact of sustained engagement on cognitive function in older adults: The Synapse Project. Psychological Science, 25(1), 103–112. 10.1177/0956797613499592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R., Dodge, H. H., James, S., Jicha, G. A., Meyer, P. F., Richards, M., Smith, A. D., Yassine, H. N., Abner, E., Hainsworth, A. H., Kehoe, P. G., Beckett, N., Anderson, C. S., & Anstey, K. J. (2022). The epidemiology is promising, but the trial evidence is weak. Why pharmacological dementia risk reduction trials haven’t lived up to expectations, and where do we go from here? Alzheimer’s & Dementia, 18(3), 507–512. 10.1002/alz.12393 [DOI] [PubMed] [Google Scholar]
- Piolatto, M., Bianchi, F., Rota, M., Marengoni, A., Akbaritabar, A., & Squazzoni, F. (2022). The effect of social relationships on cognitive decline in older adults: An updated systematic review and meta-analysis of longitudinal cohort studies. BMC Public Health, 22(1), 278. 10.1186/s12889-022-12567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkala, K. H., Routasalo, P., Kautiainen, H., Sintonen, H., & Tilvis, R. S. (2011). Effects of socially stimulating group intervention on lonely, older people’s cognition: A randomized, controlled trial. American Journal of Geriatric Psychiatry, 19(7), 654–663. 10.1097/JGP.0b013e3181f7d8b0 [DOI] [PubMed] [Google Scholar]
- Reger, M. A., Welsh, R. K., Watson, G. S., Cholerton, B., Baker, L. D., & Craft, S. (2004). The relationship between neuropsychological functioning and driving ability in dementia: A meta-analysis. Neuropsychology, 18(1), 85–93. 10.1037/0894-4105.18.1.85 [DOI] [PubMed] [Google Scholar]
- Richardson, M., Garner, P., & Donegan, S. (2019). Interpretation of subgroup analyses in systematic reviews: A tutorial. Clinical Epidemiology and Global Health, 7(2), 192–198. 10.1016/j.cegh.2018.05.005 [DOI] [Google Scholar]
- Shin, J. H. (2022). Dementia epidemiology fact sheet 2022. Annals of Rehabilitation Medicine, 46(2), 53–59. 10.5535/arm.22027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P. J., Blumenthal, J. A., Hoffman, B. M., Cooper, H., Strauman, T. A., Welsh-Bohmer, K., Browndyke, J. N., & Sherwood, A. (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72(3), 239–252. 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier, W. M., & Scheltens, P. (2005). Epidemiology and risk factors of dementia. Journal of Neurology, Neurosurgery and Psychiatry, 76(Suppl 5), v2–v7. 10.1136/jnnp.2005.082867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky, L. S. (1978). Mind in society: Development of higher psychological processes. Harvard University Press. 10.2307/j.ctvjf9vz4 [DOI] [Google Scholar]
- World Health Organization. (2020). Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health(2022, October 1, date accessed). [Google Scholar]
- Ybarra, O., Winkielman, P., Yeh, I., Burnstein, E., & Kavanagh, L. (2011). Friends (and Sometimes Enemies) with cognitive benefits: What types of social interactions boost executive functioning? Social Psychological and Personality Science, 2, 253–261. 10.1177/1948550610386808 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supplementary Information. Our review was preregistered in PROSPERO and can be accessed by searching the PROSPERO database for registration number CRD42022367828.