Abstract
Millions of citrus products are wasted every year due to postharvest fungal infections. To minimize fungal infections, packhouses utilize aqueous applications of fungicides to prevent infections that occur during harvest. The most prominent fungal pathogens of citrus fruit are commonly treated with imazalil sulfate (IMZ) due to its efficacy for controlling these pathogens at low cost and ease of handling. However, little is known on how it alters the tissues in the citrus fruit physiology. In this study, a nuclear magnetic resonance (NMR)-based metabolomics study is utilized to investigate the role of IMZ treatment in the juice, albedo, and flavedo tissues of two citrus commodities (navels and clementines). The experimental design consists of (a) fresh fruits at harvest, (b) raw fruits stored at 4 °C for 10 days, and (c) raw fruits treated with IMZ and stored at 4 °C for 10 days. Twenty-seven metabolites were identified, and several changes of metabolite composition due to either cold storage or IMZ treatment for both the spatial (albedo, flavedo, or juice) and temporal levels (days and storage) were found. The results show a notable difference between metabolomics profiles across the types and tissues, particularly significant changes on the albedo tissues of clementine. Furthermore, the pathways derived from the metabolomics profiles of the cold storage and the IMZ treatment are complementary to each other. Thus, the utility of metabolomics as a quality control tool in the citrus industry has the potential for broader applications to understand fruit growth and development.
Keywords: citrus, metabolomics, nuclear magnetic resonance (NMR), imazalil sulfate (IMZ), fungicide
1. INTRODUCTION
The citrus industry is a major contributor to the agricultural economy. It has been reported that nearly 100 million tons of citrus are produced annually throughout the world, with oranges and mandarins at the highest consumption rate.1 Unfortunately, the citrus industry is susceptible to many threats that can drastically reduce citrus production every year. These threats include preharvest diseases such as Diplodia natalensis and Phomopsis citri, postharvest disease including Penicillium digitatum and Penicillium italicum, overmature fruit, insect damage, and a variety of physiological disorders.2 The citrus season typically lasts from early winter to late spring for most varieties in both hemispheres; therefore, both have to rely on importing products during the off-season and exporting products during the season to keep the citrus products in the market. Exporting fruit overseas takes a considerable amount of time, so the products must maintain shelf life. Therefore, adequate chemical application prior to shipping is crucial for the process.
There are many preharvest and postharvest pathogens, most of which can be controlled with fungicides. P. digitatum and P. italicum are the most abundant postharvest diseases. P. digitatum is the more virulent of the two and is predicted to contribute nearly 50% of total postharvest losses.3 P. digitatum, also referred to as green mold, is the most prevalent postharvest pathogen for citrus fruit that is capable of infecting any citrus species in wounds inflicted during harvest. Wounds also occur as fruit enters the packhouse as it is processed to be distributed to the market.4 Not only does the fruit provides nutrients that allow P. digitatum to thrive, but the rind of the fruit also contains aromatics that induce germination.5 P. italicum, also known as blue mold, is similar to P. digitatum in many regards. To reduce product loss due to P. digitatum and P. italicum infections, packhouses utilize aqueous fungicide applications to prevent and reduce infections that occur during harvest. Imazalil, also known as enilconazole (1-[2-(2,4-dichlorophenyl)-2(2-propenyloxy)ethyl]-1H-imidazole, IMZ), is the most often used fungicide to treat P. digitatum and P. italicum infections due to its low cost, efficacy, and ease of application.
The noninvasive nature of nuclear magnetic resonance (NMR) has been applied to study many aspects of citrus metabolomics at varying experimental conditions.6–11 This work focuses on the effect of fungicide treatment on the metabolomics alterations in citrus fruits. Although imazalil sulfate (IMZ) is a widely utilized fungicide in the citrus industry, little is known about how it alters the chemical composition of the tissues or juice of the citrus fruit. This study aims to identify how IMZ treatment alters metabolites and induces or prevents biochemical pathways in the flavedo, albedo, and juice of Fukumoto navel oranges and clementine mandarins. According to the Environmental Protection Agency (EPA), IMZ is likely to be a human carcinogen (moderately hazardous). Therefore, understanding the metabolomics profiles of its effect on the citrus assists in both quality control of the packhouse fruit processing and the development of better and greener alternative fungicides.
2. MATERIALS AND METHODS
2.1. Experimental Design.
Twenty-four Fukumoto navel oranges and 60 clementine mandarin fruits were harvested from California State University Orchards in November 2020. Each commodity was randomized and equally divided into three treatment groups: a control, fruit to be kept in cold storage, and fruit treated with IMZ and kept in cold storage. The tissues from the control groups were isolated on the day of harvest. The cold storage group was placed in a cold room at 4 °C (~40 °F), 10 days before tissue isolation. The final group was dipped in a 500 ppm IMZ solution for 30 s and then placed in a cold room at 4 °C for 10 days before tissue isolation. The time spent in cold storage after harvest can vary depending on market conditions, the amount of product in inventory, or even the quality of the product. Ten days in storage represent ideal conditions in which fruit is processed and sent to the market with minimal delays. The concentration used for the experiment was based on the maximum label rate, 500 ppm, which is commonly used in the industry.
2.2. Sample Preparation.
All chemical reagents were purchased from Fisher Scientific except for the Fungaflor 75 WSG, IMZ, which was donated by JBT Corporation in Visalia, CA.
Tissue isolation was performed by randomly dividing fruit into five replicates from each treatment group. One gram of flavedo and albedo tissues was collected from all of the fruit in each replicate. Each tissue was homogenized and ground in a mortar and pestle with the aid of liquid nitrogen. Two hundred and forty milligrams of each tissue from each replicate was collected in a 5 mL Eppendorf tube to be further processed for metabolite identification and quantification. Fifty milliliters of juice was collected from all fruit in one replicate to be further processed for metabolite identification and quantification.
Tissue sample preparation was adapted from methods established by Slisz and Kim with some slight modifications.12,13 A total of 3 mL of methanol were aliquoted into the 5 mL Eppendorf tubes containing the tissue. The samples containing the methanol were sonicated for 15 min. The samples were centrifuged at 18 000g for 20 min. The supernatant was transferred to a clean 5 mL Eppendorf tube with two 1 mm holes drilled on the cap to serve as a means of solvent evaporation during lyophilization. Samples were snap-frozen using liquid nitrogen and lyophilized for 48 h.
Juice sample preparation was adapted by methods established by Slisz with some slight modifications.13 Fifty milliliters of the juice was centrifuged at 14 000g and ~4 °C for 20 min. Four milliliters of supernatant were transferred to 3000 kDa molecular weight cutoff filters. The samples were centrifuged at 5000g and ~4 °C for 10 min. One milliliter of the filtrate was transferred to clean 1.5 mL Eppendorf tubes with a 1 mm hole drilled on the cap to serve as a means for solvent evaporation during lyophilization. Samples were frozen and lyophilized for 48 h.
After lyophilization, all of the samples were resuspended in 650 μL of a solution containing 90 mM KH2PO4, 0.2 mM imidazole, and 0.05 mM sodium trimethylsilyl [2,2,3,3-d4] propionate (TSP) D2O solution with the pH adjusted to 6.8. Samples were vortexed for 20 s and were centrifuged at 18 000g for 20 min. Finally, 600 μL of supernatant was transferred to clean, 5 mm prelabeled NMR tubes. All control samples were prepared on the same day; the treated samples were prepared over 2 days, and samples were subsequently stored at 4 °C before the NMR experiments.
2.3. NMR Experiments.
Samples were analyzed using a 400 MHz Varian-Agilent NMR spectrometer equipped with double-resonance (one-NMR) and Z-axis pulsed field gradients. Each spectrum was collected using a one-dimensional experiment, with a 70° pulse width, an acquisition time of 1.64 s per transient, over 1024 transients, and a recycling delay of 2 s between the transients. All of the NMR experiments were performed at 30 °C.
Metabolites were identified and quantified using Chenomx NMR Suite 8.1 software (Chenomx Inc., 2014). Each spectrum was zero-filled once with an exponential apodization of 0.5 Hz prior to Fourier transformation. All of the spectra were phase-corrected and baseline-corrected. The spectral data were further calibrated for the pH of the sample with reference to imidazole and chemical shift and concentration calibrated with respect to the TSP peak. Metabolites were identified by comparing the spectra to the Chenomx library, utilizing the list of metabolites identified from the literature.
2.4. Statistical Analysis of NMR Spectroscopy Data Sets.
A multivariate statistical analysis approach was employed to identify differentially altered metabolites under the experimental design described previously. These statistical methods were based on established protocols within the R-statistical methods and were applied previously for metabolomics and other analyses.14–16 A brief description is given below.
Using linear modeling (within the LIMMA package), metabolites differentially altered between the groups are determined. The control group consists of the albedo, flavedo, and the juice of the two commodities soon after the harvest, and the other groups consist of the same tissues and juice stored at a cold temperature for 10 days with or without the treatment of IMZ. Using a two-step process, the differentially altered metabolites within a single feature (e.g., control albedo vs cold storage albedo of the navel) selected were combined with other comparisons combined. The combined set was used to detect metabolites that were differentially altered using an F-test, and the p-values were transformed for multiple comparisons using the Benjamini–Hochberg procedure for false-discovery rate (FDR) adjustment. Metabolites are considered significant within a given comparison if they pass the threshold of both fold-change (>1.5) and FDR-adjusted p-value (<0.05). All of the analyses and the plots were performed using the R-statistical environment.17
3. RESULTS
3.1. Overview on the Detection of Metabolites.
Twenty-seven metabolites were identified and quantified using the experimental data collected at 400 MHz. The supporting figure (Figure S1) shows the representative example of metabolite identification. The metabolites fall under the broader classification with 4 sugar molecules, 10 amino acids, 7 organic acids, and 6 other metabolites. Supporting tables list the concentration of the metabolites for all of the detected metabolites for albedo tissues (Table S1), flavedo tissues (Table S2), and juice (Table S3) for both the clementine and navel oranges under various experimental conditions. Though most of the metabolites were detected across the two types of citrus and under the various experimental conditions, some exceptions were noted due to detection limits. Asparagine was not detected in the albedo or juice samples, aspartate and 2-phosphoglycerate were not detected in albedo and flavedo tissues, while 4-aminobutyrate and malate were not detected in the juice samples, in either navel or clementine varieties.
The overall distribution of metabolite concentrations across the experimental conditions is depicted in Figure 1. The hierarchical clustering (Euclidean distant metrics) across the experimental conditions of the samples (horizontal labels) and all of the metabolites (vertical labels) are shown. The sample types are clusters together in the navel oranges (Figure 1A) and clementine mandarins (Figure 1B). The similarities include sugars (glucose, fructose, sucrose, and myo-inositol) from the major cluster in the juice samples. Similarly, asparagine, 4-amino-butyrate, and malate are relatively low (or not detected) in the juice samples. The concentration levels of the sugars in the juice have a high dynamic range compared to those of albedo and flavedo tissues. The juice of both commodities contains a higher concentration of many amino acids, including arginine, aspartate, leucine, isoleucine, and valine. The composition of citrate is the highest in the juice of both commodities as well. There are some notable differences of some metabolites in the tissues between the two commodities. Synephrine is more abundant in the albedo and flavedo tissues in clementine compared to the navel. Phenylalanine is more abundant in the albedo and flavedo tissues in navels compared to clementine.
Figure 1.
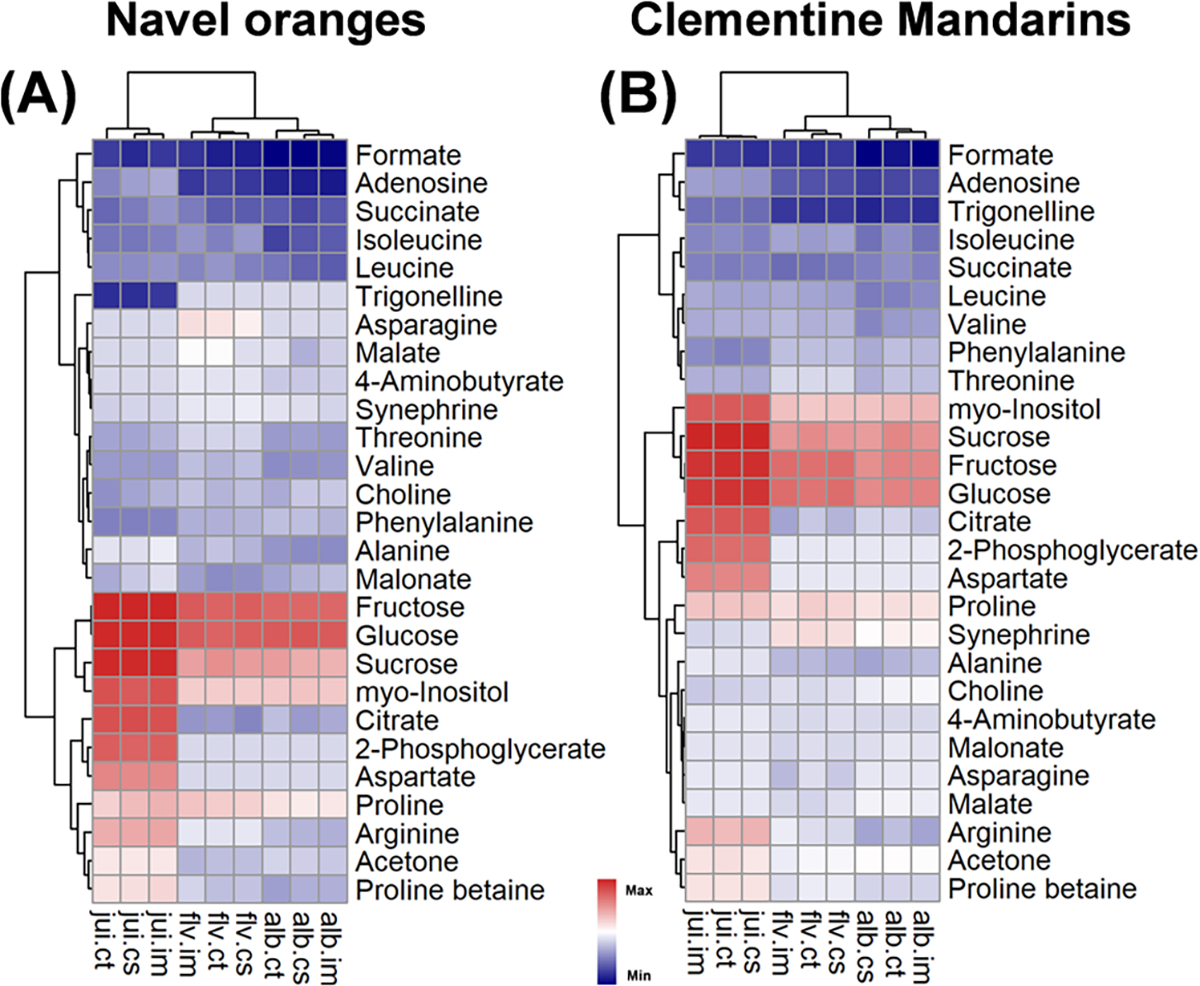
Distribution of detected metabolite concentrations. Hierarchical clustering of the metabolites shown as a heatmap for the (A) navel oranges and (B) clementine mandarins. Both the metabolites and experimental conditions are clustered on a Euclidean distance metric, and the final concentrations (mM) are scaled for visual presentation. The samples are juice (jui), flavedo (flv), and albedo (alb), and experimental conditions are control, fresh after harvest (ct), cold storage for 10 days (cs), and cold storage for 10 days with IMZ treatment (im).
The discriminations between the different treatment conditions (control, cold storage for 10 days, and cold storage with fungicide treatment) using a partial least-squares discrimination analysis (PLS-DA) are shown in Figure 2. The control sample (fresh after harvesting) group has more considerable discrimination (red symbols) from either cold storage (green symbols) or cold storage with IMZ treatment (blue symbols) in most cases. The cold storage and cold storage with IMZ treatment tend to group close to each other in most of the conditions. The fresh albedo samples of clementine mandarins show one of the largest variations within the replicates (green symbols, Figure 2B). Most of the samples have more than 20% discrimination along with either of the first two components of the PLS-DA classification.
Figure 2.
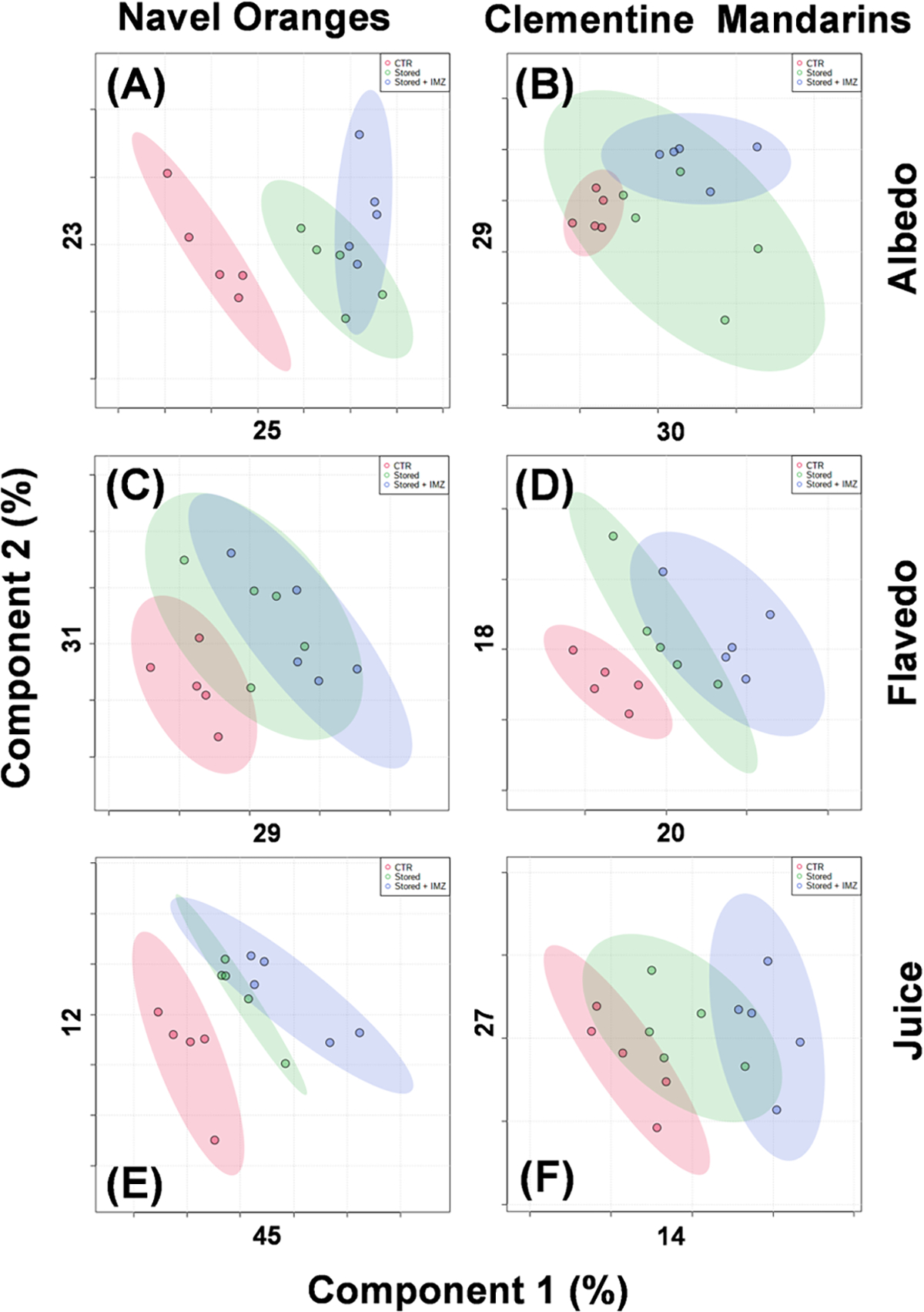
Classification of metabolomics profiles. Partial least-squares discrimination analysis (PLS-DA) of the navel oranges (left panels) and clementine mandarins (right panels) for the albedo (A, B), flavedo (C, D) tissues, and juice (E, F). The control samples are in red, cold storage samples are in green, and cold storage with IMZ treatment are in blue. The shaded ellipses show the 90% confidence region on the classification. The percentage component of discrimination along the first two dimensions is given below each panel.
3.2. Effect of Cold Storage on the Metabolomics Profiles.
With general discriminations identified by the PLS-DA (Figures 1 and 2), specific metabolites altered due to changes in the experimental conditions for the different tissues/juice are determined using multivariate statistics. Metabolites are considered significant if the statistical measures fit the criteria that the |fold-change| (>1.5) and FDR-adjusted p-value (<0.05). Twelve different metabolites are differentially altered in the flavedo or albedo tissues in either the navel oranges or the clementine mandarins. Figure 3 shows the box–whisker plots of the concentrations of these metabolites, and Table S4a lists them. Suppose any of these metabolites are considered significant (FC > 1.5 and p-value < 0.05), the respective conditions are marked in the plots with a horizontal bar between the conditions. In general, the metabolite levels are downregulated in cold storage. The effect of cold storage shows a relatively more significant number of metabolites altered in clementine mandarins than in the navel oranges. Eight metabolites are differentially altered in either albedo or flavedo tissues of clementine mandarins (sucrose, synephrine, phenylalanine, isoleucine, valine, arginine, formate, and trigonelline). In comparison, five metabolites are differentially altered (choline, leucine, malate, citrate, and isoleucine) in the navel orange. Cold storage reduces the level of leucine in both the albedo (FC = −1.5) and flavedo (FC = −1.8) tissues of the navel oranges. Within the albedo tissues, isoleucine shows an increase (FC = 1.5) in navel oranges and a decrease (FC = −1.9) in the clementine due to cold storage.
Figure 3.
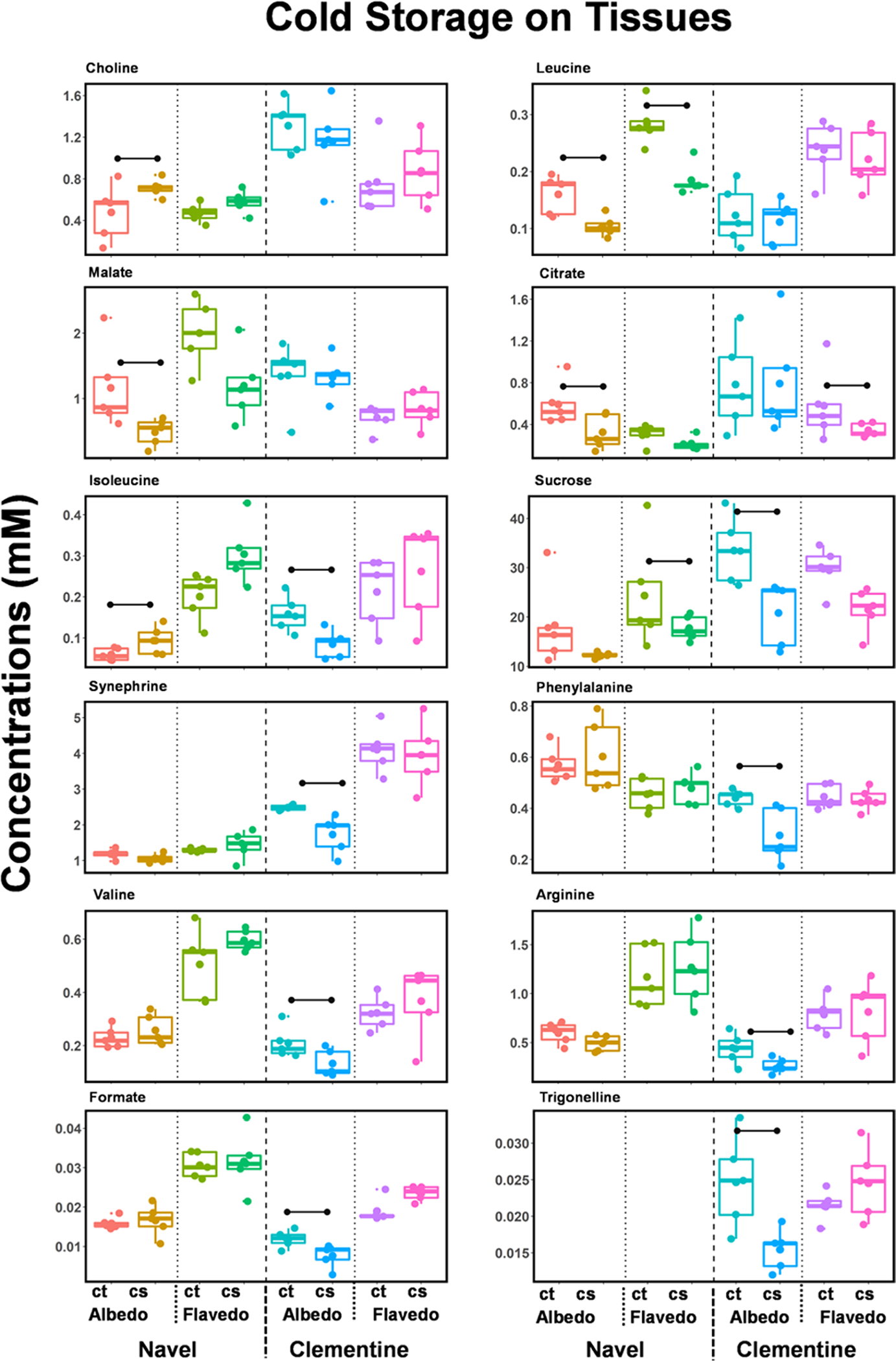
Metabolites altered in the albedo and flavedo tissues due to cold storage. Box–whisker plots of the metabolites significantly altered in at least one of the tissues (albedo or flavedo) in either navel oranges or clementine mandarins. The metabolites are identified at the top of each panel, and the horizontal bar indicates a significant change in both |fold-change| (>1.5) and p-value (<0.05)—control: ct, cold storage: cs.
The concentration changes due to the cold storage of juice samples for the navel oranges and the clementine mandarins are in Figure 4, with the fold changes listed in Table S4 (panel a). Cold storage has an almost negligible effect on the metabolomics levels of the juice. No metabolites are significantly altered in the clementine mandarins, while adenosine (FC = 1.6), malonate (FC = 1.84), and proline (FC = 1.54) are altered in the navel oranges (Table S4a).
Figure 4.
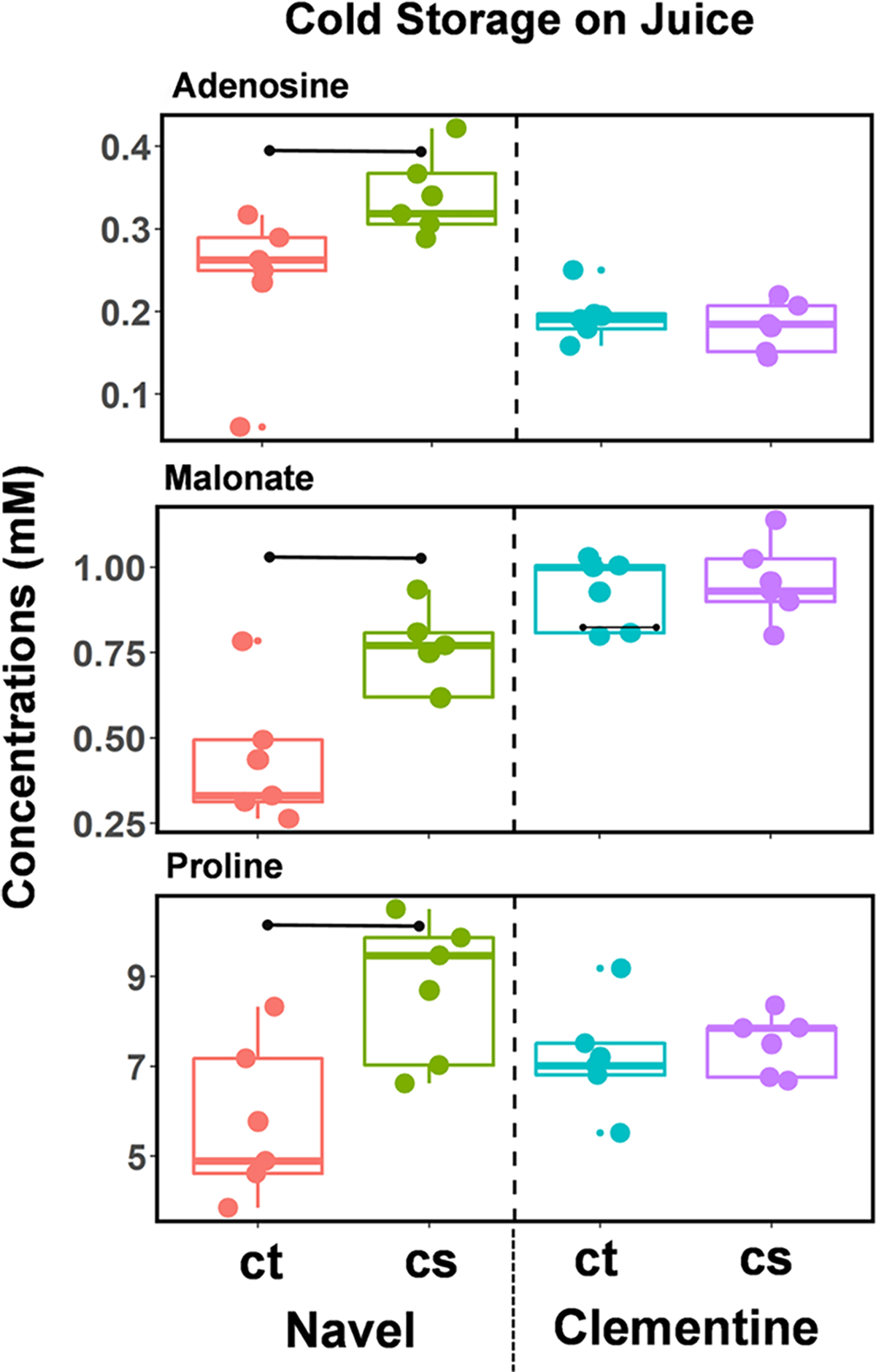
Metabolites altered in the juice due to cold storage. Box–whisker plots of the metabolites that are significantly in the juice sample of navel oranges or clementine mandarins. The metabolites are identified at the top of each panel, and the horizontal bar indicates a significant change in both |fold-change| (>1.5) and p-value (<0.05).
3.3. Effect of IMZ Treatment on the Metabolomics Profiles.
The IMZ-treated fruits were stored in the cold room. Therefore, to eliminate the effect of cold storage on the metabolomics profiles, a comparison between the fruits stored in the cold room with or without fungicide treatment is considered. Figure 5 shows the metabolite changes exclusively due to IMZ, and Table S4b lists the fold changes of all of the metabolites. Under the same criterion for significance (|fold-change| > 1.5 and p-value < 0.05), three metabolites show differential changes: alanine, valine, and succinate. Succinate shows an increase of 1.78 times due to the treatment in the flavedo tissues of navel oranges, while alanine and valine show FC of 1.66 and 1.79, respectively, in the albedo tissues of clementine mandarins. Most of the metabolites measured in the juice do not show any significant changes, except for succinate, which is upregulated in the navel oranges.
Figure 5.
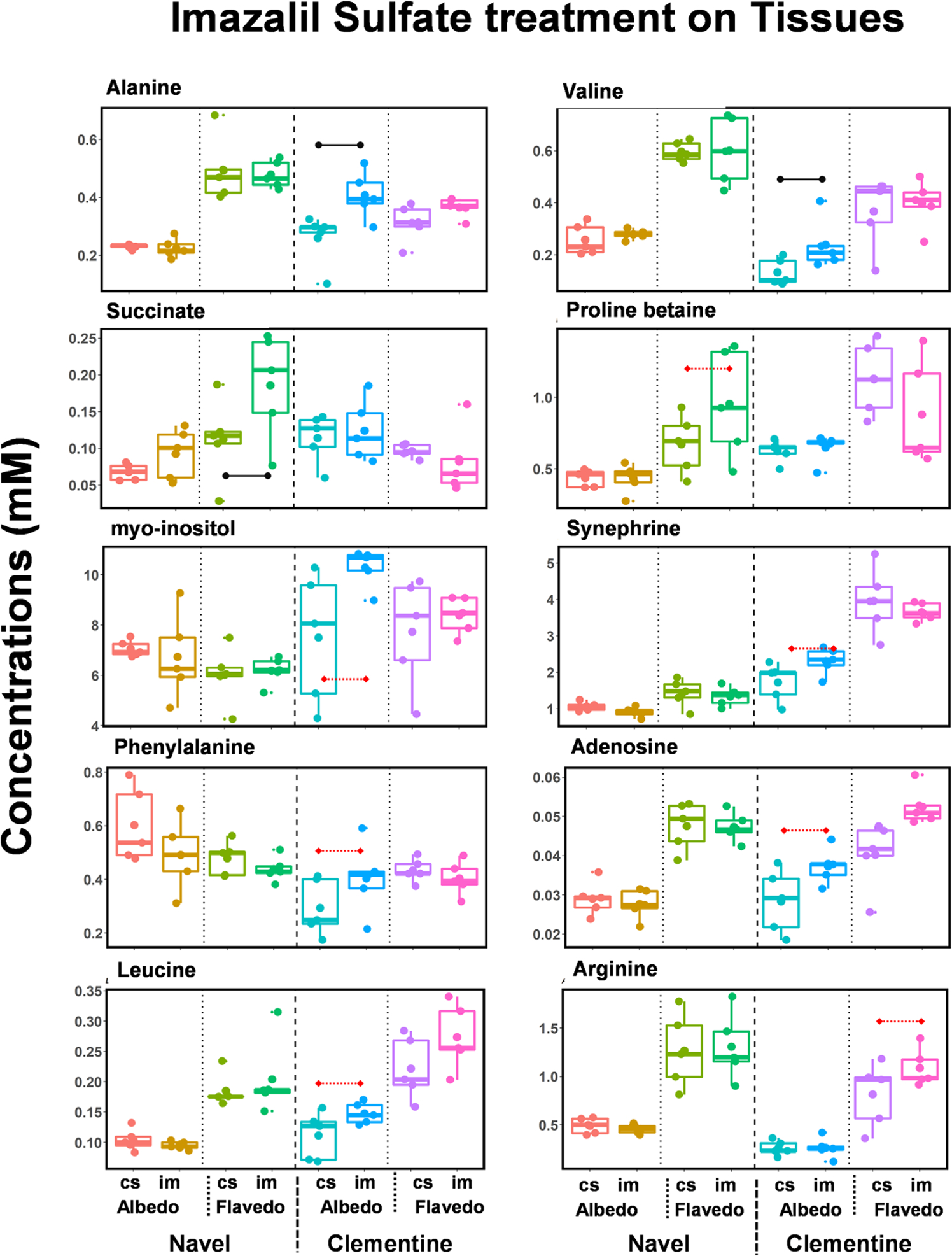
Metabolites altered in the albedo and flavedo tissues due to IMZ treatment. Box–whisker plots of the metabolites significantly altered in at least one of the tissues (albedo or flavedo) in either navel oranges or clementine mandarins. The metabolites are identified at the top of each panel, and the horizontal bar indicates a significant change in both |fold-change| (>1.5) and p-value (<0.05). Horizontal bars (dotted lines, red) show the metabolites with |fold-change| (>1.3) and p-value (<0.05). Cold storage: cs, IMZ treatment in cold storage: im.
When the fold-change definition of metabolites that are altered is relaxed |FC| > 1.35 (with p-value < 0.05), additional metabolites are found to be significant (Table S4b). These include proline betaine (navel flavedo, FC = 1.38), adenosine (clementine, albedo, FC = 1.36), leucine (clementine, albedo, FC = 1.39), phenylalanine (clementine, albedo, FC = 1.38), synephrine (clementine, albedo, FC = 1.38), myo-inositol (clementine, albedo, FC = 1.44), and arginine (clementine, flavedo, FC = 1.44). The metabolites altered due to IMZ treatment are uniquely different from those altered due to cold storage. No metabolites are altered with a significant measure of |fold-change| > 1.5 and p-value < 0.05. However, arginine is upregulated by 1.44 times in clementine mandarin juice samples (data not shown).
3.4. Metabolomics Profiles of Cold Storage and IMZ Treatment in Cold Storage Differ.
Metabolomics analysis of navel oranges and clementine mandarins at postharvest conditions of cold storage or IMZ treatment in the cold storage showed similarities between the two varieties (Figure 1) and differences due to treatment (Figure 2). Multivariate analysis identified several potential metabolites (Figures 3–5) under different experimental conditions. Under the condition of significance (|fold-change| > 1.5 and p-value < 0.05), 16 metabolites were identified to be affected by either cold storage or IMZ treatment in cold storage. Under the slightly relaxed condition of significance (|fold-change| > 1.35 and p-value < 0.05), 22 metabolites were found to be differentially altered under either of the same conditions (Figures 3–5). The 12 metabolites that are altered due to cold storage in either of the two varieties at either albedo, flavedo, or juice have sugar (sucrose), amino acids (isoleucine, leucine, phenylalanine, valine, arginine, and proline), organic acids (malate, citrate, and formate), and other metabolites (choline and trigonelline). In comparison, the three metabolites altered due to cold storage treatment consist of the amino acid proline, organic acid malonate, and adenosine. The 10 metabolites altered due to cold storage and IMZ treatment are the sugar (myo-inositol), amino acids (leucine, phenylalanine, valine, arginine, and alanine), organic acid (succinate), and others (proline betaine, synephrine, and adenosine).
Under the cold storage conditions, 12 metabolites are differentially altered with respect to the control samples in the clementine mandarins (in albedo, flavedo, or juice) and 9 metabolites in navel oranges, 2 metabolites that are common between them (sucrose and isoleucine). With IMZ treatment of the 10 metabolites that are differentially expressed, two are in the navel oranges and eight in the clementine mandarins, with no common metabolites. Taken together, 12 metabolites are altered uniquely due to cold storage conditions, 7 metabolites change in both cold storage and IMZ treatment, while 3 metabolites are altered only due to IMZ treatment. Figure 6 shows the summary of the functional enrichment analysis of these select metabolites using the citrus clementine as the model system available at the CitrusCyc.18,19 The pathway enrichment plots show three major pathways, biosynthesis, energy metabolism, and degradation, that have distinct differences between metabolites’ subsets. In general, the 10 metabolites that are differentially altered due to IMZ treatment (Figure 7, red and orange bars) and the 12 metabolites that respond to cold storage (blue bars) are opposite to each other (based on the fold changes). Biosynthesis of amino acids (AA Syn), nucleosides (Nucleo Syn), cell-structure (Cell-Struct Syn), and cofactors (Cofactor Syn) are upregulated due to cold storage and downregulated due to IMZ treatment. In contrast, amine/polyamine biosynthesis (Amine Syn) has an opposite trend (Figure 7). The secondary metabolites (Sec Metab Syn) and metabolic regulator (Metab Reg Syn) biosynthesis are altered only due to cold storage.
Figure 6.
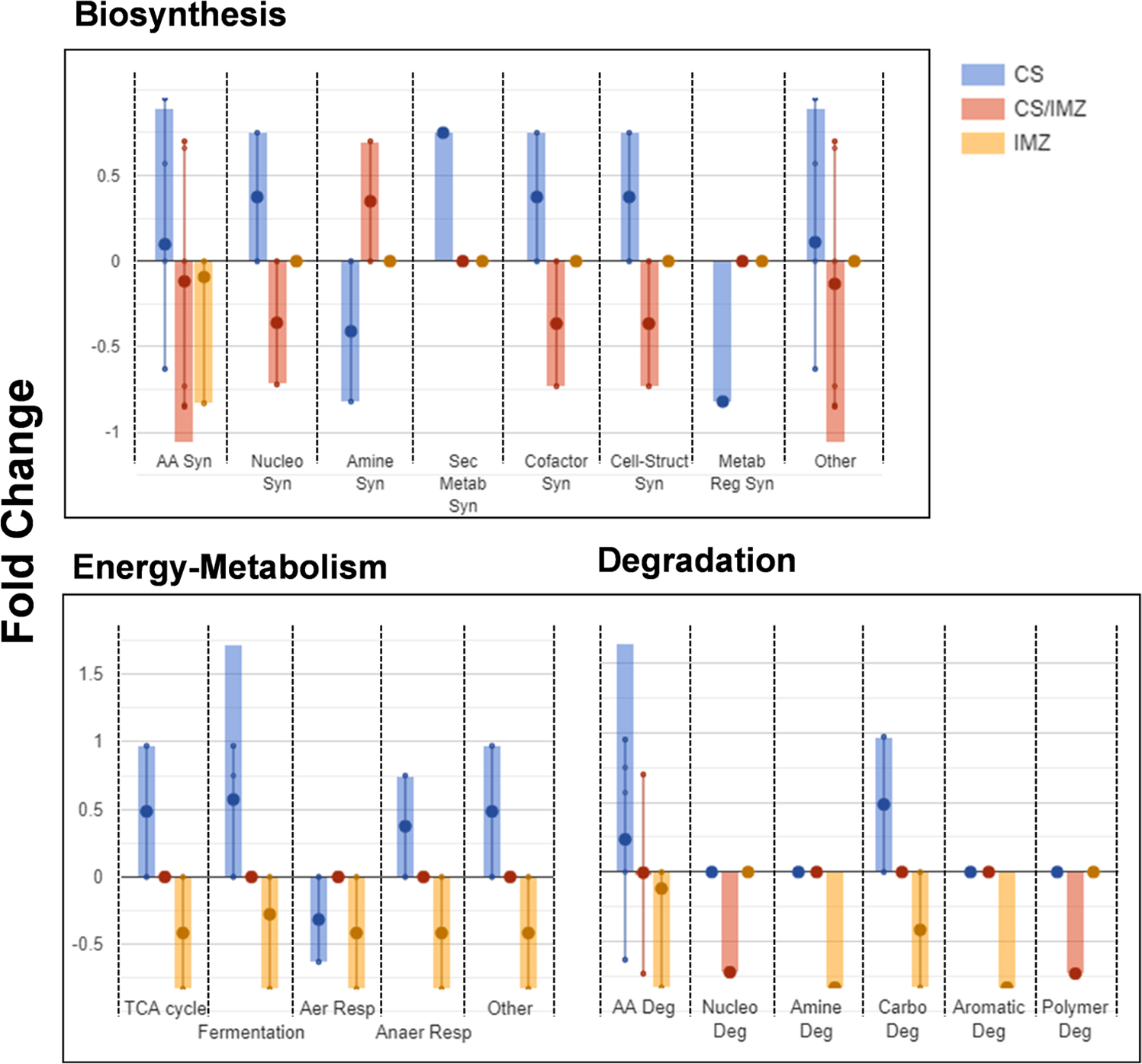
Pathway enrichment due to metabolomics changes. Three major pathways related to citrus clementine were plotted based on the relative changes in the metabolomics due to either cold storage (CS, blue), IMZ treatment (IMZ, orange), or common between both CS and IMZ (CS/IMZ, red). Pathway enrichment for three major pathways, biosynthesis, energy, and degradation, was plotted along with bar plots highlighting the more specific pathways. The y-axis of the plots are the relative fold-change in each experimental condition, with the error bars’ relative representation generated by the CitrusCyc database. Biosynthesis pathways include amino acid synthesis (AA Syn), nucleotide synthesis (Nucleo Syn), amine synthesis (Amine Syn), secondary metabolite synthesis (Sec Metab Syn), cofactor synthesis (Cofactor Syn), cell-structure synthesis (Cell-Struct Syn), metabolite regulation synthesis (Metab Reg Syn), and others. Energy metabolism pathways include tricarboxylic acid cycle (TCA cycle), fermentation, aerobic respiration (Aer Resp), anaerobic respiration (Anaer Resp), and others. Degradation pathways include amino acid degradation (AA Deg), nucleotide degradation (Nucleo Deg), amine degradation (Amine Deg), carbohydrate degradation (Carbo Deg), aromatic degradation (Aromatic Deg), and polymer degradation (Polymer Deg).
Figure 7.
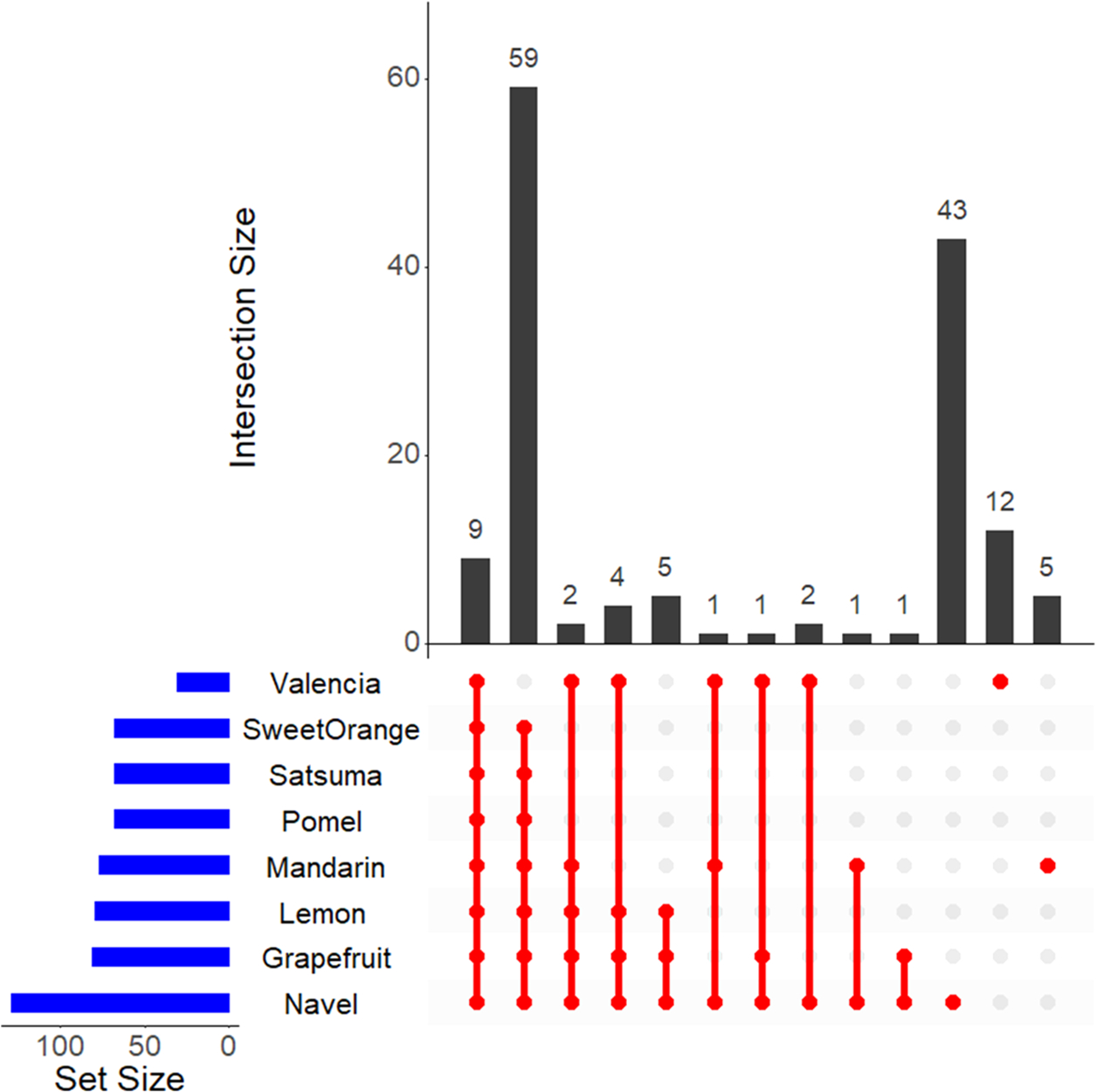
Venn diagram of metabolite distribution among the citrus variety. The horizontal bars (blue) show the number of metabolites identified in each variety of the citrus listed, vertical red lines/circles show the number of overlapping metabolites across the different varieties, and the bar graph (black) shows the total counts in each. Nine metabolites overlap across all of the varieties (leftmost bar), while five metabolites are uniquely identified in mandarins.
Cold storage and IMZ treatment have opposite trends within the energy metabolism pathways, particularly involving unique metabolites. TCA cycle, fermentation, and anaerobic respiration (Anaer Resp) pathways are upregulated due to cold storage but downregulated due to IMZ treatment, while aerobic respiration (Aer Resp) is downregulated in both (Figure 7). The degradation pathways all do show a downregulation when IMZ treatment is considered, with additional upregulation of degradation of amino acids (AA Deg) and carbohydrate (Carbo Deg) during the cold storage (Figure 7). Other pathways (data not shown) that have a similar trend include C1 compound (CO2 fixation, as well as pathways for utilization of carbon monoxide, formaldehyde, and methanol), utilization and assimilation (upregulated only in cold storage), non-carbon nutrients and macromolecule-modification (upregulated in cold storage and downregulated in IMZ treatment), and detoxification (upregulated in both cold storage and IMZ treatment).
Notably, the metabolomics pathways differentiate between the major experimental conditions, cold storage, and IMZ treatment, even though the fruits are stored at a cold temperature under treatment. Besides, most of the three categories’ pathways due to IMZ treatment are downregulated regarding the respective controls, while the cold storage upregulates the metabolites in the same pathway categories.
4. DISCUSSION
In the last decade, several notable applications of metabolomics to the citrus industry have been published under a specific combination of experimental conditions that employ one of the three popular techniques, NMR, gas chromatography-mass spectroscopy (GCMS), or high-performance liquid chromatography-mass spectroscopy (HPLC-MS).11,20–32 In the NMR-based metabolomics applications, Slupsky’s group has presented many studies ranging from nutrient composition to extended multiomics studies on graft inoculated with Candidatus Liberibacter asiaticus.13,20,21,30,31,33 Villa–Ruano et al. have presented a comprehensive study on the citrus juices produced in Veracruz, Mexico, including lemons, tangerines, oranges, and grapefruits.11 Several other studies on the NMR-based metabolomics on the juice samples have also been published, which include the degradation of fresh orange juice,34 nonthermal processing of orange juice,8 and profiles of Argentine citrus (orange) juice.10 While most studies focused on solution-phase metabolomics studies, high-resolution magic angle spinning (HR-MAS) NMR spectroscopy on semisolid samples has also been presented.6,35,36
In general, MS-based methods identify much more metabolites than the NMR-based approaches. Collectively, both the techniques have identified 155 metabolites, of which MS-based methods uniquely identified 96 metabolites, 22 metabolites by NMR, and the rest of 27 metabolites were shared between them.22,23,25–27,37–39 Figure 7 shows a matrix-based visualization of interacting sets (similar to a Venn diagram) of the distribution of the various metabolites across the variety of citrus fruits using the UpSetR (R-package).40 The largest number of metabolites were found in navel oranges (>100); perhaps, it is the most studied variety. The nine metabolites shared across all of the eight varieties of the citrus fall under the class of amino acids (alanine, asparagine, isoleucine, leucine, phenylalanine, proline, threonine, and valine) along with sugar and sucrose.
In our study, we have identified all of the metabolites in at least one of the conditions. The analysis also revealed that the mandarins and valencia (oranges) have a unique set of 5 and 12 metabolites, respectively. The five unique metabolites of mandarins are 2-ketoglutaric acid, galacturonic acid, ornithine, scyllo-inositol, and xylose, and none of them were identified in this study. Under the broad classification of the identified metabolites, 42 of them fall under the category of organic acids, followed by 23 in the amino acids, with 9 in the flavonoids and 6 in the terpene categories. All of the metabolites presented in the study are a subset of the NMR-identified metabolites.
Based on the results presented here (Figure 1–5 and Tables S1–S4), there were considerable commonalities and differences between Fukumoto navel oranges and the clementine mandarins. There is a clustering of the four sugars identified for both species, but fructose and glucose are more abundant in the albedo and flavedo of navel oranges as opposed to clementine (Figure 1). Both commodities have a similar composition of organic acids within a respective tissue, excluding malonate and 4-aminobutyrate. Malonate concentration is higher in all clementine tissues than that in navel tissues, while 4-aminobutyrate is more abundant in the albedo and flavedo of the navel than in clementine (Figures 3, 4 and Tables S1–S4). Amino acid levels had similar compositions in their respective tissues between the two commodities, with some notable exceptions. Arginine, phenylalanine, threonine, and valine were more prevalent in all navel tissues as compared to clementine. Asparagine levels are higher in navel flavedo compared to those in clementine flavedo. Other notable metabolites that were distinct between the commodities include synephrine and trigonelline. Synephrine composition was higher in the albedo and flavedo tissues for the navel, but the clementine juice had a higher composition than navel juice. Trigonelline was detected in all clementine tissue but only detected in navel juice.
The study also finds that clementine mandarins, particularly the albedo tissues, have a relatively large number of metabolites downregulated due to cold storage and IMZ treatment in cold storage (Table S4). It is interesting to note that albedo tissues are more influenced by either the cold storage or the IMZ treatment (in terms of the number of differentially altered metabolites) than the flavedo tissues on the outer layer of the fruit. Cabras et al. have studied the factors such as the concentration, temperature, and length of the treatment of IMZ on its uptake and persistence in citrus.41 The results suggest that in addition to the dependence on treatment duration, treatments at higher temperatures (50 °C) tend to increase the deposition significantly (~8 times) than those close to room temperature (20 °C). Further studies suggest that experimental conditions such as storage, concentration, and temperatures may lead to differential permeation of IMZ through the rind to albedo tissues.42,43 Analysis of the IMZ distribution using laser-ablation electrospray ionization (LAESI) mass spectrometry imaging (MSI) in supermarket-bought citrus fruits indicates that the penetration depth of IMZ into the peel has a significant local variation.44 Matrix-assisted laser desorption ionization (MALDI) imaging studies on IMZ-treated apples indicate that it can penetrate at a rate of ~1 mm/day.45 Although the manual separation of albedo from the flavedo part could also lead to variations, PLS-DA analysis between the flavedo and albedo tissues of the fruits is discriminated (Figure S2). The half-life of IMZ is between 15 and 18 days at cold storage and under the standard industrial treatment of IMZ (~500 ppm).46 Furthermore, the relative changes in the levels of measured metabolites are more (both in the number of metabolites and in the differential effect) on the albedo tissues of clementine mandarins than those on the navel oranges (Tables S1–S4). Taken together, these observations strongly suggest that measured metabolomics are tissue-specific, with the albedo tissues being much more sensitive to the IMZ treatment.
IMZ is a highly effective fungicide as it functions by inhibiting the synthesis of the fungal cell wall. Therefore, it is the most utilized fungicide used widely in a postharvest stage in fruits and vegetables to increase the shelf life of produce. The substantial use of fungicides affects human health, particularly for workers coming in close contact at both pre- and postharvest processing. In mice and other experimental model systems, many toxic effects of IMZ have been reported.47–49 The biological effects of IMZ may also include the potential to affect the endocrine system by interacting with steroidogenesis, cytochrome P450 isoforms, antiandrogenic activity, and severe eye irritation in animals.50–53
IMZ treatment affecting the energy metabolism pathway primarily, but not the cold storage, is perhaps indicative of its effect in citrus (Figure 7). Use of citrus peels in food preparation (e.g., marmalade or cakes) tends to increase the relative concentration of IMZ in humans54 and that extensive washing of the fruits removes only a moderate amount (~30%) of the IMZ from the peels.55 Because of these factors, alternatives to IMZ treatments, such as the deployment of fludioxonil (FLU), a synthetic analogue of the bacterial metabolite of pyrrolnitrin, as well as other strategies and their efficacies could be made fruitful.56
There are many opportunities for growth regarding citrus metabolomics. IMZ is a widely used fungicide in the citrus industry, but it is only one of many fungicides that are also used, all of which have the potential to investigate further. Investigating fruit inoculated with other plant pathogens such as P. italicum and Geotrichum candidum can identify common or distinct biomarkers within these diseases.
5. CONCLUSIONS
This work demonstrates a viable metabolomics approach to assessing the quality of citrus fruit. Although quality control standards are established within the industry, the results are often subjective or ambiguous. Utilizing a metabolomics approach as a quality control practice enables companies to identify all detectable metabolites within their product, objectively quantify those metabolites, and perhaps even target critical metabolites associated with specific interest criteria. This work demonstrates that the metabolome of the two commodities and their respected tissues are distinct in many regards. The majority of the literature regarding citrus metabolomics studies is observational, with no treatment effects added. This work demonstrates that the two treatments, cold storage fruit and IMZ-treated cold storage fruit, altered the metabolome of the citrus tissues.
Although many chemicals are applied to citrus products to prevent certain diseases or combat pests, there is little literature available regarding how these treatments alter the physiology of the fruit. Typically, packhouses only analyze the level of treatments on their products. There is a little phenotypic distinction between chemically processed and treated fruit, excluding wax treatments. Metabolomics provides a tool to enable citrus companies to assess how their products respond at the molecular level. This work provided 11 metabolites significantly altered due to IMZ treatment that can serve as quality indices.
Furthermore, each treatment had a distinct effect on the metabolome of the citrus tissues. Many metabolites were identified that could potentially serve as indices that can be used for prediction or verification. Potential metabolic pathways were also identified with altered metabolites in the fruit treated with IMZ, providing insight into how IMZ influences fruit quality. These identified pathways can provide support in the development of future citrus fungicides.
Supplementary Material
ACKNOWLEDGMENTS
K.H.M. thanks the faculty-supported student research award (FSSRA) for partial funding. The authors thank JBT Corporation in Visalia, CA, for the sample of IMZ and Fresno State Citrus orchard staff. The work was, in part, by the U.S. National Institutes of Health grant SC3-GM125546 to V.V.K.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsagscitech.1c00169.
Identification of metabolites from the NMR spectrum; representative NMR spectrum of the albedo tissue with detected metabolites (Figure S1); partial least-square discrimination analysis (PLS-DA) of control, cold storage, and cold storage+ IMZ (Figure S2); average metabolite concentrations in albedo tissues (Table S1); average metabolite concentrations in flavedo tissues (Table S2); average metabolite concentrations in juice (Table S3); metabolite fold-change and p-values of clementine control fruit vs cold storage fruit (Table S4a); metabolite fold-change and p-values of clementine cold storage vs cold storage + treatment (Table S4b) (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsagscitech.1c00169
Contributor Information
Keeton H. Montgomery, Department of Chemistry & Biochemistry, California State University, Fresno, California 93740, United States
Gurreet Brar, Department of Plant Science, California State University, Fresno, California 93740, United States.
Viswanathan V. Krishnan, Department of Chemistry & Biochemistry, California State University, Fresno, California 93740, United States; Department of Pathology and Laboratory Medicine, School of Medicine, University of California, Davis, California 95616, United States.
REFERENCES
- (1).USDA Citrus: World Markets and Trade | USDA Foreign Agricultural Service. 2021; Available from: https://www.fas.usda.gov/data/citrus-world-markets-and-trade (accessed 2021-02-21). [Google Scholar]
- (2).Ismail M; Zhang J Post-harvest Citrus Diseases and their control. Outlooks Pest Manage. 2004, 15, 29–35. [Google Scholar]
- (3).Ghanei Ghooshkhaneh N; Golzarian MR; Mamarabadi M Detection and classification of citrus green mold caused by Penicillium digitatum using multispectral imaging. J. Sci. Food Agric. 2018, 98, 3542–3550. [DOI] [PubMed] [Google Scholar]
- (4).Perez MF; Perez Ibarreche J; Isas AS; Sepulveda M; Ramallo J; Dib JR Antagonistic yeasts for the biological control of Penicillium digitatum on lemons stored under export conditions. Biol. Control 2017, 115, 135–140. [Google Scholar]
- (5).Droby S; Eick A; Macarisin D; Cohen L; Rafael G; Stange R; McColum G; Dudai N; Nasser A; Wisniewski M; Shapira R Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar]
- (6).Mucci A; Parenti F; Righi V; Schenetti L Citron and lemon under the lens of HR-MAS NMR spectroscopy. Food Chem. 2013, 141, 3167–3176. [DOI] [PubMed] [Google Scholar]
- (7).Freitas DDS; Carlos EF; Gil MC; Vieira LG; Alcantara GB NMR-Based Metabolomic Analysis of Huanglongbing-Asymptomatic and -Symptomatic Citrus Trees. J. Agric. Food Chem. 2015, 63, 7582–7588. [DOI] [PubMed] [Google Scholar]
- (8).Alves Filho EG; Almeida FDL; Cavalcante RS; de Brito ES; Cullen PJ; Frias JM; Bourke P; Fernandes FAN; Rodrigues S (1)H NMR spectroscopy and chemometrics evaluation of non-thermal processing of orange juice. Food Chem. 2016, 204, 102–107. [DOI] [PubMed] [Google Scholar]
- (9).do Prado Apparecido R; Carlos EF; Liao LM; Vieira LGE; Alcantara GB NMR-based metabolomics of transgenic and non-transgenic sweet orange reveals different responses in primary metabolism during citrus canker development. Metabolomics 2017, 13, No. 20. [Google Scholar]
- (10).Salazar MO; Pisano PL; Sierra MG; Furlan RLE NMR and multivariate data analysis to assess traceability of argentine citrus. Microchem. J. 2018, 141, 264–270. [Google Scholar]
- (11).Villa-Ruano N; Perez-Hernandez N; Zepeda-Vallejo LG; Quiroz-Acosta T; Mendieta-Moctezuma A; Montoya-Garcia C; Garcia-Nava ML; Becerra-Martinez E (1) H-NMR Based Metabolomics Profiling of Citrus Juices Produced in Veracruz, Mexico. Chem. Biodiversity 2019, 16, No. e1800479. [DOI] [PubMed] [Google Scholar]
- (12).Kim HK; Choi YH; Verpoorte R NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [DOI] [PubMed] [Google Scholar]
- (13).Slisz AM; Breksa AP 3rd; Mishchuk DO; McCollum G; Slupsky CM Metabolomic analysis of citrus infection by ‘Candidatus Liberibacter’ reveals insight into pathogenicity. J. Proteome Res. 2012, 11, 4223–4230. [DOI] [PubMed] [Google Scholar]
- (14).Krishnan VV; Ravindran R; Wun T; Luciw PA; Khan IH; Janatpour K Multiplexed measurements of immunomodulator levels in peripheral blood of healthy subjects: Effects of analytical variables based on anticoagulants, age, and gender. Cytometry, Part B 2014, 86, 426–435. [DOI] [PubMed] [Google Scholar]
- (15).Mani A; Ravindran R; Mannepalli S; Vang D; Luciw PA; Hogarth M; Khan IH; Krishnan VV Data mining strategies to improve multiplex microbead immunoassay tolerance in a mouse model of infectious diseases. PLoS One 2015, 10, No. e0116262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wilkop TE; Wang M; Heringer A; Singh J; Zakharov F; Krishnan VV; Drakakaki G NMR spectroscopy analysis reveals differential metabolic responses in arabidopsis roots and leaves treated with a cytokinesis inhibitor. PLoS One 2020, 15, No. e0241627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Team, R. C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- (18).Wu GA; Prochnik S; Jenkins J; Salse J; Hellsten U; Murat F; Perrier X; Ruiz M; Scalabrin S; Terol J; Takita MA; Labadie K; Poulain J; Couloux A; Jabbari K; Cattonaro F; Del Fabbro C; Pinosio S; Zuccolo A; Chapman J; Grimwood J; Tadeo FR; Estornell LH; Munoz-Sanz JV; Ibanez V; Herrero-Ortega A; Aleza P; Perez-Perez J; Ramon D; Brunel D; Luro F; Chen C; Farmerie WG; Desany B; Kodira C; Mohiuddin M; Harkins T; Fredrikson K; Burns P; Lomsadze A; Borodovsky M; Reforgiato G; Freitas-Astua J; Quetier F; Navarro L; Roose M; Wincker P; Schmutz J; Morgante M; Machado MA; Talon M; Jaillon O; Ollitrault P; Gmitter F; Rokhsar D Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014, 32, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).CitrusCyc. CitrusCyc Pathways Database 2020. [cited 2021; Available from: 2021, http://ptools.citrusgenomedb.org/ (accessed 2021/02/21). [Google Scholar]
- (20).Zhang X; Breksa AP 3rd; Mishchuk DO; Slupsky CM Elevation, rootstock, and soil depth affect the nutritional quality of mandarin oranges. J. Agric. Food Chem. 2011, 59, 2672–2679. [DOI] [PubMed] [Google Scholar]
- (21).Chin EL; Mishchuk DO; Breksa AP; Slupsky CM Metabolite signature of Candidatus Liberibacter asiaticus infection in two citrus varieties. J. Agric. Food Chem. 2014, 62, 6585–6591. [DOI] [PubMed] [Google Scholar]
- (22).Wang S; Tu H; Wan J; Chen W; Liu X; Luo J; Xu J; Zhang H Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chem. 2016, 199, 8–17. [DOI] [PubMed] [Google Scholar]
- (23).Wang S; Yang C; Tu H; Zhou J; Liu X; Cheng Y; Luo J; Deng X; Zhang H; Xu J Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, No. 10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Asai T; Matsukawa T; Kajiyama S Metabolomic analysis of primary metabolites in citrus leaf during defense responses. J. Biosci. Bioeng. 2017, 123, 376–381. [DOI] [PubMed] [Google Scholar]
- (25).Tang N; Chen N; Hu N; Deng W; Chen ZX; Li ZG Comparative metabolomics and transcriptomic profiling reveal the mechanism of fruit quality deterioration and the resistance of citrus fruit against Penicillium digitatum. Postharvest Biol. Technol. 2018, 145, 61–73. [Google Scholar]
- (26).Feng S; Niu L; Suh JH; Hung WL; Wang Y Comprehensive Metabolomics Analysis of Mandarins (Citrus reticulata) as a Tool for Variety, Rootstock, and Grove Discrimination. J. Agric. Food Chem. 2018, 66, 10317–10326. [DOI] [PubMed] [Google Scholar]
- (27).Hung WL; Wang Y Metabolite Profiling of Candidatus Liberibacter Infection in Hamlin Sweet Oranges. J. Agric. Food Chem. 2018, 66, 3983–3991. [DOI] [PubMed] [Google Scholar]
- (28).Tsujimoto T; Yoshitomi T; Maruyama T; Yamamoto Y; Hakamatsuka T; Uchiyama N High-Resolution Liquid Chromatography-Mass Spectrometry-Based Metabolomic Discrimination of Citrus-Type Crude Drugs and Comparison with Nuclear Magnetic Resonance Spectroscopy-Based Metabolomics. J. Nat. Prod. 2019, 82, 2116–2123. [DOI] [PubMed] [Google Scholar]
- (29).Wan C; Shen Y; Nisar MF; Qi W; Chen C; Chen J The Antifungal Potential of Carvacrol against Penicillium Digitatum through 1H-NMR Based Metabolomics Approach. Appl. Sci. 2019, 9, 2240. [Google Scholar]
- (30).Ramsey JS; Chin EL; Chavez JD; Saha S; Mischuk D; Mahoney J; Mohr J; Robison FM; Mitrovic E; Xu Y; Strickler SR; Fernandez N; Zhong X; Polek M; Godfrey KE; Giovannoni JJ; Mueller LA; Slupsky CM; Bruce JE; Heck M Longitudinal Transcriptomic, Proteomic, and Metabolomic Analysis of Citrus limon Response to Graft Inoculation by Candidatus Liberibacter asiaticus. J. Proteome Res. 2020, 19, 2247–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chin EL; Ramsey JS; Mishchuk DO; Saha S; Foster E; Chavez JD; Howe K; Zhong X; Polek M; Godfrey KE; Mueller LA; Bruce JE; Heck M; Slupsky CM Longitudinal Transcriptomic, Proteomic, and Metabolomic Analyses of Citrus sinensis (L.) Osbeck Graft-Inoculated with ″Candidatus Liberibacter asiaticus″. J. Proteome Res. 2020, 19, 719–732. [DOI] [PubMed] [Google Scholar]
- (32).Muccilli V; Vitale A; Sheng L; Gentile A; Cardullo N; Tringali C; Oliveri C; La Rosa R; Di Guardo M; La Malfa S; Deng Z; Distefano G Substantial Equivalence of a Transgenic Lemon Fruit Showing Postharvest Fungal Pathogens Resistance. J. Agric. Food Chem. 2020, 68, 3806–3816. [DOI] [PubMed] [Google Scholar]
- (33).Zhang X; Breksa AP 3rd; Mishchuk DO; Fake CE; O’Mahony MA; Slupsky CM Fertilisation and pesticides affect mandarin orange nutrient composition. Food Chem. 2012, 134, 1020–1024. [DOI] [PubMed] [Google Scholar]
- (34).de Oliveira CR; Carneiro RL; Ferreira AG Tracking the degradation of fresh orange juice and discrimination of orange varieties: an example of NMR in coordination with chemometrics analyses. Food Chem. 2014, 164, 446–453. [DOI] [PubMed] [Google Scholar]
- (35).de Oliveira CS; Carlos EF; Vieira LG; Liao LM; Alcantara GB HR-MAS NMR metabolomics of ‘Swingle’ citrumelo rootstock genetically modified to overproduce proline. Magn. Reson. Chem. 2014, 52, 422–429. [DOI] [PubMed] [Google Scholar]
- (36).Cicero N; Corsaro C; Salvo A; Vasi S; Giofre SV; Ferrantelli V; Di Stefano V; Mallamace D; Dugo G The metabolic profile of lemon juice by proton HR-MAS NMR: the case of the PGI Interdonato Lemon of Messina. Nat. Prod. Res. 2015, 29, 1894–1902. [DOI] [PubMed] [Google Scholar]
- (37).Asai T; Matsukawa T; Kajiyama S Metabolomic analysis of primary metabolites in citrus leaf during defense responses. J. Biosci. Bioeng. 2017, 123, 376–381. [DOI] [PubMed] [Google Scholar]
- (38).Migues I; Hodos N; Moltini AI; Gámbaro A; Rivas F; Moyna G; Heinzen H 1H NMR metabolic profiles as selection tools of new mandarin cultivars based on fruit acceptability. Sci. Hortic. 2021, 287, No. 110262. [Google Scholar]
- (39).Slisz AM; Breksa AP; Mishchuk DO; McCollum G; Slupsky CM Metabolomic Analysis of Citrus Infection by ‘Candidatus Liberibacter’ Reveals Insight into Pathogenicity. J. Proteome Res. 2012, 11, 4223–4230. [DOI] [PubMed] [Google Scholar]
- (40).Conway JR; Lex A; Gehlenborg N UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Cabras P; Schirra M; Pirisi FM; Garau VL; Angioni A Factors affecting imazalil and thiabendazole uptake and persistence in citrus fruits following dip treatments. J. Agric. Food Chem. 1999, 47, 3352–3354. [DOI] [PubMed] [Google Scholar]
- (42).Dore A; Molinu MG; Venditti T; D’Hallewin G Immersion of lemons into imazalil mixtures heated at 50 degrees C alters the cuticle and promotes permeation of imazalil into rind wounds. J. Agric. Food Chem. 2009, 57, 623–631. [DOI] [PubMed] [Google Scholar]
- (43).Dore A; Molinu MG; Venditti T; D’Hallewin G Sodium bicarbonate induces crystalline wax generation, activates host-resistance, and increases imazalil level in rind wounds of oranges, improving the control of green mold during storage. J. Agric. Food Chem. 2010, 58, 7297–7304. [DOI] [PubMed] [Google Scholar]
- (44).Nielen MW; van Beek TA Macroscopic and microscopic spatially-resolved analysis of food contaminants and constituents using laser-ablation electrospray ionization mass spectrometry imaging. Anal. Bioanal. Chem. 2014, 406, 6805–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Pereira I; Banstola B; Wang K; Donnarumma F; Vaz BG; Murray KK Matrix-Assisted Laser Desorption Ionization Imaging and Laser Ablation Sampling for Analysis of Fungicide Distribution in Apples. Anal. Chem. 2019, 91, 6051–6056. [DOI] [PubMed] [Google Scholar]
- (46).Besil N; Perez-Parada A; Cesio V; Varela P; Rivas F; Heinzen H Degradation of imazalil, orthophenylphenol and pyrimethanil in Clementine mandarins under conventional post-harvest industrial conditions at 4 degrees C. Food Chem. 2016, 194, 1132–1137. [DOI] [PubMed] [Google Scholar]
- (47).Tanaka T Reproductive and neurobehavioral effects of imazalil administered to mice. Reprod. Toxicol. 1995, 9, 281–288. [DOI] [PubMed] [Google Scholar]
- (48).Tanaka T; Ogata A; Inomata A; Nakae D Effects of maternal exposure to imazalil on behavioral development in F(1)-generation mice. Birth Defects Res., Part B 2013, 98, 334–342. [DOI] [PubMed] [Google Scholar]
- (49).Jin C; Luo T; Zhu Z; Pan Z; Yang J; Wang W; Fu Z; Jin Y Imazalil exposure induces gut microbiota dysbiosis and hepatic metabolism disorder in zebrafish. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2017, 202, 85–93. [DOI] [PubMed] [Google Scholar]
- (50).Muto N; Hirai H; Tanaka T; Itoh N; Tanaka K Induction and inhibition of cytochrome P450 isoforms by imazalil, a food contaminant, in mouse small intestine and liver. Xenobiotica 1997, 27, 1215–1223. [DOI] [PubMed] [Google Scholar]
- (51).Zarn JA; Bruschweiler BJ; Schlatter JR Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ. Health Perspect. 2003, 111, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Orton F; Rosivatz E; Scholze M; Kortenkamp A Widely used pesticides with previously unknown endocrine activity revealed as in vitro antiandrogens. Environ. Health Perspect. 2011, 119, 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Faniband MH; Littorin M; Ekman E; Jonsson BA; Lindh CH LC-MS-MS Analysis of Urinary Biomarkers of Imazalil Following Experimental Exposures. J. Anal. Toxicol. 2015, 39, 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Friar PM; Reynolds SL The effect of home processing on postharvest fungicide residues in citrus fruit: residues of imazalil, 2-phenylphenol and thiabendazole in ‘home-made’ marmalade, prepared from late Valencia oranges. Food Addit. Contam. 1994, 11, 57–70. [DOI] [PubMed] [Google Scholar]
- (55).Vass A; Korpics E; Dernovics M Follow-up of the fate of imazalil from post-harvest lemon surface treatment to a baking experiment. Food Addit. Contam., Part A 2015, 32, 1875–1884. [DOI] [PubMed] [Google Scholar]
- (56).Schirra M; D’Aquino S; Palma A; Marceddu S; Angioni A; Cabras P; Scherm B; Migheli Q Residue level, persistence, and storage performance of citrus fruit treated with fludioxonil. J. Agric. Food Chem. 2005, 53, 6718–6724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


