Abstract
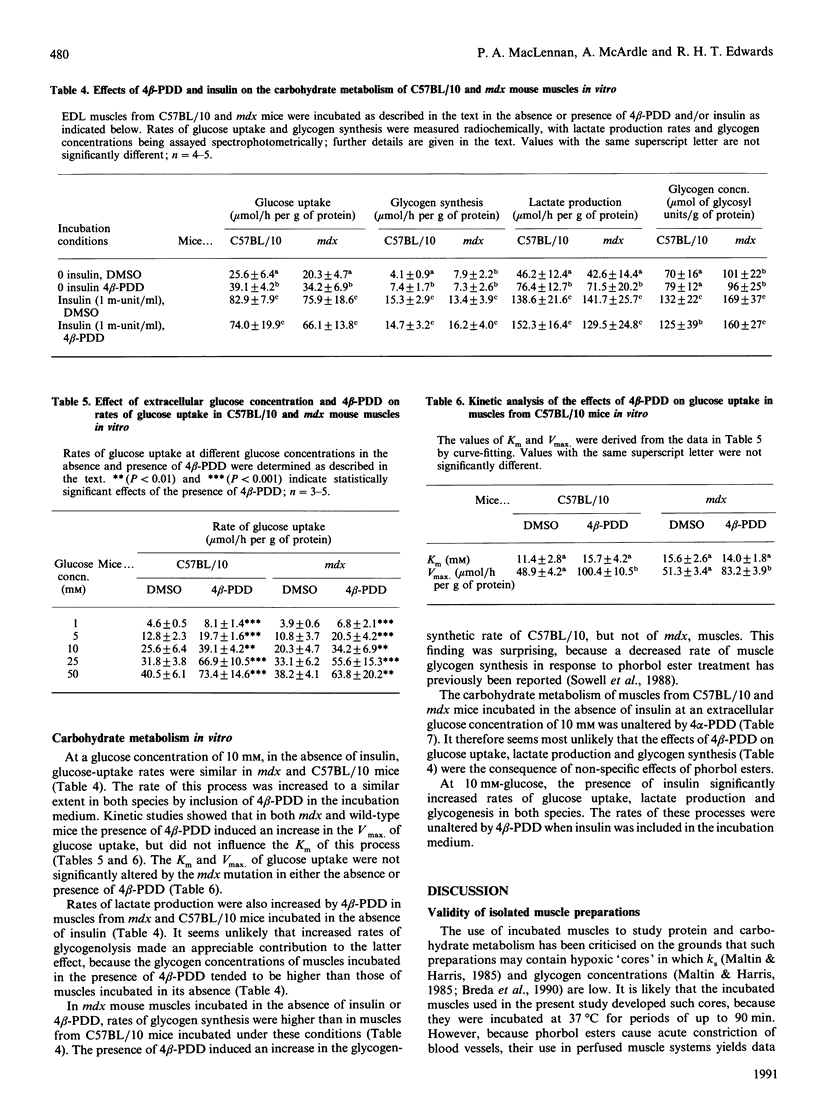
1. mdx mice do not express dystrophin, the product of the gene which is defective in Duchenne and Becker muscular dystrophy. We have previously shown that protein-synthetic rates (ks) are increased in mdx mouse muscles [MacLennan & Edwards (1990) Biochem. J. 268, 795-797]. 2. The tumour-promoting stereoisomer of phorbol 12,13-didecanoate (4 beta-PDD) acutely increased the ks of muscles from mdx and wild-type (C57BL/10) mice incubated in vitro in the absence of insulin. The effects of 4 beta-PDD are presumably mediated by activation of protein kinase C (PKC). 3. The muscle glycogen concentrations of mdx mice were higher than those of C57BL/10 mice. Studies performed in vivo and in vitro suggested that the effect might be at least partially due to increased rate of glycogen synthesis in mdx muscle. 4. 4 beta-PDD increased the glycogen-synthetic rates rates of C57BL/10, but not mdx, muscles incubated in vitro in the absence of insulin. 5. In muscles from both species incubated in the absence of insulin, treatment with 4 beta-PDD also induced increased rates of glucose uptake and lactate production. Kinetic studies of C57BL/10 and mdx muscles suggested that 4 beta-PDD raised the Vmax. of glucose uptake, but did not alter the Km for the process. 6. The possible role of PKC in controlling the protein and carbohydrate metabolism of normal and mdx mouse muscles is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Babij P., Booth F. W. Biochemistry of exercise. Advances in molecular biology relevant to adaptation of muscle to exercise. Sports Med. 1988 Mar;5(3):137–143. doi: 10.2165/00007256-198805030-00001. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius A. C., Ojamaa K. M., Flaim K. E., Li J. B., Wassner S. J., Jefferson L. S. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol. 1984 Apr;246(4 Pt 1):E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Cherqui G., Caron M., Wicek D., Lascols O., Capeau J., Picard J. Insulin stimulation of glucose metabolism in rat adipocytes: possible implication of protein kinase C. Endocrinology. 1986 May;118(5):1759–1769. doi: 10.1210/endo-118-5-1759. [DOI] [PubMed] [Google Scholar]
- Cleland P. J., Abel K. C., Rattigan S., Clark M. G. Long-term treatment of isolated rat soleus muscle with phorbol ester leads to loss of contraction-induced glucose transport. Biochem J. 1990 May 1;267(3):659–663. doi: 10.1042/bj2670659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland P. J., Appleby G. J., Rattigan S., Clark M. G. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J Biol Chem. 1989 Oct 25;264(30):17704–17711. [PubMed] [Google Scholar]
- Cullen M. J., Jaros E. Ultrastructure of the skeletal muscle in the X chromosome-linked dystrophic (mdx) mouse. Comparison with Duchenne muscular dystrophy. Acta Neuropathol. 1988;77(1):69–81. doi: 10.1007/BF00688245. [DOI] [PubMed] [Google Scholar]
- Dangain J., Vrbova G. Muscle development in mdx mutant mice. Muscle Nerve. 1984 Nov-Dec;7(9):700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Barnes D. E., Davis J. S., Standaert M. L., Pollet R. J. Effects of insulin and protein synthesis inhibitors on phospholipid metabolism, diacylglycerol levels, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. J Biol Chem. 1984 Jun 10;259(11):7094–7100. [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaim K. E., Copenhaver M. E., Jefferson L. S. Effects of diabetes on protein synthesis in fast- and slow-twitch rat skeletal muscle. Am J Physiol. 1980 Jul;239(1):E88–E95. doi: 10.1152/ajpendo.1980.239.1.E88. [DOI] [PubMed] [Google Scholar]
- Fuller S. J., Gaitanaki C. J., Sugden P. H. Effects of catecholamines on protein synthesis in cardiac myocytes and perfused hearts isolated from adult rats. Stimulation of translation is mediated through the alpha 1-adrenoceptor. Biochem J. 1990 Mar 15;266(3):727–736. doi: 10.1042/bj2660727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. J., Sugden P. H. Protein synthesis in rat cardiac myocytes is stimulated at the level of translation by phorbol esters. FEBS Lett. 1989 Apr 24;247(2):209–212. doi: 10.1016/0014-5793(89)81336-5. [DOI] [PubMed] [Google Scholar]
- Furuno K., Goldberg A. L. The activation of protein degradation in muscle by Ca2+ or muscle injury does not involve a lysosomal mechanism. Biochem J. 1986 Aug 1;237(3):859–864. doi: 10.1042/bj2370859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. N. Acute alterations in sodium flux in vitro lead to decreased myofibrillar protein breakdown in rat skeletal muscle. Biochem J. 1987 Oct 1;247(1):151–156. doi: 10.1042/bj2470151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumà A., Camps M., Palacín M., Testar X., Zorzano A. Protein kinase C activators selectively inhibit insulin-stimulated system A transport activity in skeletal muscle at a post-receptor level. Biochem J. 1990 Jun 15;268(3):633–639. doi: 10.1042/bj2680633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Knudson C. M., Campbell K. P., Kunkel L. M. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987 Dec 24;330(6150):754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Holness M. J., MacLennan P. A., Palmer T. N., Sugden M. C. The disposition of carbohydrate between glycogenesis, lipogenesis and oxidation in liver during the starved-to-fed transition. Biochem J. 1988 Jun 1;252(2):325–330. doi: 10.1042/bj2520325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson M. M., Pell J. M., Bates P. C., Millward D. J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986 Apr 15;235(2):329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Jackson M. J., Edwards R. H. Release of intracellular enzymes from an isolated mammalian skeletal muscle preparation. Clin Sci (Lond) 1983 Aug;65(2):193–201. doi: 10.1042/cs0650193. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T. Protein kinase C is not required for insulin stimulation of hexose uptake in muscle cells in culture. Biochem J. 1987 Feb 15;242(1):131–136. doi: 10.1042/bj2420131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Membrane effects of tumor promoters: stimulation of sugar uptake in mammalian cell cultures. J Cell Physiol. 1979 Jun;99(3):451–460. doi: 10.1002/jcp.1040990319. [DOI] [PubMed] [Google Scholar]
- Leighton B., Foot E. The effects of amylin on carbohydrate metabolism in skeletal muscle in vitro and in vivo. Biochem J. 1990 Jul 1;269(1):19–23. doi: 10.1042/bj2690019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan P. A., Edwards R. H. Protein turnover is elevated in muscle of mdx mice in vivo. Biochem J. 1990 Jun 15;268(3):795–797. doi: 10.1042/bj2680795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin C. A., Harris C. I. Morphological observations and rates of protein synthesis in rat muscles incubated in vitro. Biochem J. 1985 Dec 15;232(3):927–930. doi: 10.1042/bj2320927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kaiser K. E., Kipnis D. M. Epitrochlearis muscle. I. Mechanical performance, energetics, and fiber composition. Am J Physiol. 1980 Dec;239(6):E454–E460. doi: 10.1152/ajpendo.1980.239.6.E454. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G., Saladik D. Regulation of hexose transport in BALB/c 3T3 preadipose cells: effects of glucose concentration and 12-O-tetradecanoylphorbol-13-acetate. J Cell Physiol. 1982 Sep;112(3):376–384. doi: 10.1002/jcp.1041120311. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- RAFAELSEN O. J. GLYCOGEN CONTENT OF RAT DIAPHRAGM AFTER INTRAPERITONEAL INJECTION OF INSULIN AND OTHER HORMONES. Acta Physiol Scand. 1964 Aug;61:314–322. [PubMed] [Google Scholar]
- Rennie M. J., Idström J. P., Mann G. E., Scherstén T., Bylund-Fellenius A. C. A paired-tracer dilution method for characterizing membrane transport in the perfused rat hindlimb. Effects of insulin, feeding and fasting on the kinetics of sugar transport. Biochem J. 1983 Sep 15;214(3):737–743. doi: 10.1042/bj2140737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Sowell M. O., Treutelaar M. K., Burant C. F., Buse M. G. Minimal effects of phorbol esters on glucose transport and insulin sensitivity of rat skeletal muscle. Diabetes. 1988 May;37(5):499–506. doi: 10.2337/diab.37.5.499. [DOI] [PubMed] [Google Scholar]
- Tanti J. F., Rochet N., Grémeaux T., Van Obberghen E., Le Marchand-Brustel Y. Insulin-stimulated glucose transport in muscle. Evidence for a protein-kinase-C-dependent component which is unaltered in insulin-resistant mice. Biochem J. 1989 Feb 15;258(1):141–146. doi: 10.1042/bj2580141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Williams I. H., Chua B. H., Sahms R. H., Siehl D., Morgan H. E. Effects of diabetes on protein turnover in cardiac muscle. Am J Physiol. 1980 Sep;239(3):E178–E185. doi: 10.1152/ajpendo.1980.239.3.E178. [DOI] [PubMed] [Google Scholar]
- van Breda E., Keizer H. A., Glatz J. F., Geurten P. Use of the intact mouse skeletal-muscle preparation for metabolic studies. Evaluation of the model. Biochem J. 1990 Apr 1;267(1):257–260. doi: 10.1042/bj2670257. [DOI] [PMC free article] [PubMed] [Google Scholar]


