Abstract
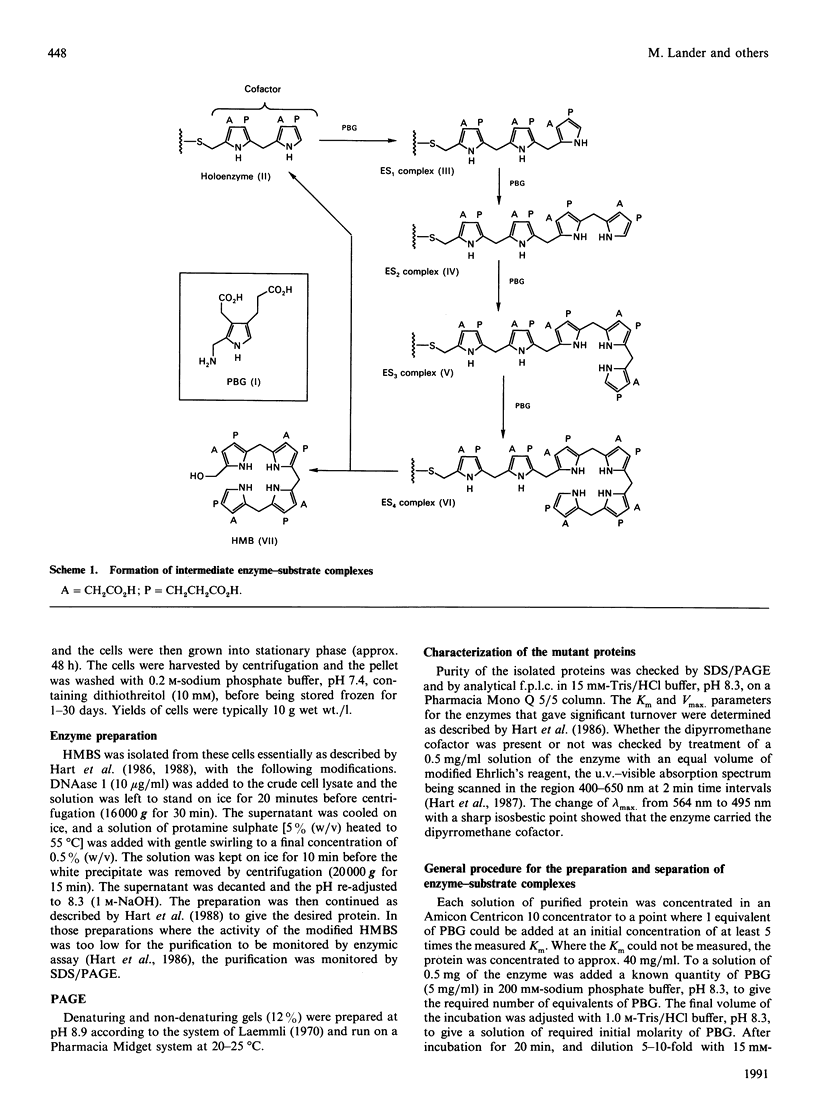
The role of conserved arginine residues in hydroxymethylbilane synthase was investigated by replacing these residues in the enzyme from Escherichia coli with leucine residues by using site-directed mutagenesis. The kinetic parameters for these mutant enzymes and studies on the formation of intermediate enzyme-substrate complexes indicate that several of these arginine residues are involved in binding the carboxylate side chains of the pyrromethane cofactor and the growing oligopyrrole chain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Abell C., Battersby A. R. The sequence of hemC, hemD and two additional E. coli genes. Nucleic Acids Res. 1988 Oct 25;16(20):9871–9871. doi: 10.1093/nar/16.20.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby A. R., Fookes C. J., Matcham G. W., McDonald E. Biosynthesis of the pigments of life: formation of the macrocycle. Nature. 1980 May 1;285(5759):17–21. doi: 10.1038/285017a0. [DOI] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Abell C., Battersby A. R. Purification, N-terminal amino acid sequence and properties of hydroxymethylbilane synthase (porphobilinogen deaminase) from Escherichia coli. Biochem J. 1986 Nov 15;240(1):273–276. doi: 10.1042/bj2400273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Miller A. D., Battersby A. R. Evidence that the pyrromethane cofactor of hydroxymethylbilane synthase (porphobilinogen deaminase) is bound through the sulphur atom of a cysteine residue. Biochem J. 1988 Jun 15;252(3):909–912. doi: 10.1042/bj2520909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Warren M. J. Evidence for a dipyrromethane cofactor at the catalytic site of E. coli porphobilinogen deaminase. FEBS Lett. 1987 Dec 10;225(1-2):87–92. doi: 10.1016/0014-5793(87)81136-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeper F. J. The biosynthesis of porphyrins, chlorophylls, and vitamin B12. Nat Prod Rep. 1985 Feb;2(1):19–47. doi: 10.52054/FVVO.16.2.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper F. J. The biosynthesis of porphyrins, chlorophylls, and vitamin B12. Nat Prod Rep. 1985 Dec;2(6):561–580. doi: 10.1039/np9850200561. [DOI] [PubMed] [Google Scholar]
- Leeper F. J. The biosynthesis of porphyrins, chlorophylls, and vitamin B12. Nat Prod Rep. 1987 Aug;4(4):441–469. doi: 10.1039/np9870400441. [DOI] [PubMed] [Google Scholar]
- Leeper F. J. The biosynthesis of porphyrins, chlorophylls, and vitamin B12. Nat Prod Rep. 1989 Apr;6(2):171–203. doi: 10.1039/np9890600171. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Packman L. C., Hart G. J., Alefounder P. R., Abell C., Battersby A. R. Evidence that pyridoxal phosphate modification of lysine residues (Lys-55 and Lys-59) causes inactivation of hydroxymethylbilane synthase (porphobilinogen deaminase). Biochem J. 1989 Aug 15;262(1):119–124. doi: 10.1042/bj2620119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Romeo P. H., Dubart A., Beaupain D., Cohen-Solal M., Goossens M. Molecular cloning and complete primary sequence of human erythrocyte porphobilinogen deaminase. Nucleic Acids Res. 1986 Aug 11;14(15):5955–5968. doi: 10.1093/nar/14.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Nepveu A., Echelard Y., Dymetryszyn J., Drolet M., Goyer C. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J Bacteriol. 1987 Sep;169(9):4257–4262. doi: 10.1128/jb.169.9.4257-4262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif A. L., Smith A. G., Abell C. Isolation and characterisation of a cDNA clone for a chlorophyll synthesis enzyme from Euglena gracilis. The chloroplast enzyme hydroxymethylbilane synthase (porphobilinogen deaminase) is synthesised with a very long transit peptide in Euglena. Eur J Biochem. 1989 Sep 15;184(2):353–359. doi: 10.1111/j.1432-1033.1989.tb15026.x. [DOI] [PubMed] [Google Scholar]
- Stubnicer A. C., Picat C., Grandchamp B. Rat porphobilinogen deaminase cDNA: nucleotide sequence of the erythropoietic form. Nucleic Acids Res. 1988 Apr 11;16(7):3102–3102. doi: 10.1093/nar/16.7.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. J., Jordan P. M. Investigation into the nature of substrate binding to the dipyrromethane cofactor of Escherichia coli porphobilinogen deaminase. Biochemistry. 1988 Dec 13;27(25):9020–9030. doi: 10.1021/bi00425a021. [DOI] [PubMed] [Google Scholar]



