Abstract
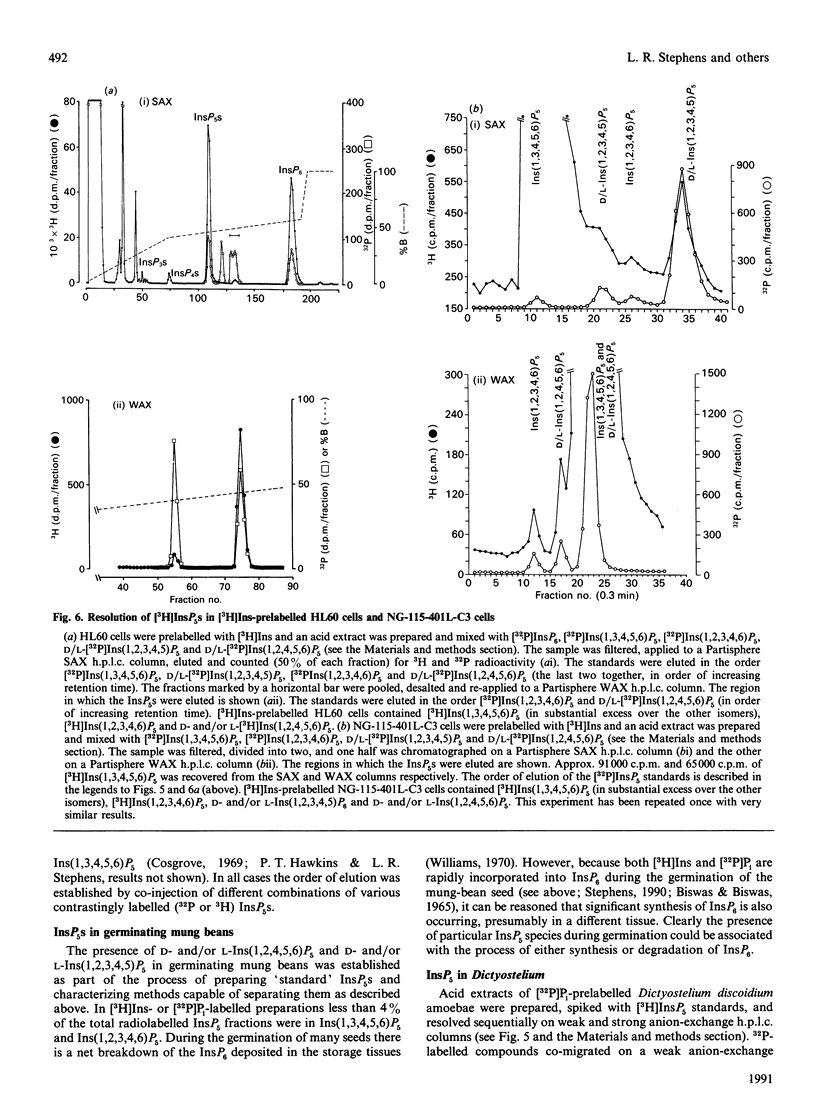
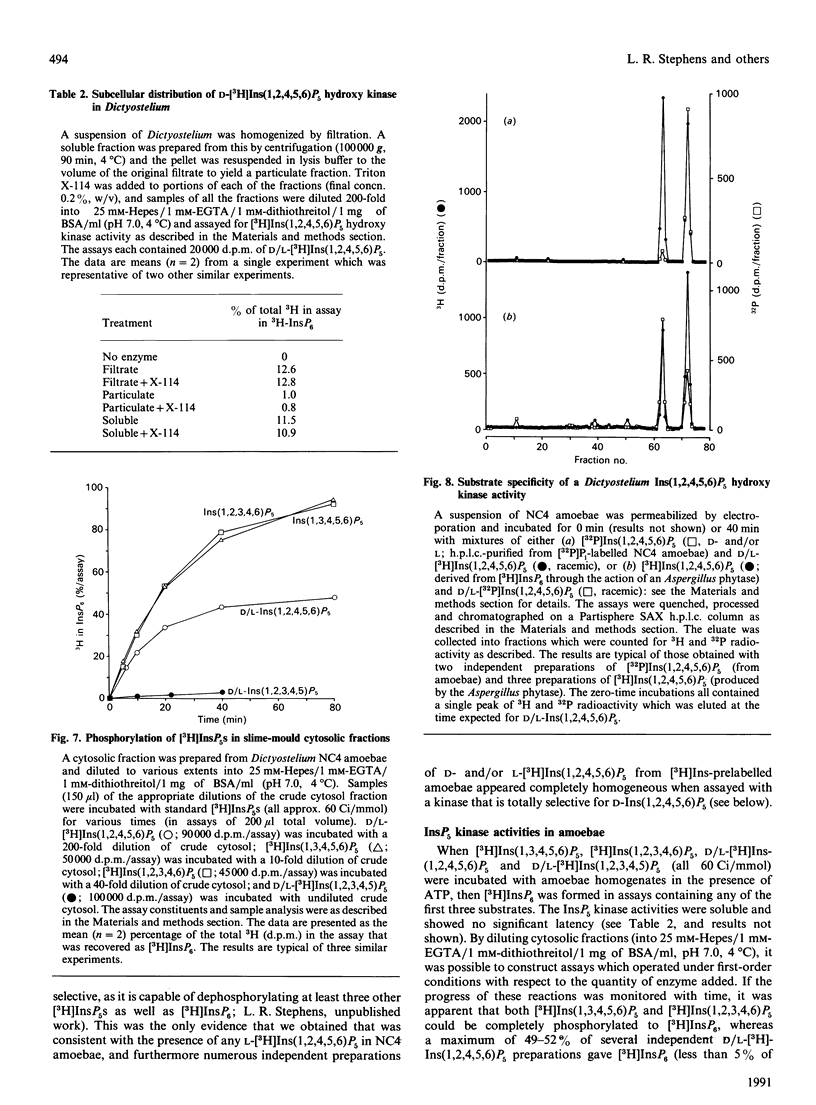
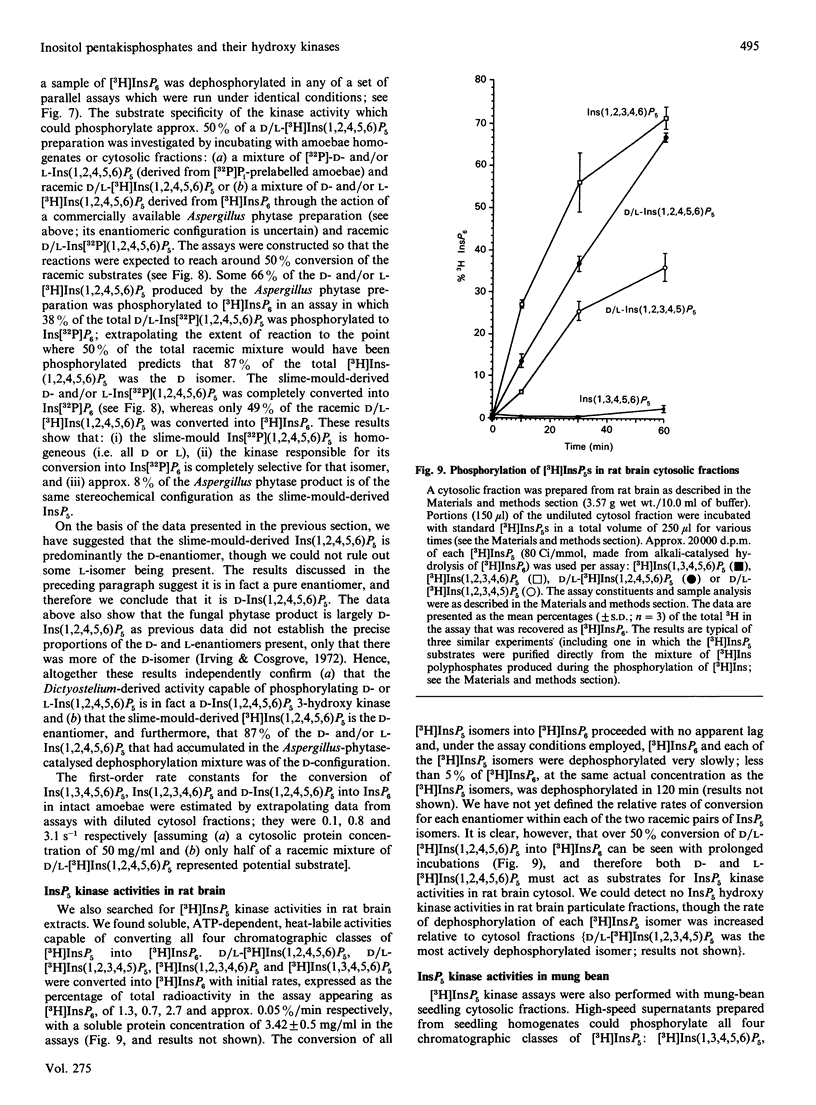
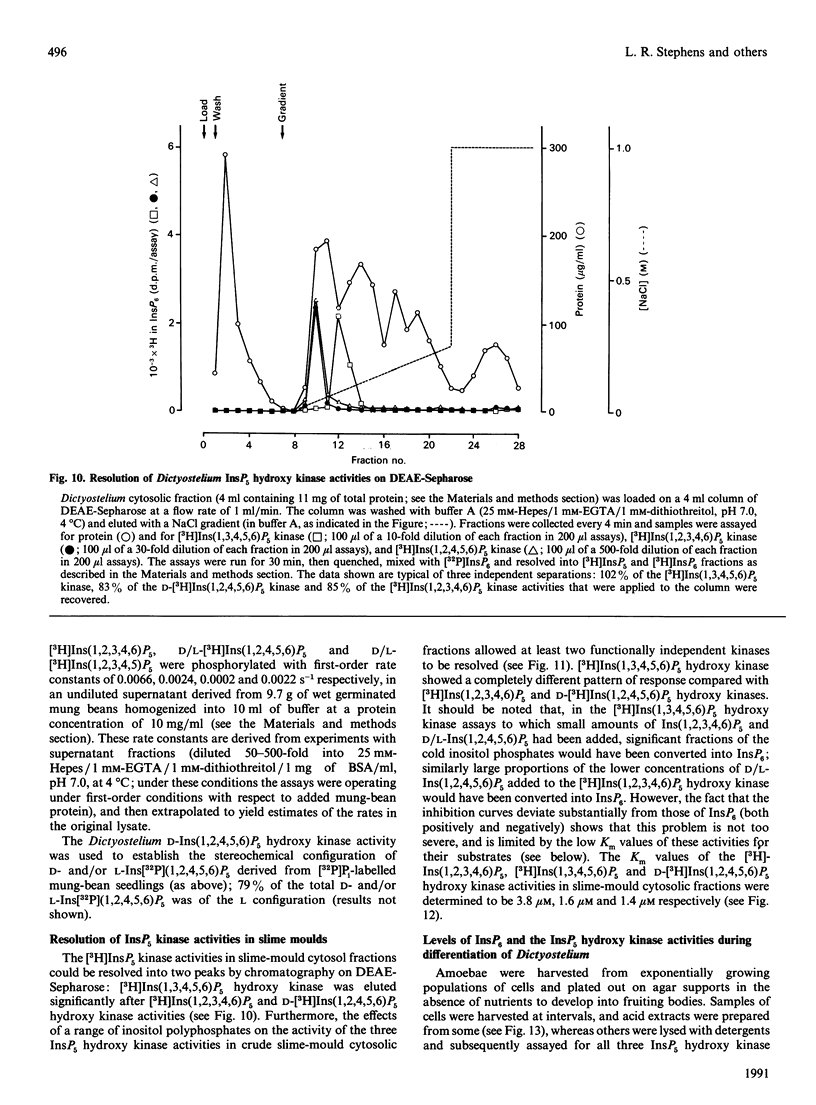
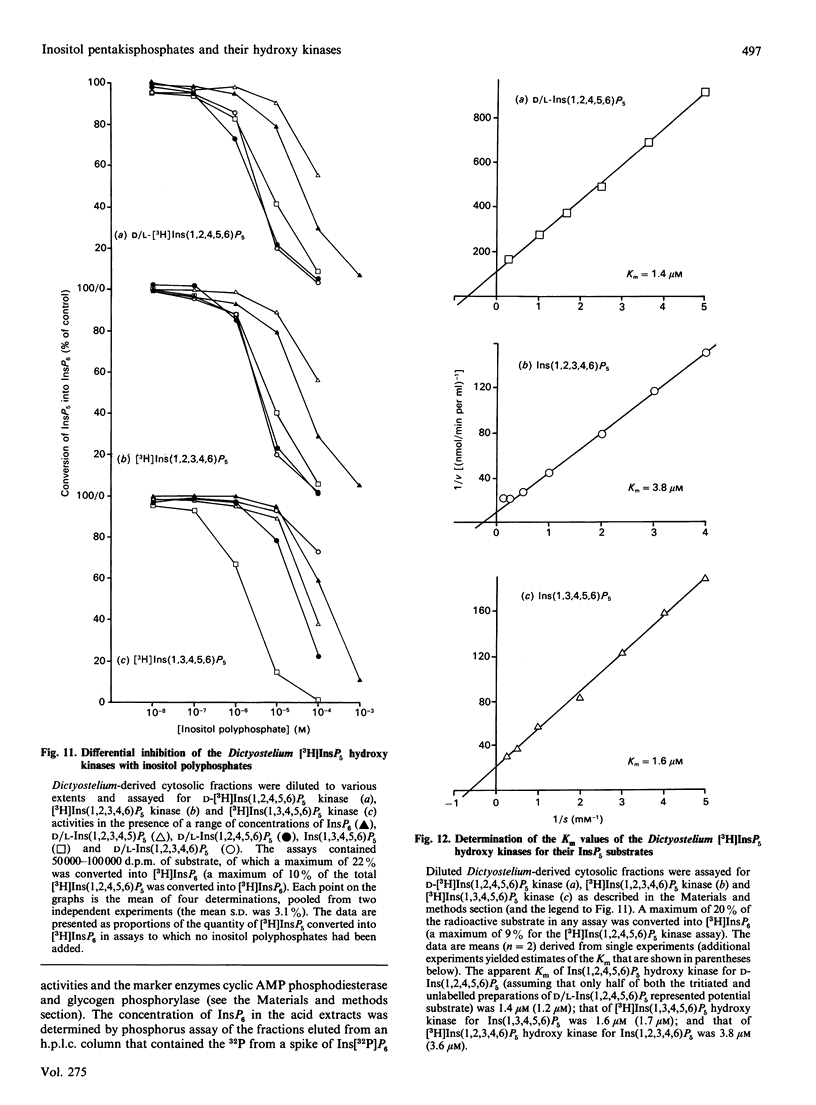
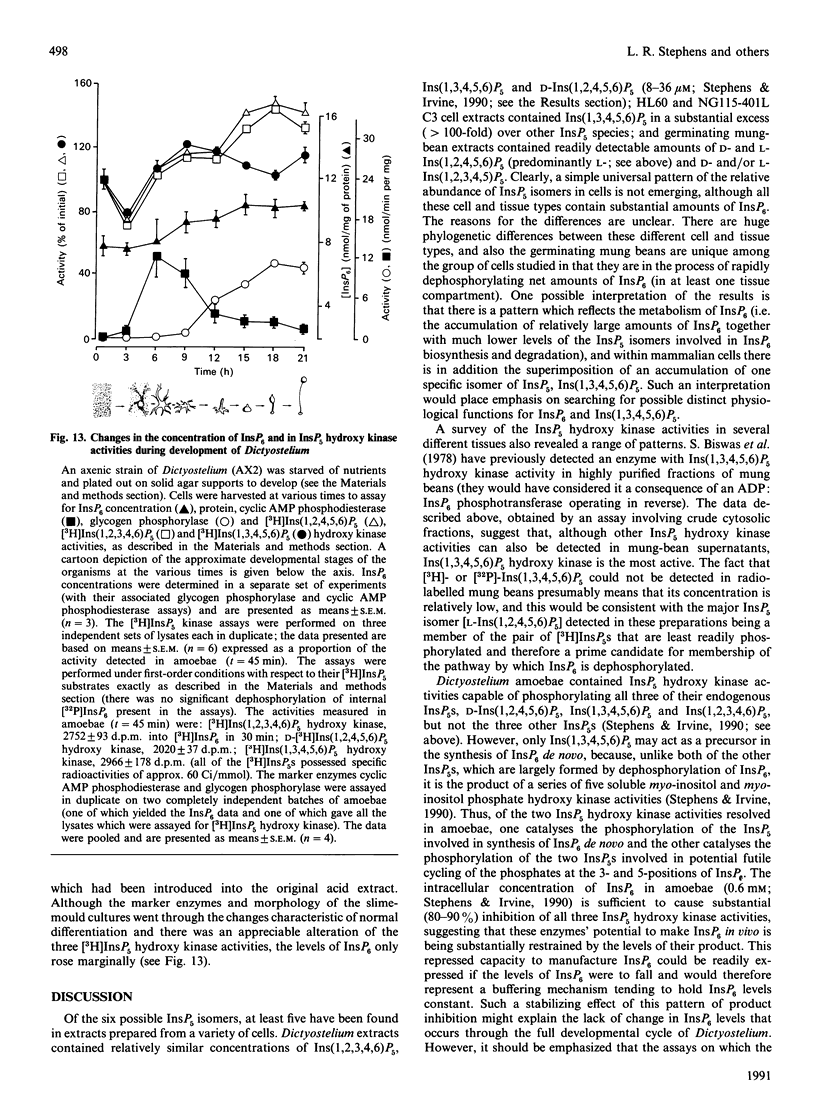
1. Standard and high-performance anion-exchange-chromatographic techniques have been used to purify myo-[3H]inositol pentakisphosphates from various myo-[3H]inositol-prelabelled cells. Slime mould (Dictyostelium discoideum) contained 8 microM-myo-[3H]inositol 1,3,4,5,6-pentakisphosphate, 16 microM-myo-[3H]inositol 1,2,3,4,6-pentakisphosphate and 36 microM-D-myo-[3H]inositol 1,2,4,5,6-pentakisphosphate [calculated intracellular concentrations; Stephens & Irvine (1990) Nature (London) 346, 580-583]; germinating mung-bean (Phaseolus aureus) seedlings contained both D- and L-myo-[3H]inositol 1,2,4,5,6-pentakisphosphate (which was characterized by 31P and two-dimensional proton n.m.r.) and D- and/or L-myo-[3H]inositol 1,2,3,4,5-pentakisphosphate; HL60 cells contained myo-[3H]inositol 1,3,4,5,6-pentakisphosphate (in a 500-fold excess over the other species), myo-[3H]inositol 1,2,3,4,6-pentakisphosphate and D- and/or L-myo-[3H]inositol 1,2,4,5,6-pentakisphosphate; and NG-115-401L-C3 cells contained myo-[3H]inositol 1,3,4,5,6-pentakisphosphate (in a 100-fold excess over the other species), D- and/or L-myo-[3H]inositol 1,2,4,5,6-pentakisphosphate, myo-[3H]inositol 1,2,3,4,6-pentakisphosphate and D- and/or L-myo-[3H]inositol 1,2,3,4,5-pentakisphosphate. 2. Multiple soluble ATP-dependent myo-inositol pentakisphosphate kinase activities have been detected in slime mould, rat brain and germinating mung-bean seedling homogenates. In slime-mould cytosolic fractions, the three myo-inositol pentakisphosphates that were present in intact slime moulds could be phosphorylated to myo-[3H]inositol hexakisphosphate: the relative first-order rate constants for these reactions were, in the order listed above, 1:8:31 respectively (with first-order rate constants in the intact cell of 0.1, 0.8 and 3.1 s-1, assuming a cytosolic protein concentration of 50 mg/ml), and the Km values of the activities for their respective inositol phosphate substrates (in the presence of 5 mM-ATP) were 1.6 microM, 3.8 microM and 1.4 microM. At least two forms of myo-inositol pentakisphosphate kinase activity could be resolved from a slime-mould cytosolic fraction by both pharmacological and chromatographic criteria. Rat brain cytosol and a soluble fraction derived from germinating mung-bean seedlings could phosphorylate myo-inositol D/L-1,2,4,5,6-, D/L-1,2,3,4,5-, 1,2,3,4,6- and 1,3,4,5,6-pentakisphosphates to myo-inositol hexakisphosphate: the relative first-order rate constants were 57:27:77:1 respectively for brain cytosol (with first-order rate constants in the intact cell of 0.0041, 0.0019, 0.0056 and 0.000073 s-1 respectively, assuming a cytosolic protein concentration of 50 mg/ml) and 1:11:12:33 respectively for mung-bean cytosol (with first-order rate constants in a supernatant fraction with a protein concentration of 10 mg/ml of 0.0002, 0.0022, 0.0024 and 0.0066 s-1 respectively).
Full text
PDF


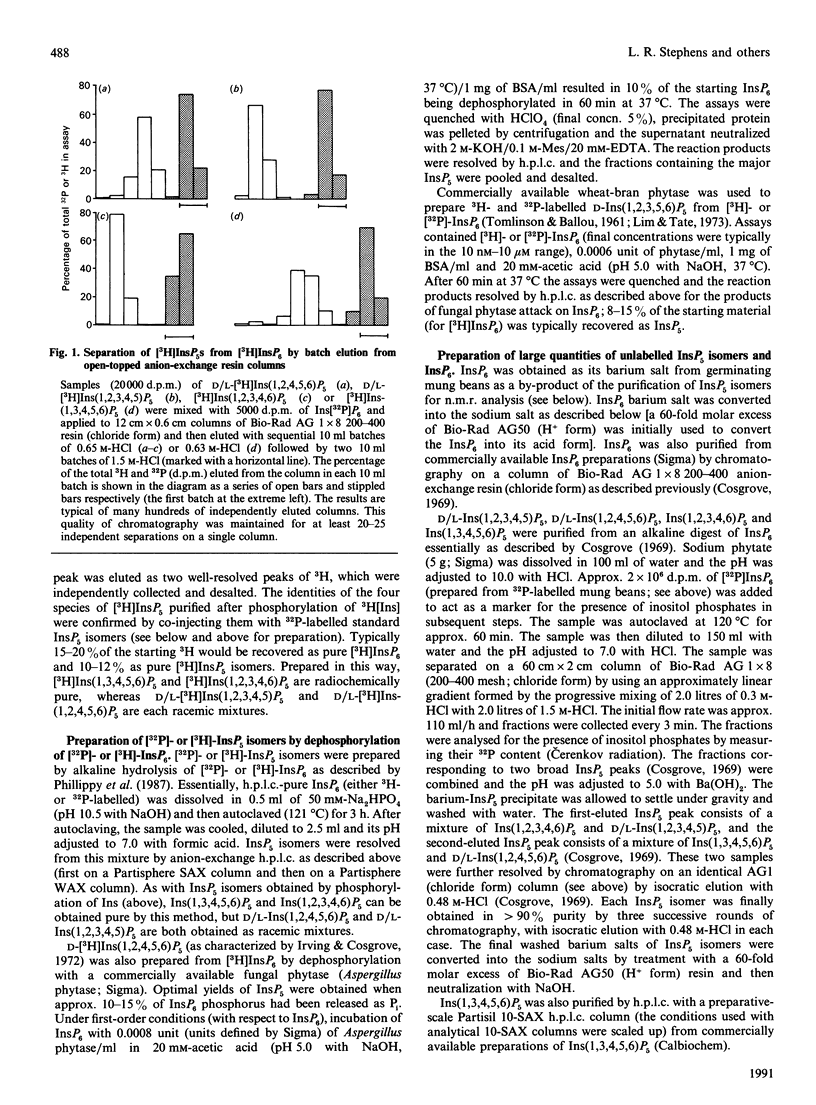
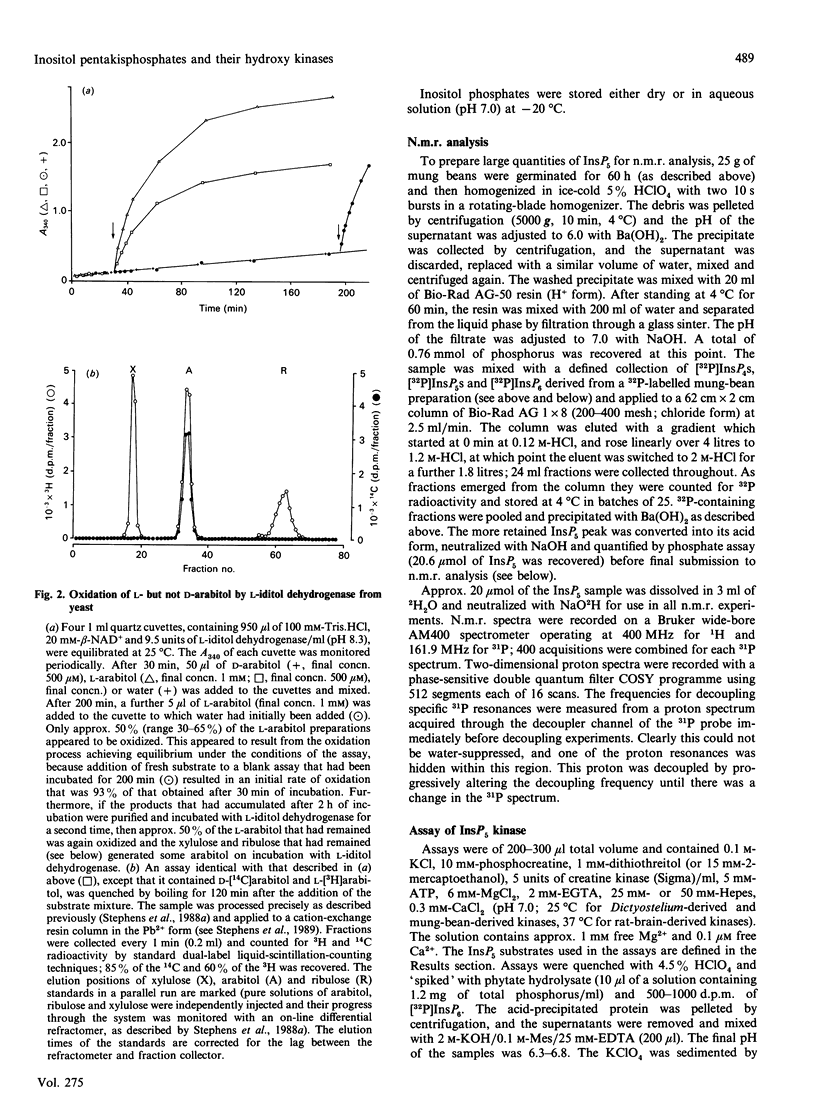
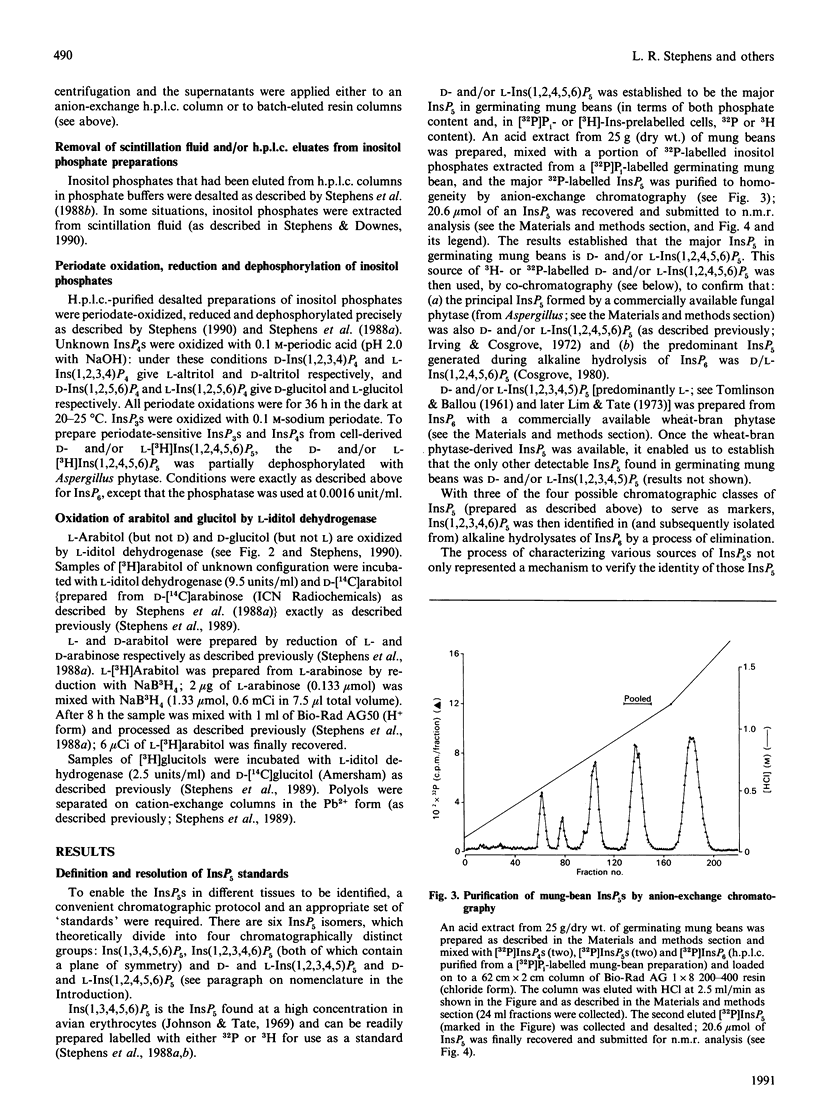
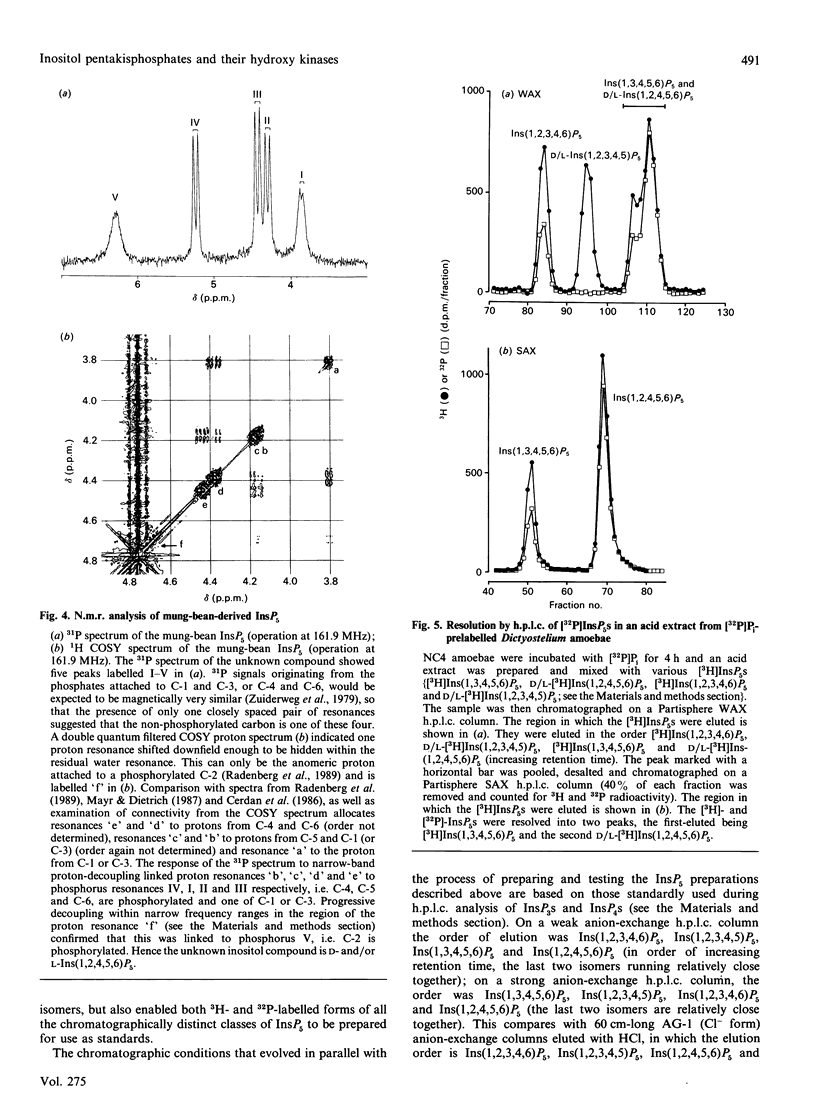


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Baginski E. S., Foà P. P., Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem. 1967 Apr;13(4):326–332. [PubMed] [Google Scholar]
- Bartlett G. R. Isolation and assay of red-cell inositol polyphosphates. Anal Biochem. 1982 Aug;124(2):425–431. doi: 10.1016/0003-2697(82)90060-4. [DOI] [PubMed] [Google Scholar]
- Biswas S., Biswas B. B. Enzymatic synthesis of guanosine triphosphate from phytin and guanosine diphosphate. Biochim Biophys Acta. 1965 Dec 9;108(4):710–713. doi: 10.1016/0005-2787(65)90069-9. [DOI] [PubMed] [Google Scholar]
- Biswas S., Maity I. B., Chakrabarti S., Biswas B. B. Purification and characterization of myo-inositol hexaphosphate-adenosine diphosphate phosphotransferase from Phaseolus aureus. Arch Biochem Biophys. 1978 Jan 30;185(2):557–566. doi: 10.1016/0003-9861(78)90201-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cerdan S., Hansen C. A., Johanson R., Inubushi T., Williamson J. R. Nuclear magnetic resonance spectroscopic analysis of myo-inositol phosphates including inositol 1,3,4,5-tetrakisphosphate. J Biol Chem. 1986 Nov 5;261(31):14676–14680. [PubMed] [Google Scholar]
- Cosgrove D. J. Ion-exchange chromatography of inositol polyphosphates. Ann N Y Acad Sci. 1969 Oct 17;165(2):677–686. [PubMed] [Google Scholar]
- Galliard T., Michell R. H., Hawthorne J. N. Incorporation of phosphate into diphosphoinositide by subcellular fractions from liver. Biochim Biophys Acta. 1965 Dec 2;106(3):551–563. doi: 10.1016/0005-2760(65)90071-8. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Reynolds D. J., Poyner D. R., Hanley M. R. Identification of a novel inositol phosphate recognition site: specific [3H]inositol hexakisphosphate binding to brain regions and cerebellar membranes. Biochem Biophys Res Commun. 1990 Mar 16;167(2):819–827. doi: 10.1016/0006-291x(90)92099-l. [DOI] [PubMed] [Google Scholar]
- Hughes P. J., Shears S. B. Inositol 1,3,4,5,6-pentakisphosphate and inositol hexakisphosphate inhibit inositol-1,3,4,5-tetrakisphosphate 3-phosphatase in rat parotid glands. J Biol Chem. 1990 Jun 15;265(17):9869–9875. [PubMed] [Google Scholar]
- Irving G. C., Cosgrove D. J. Inositol phosphate phosphatases of microbiological origin: the inositol pentaphosphate products of Aspergillus ficuum phytases. J Bacteriol. 1972 Oct;112(1):434–438. doi: 10.1128/jb.112.1.434-438.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Hallam T. J., Downes C. P., Hanley M. R. Receptor coupled events in bradykinin action: rapid production of inositol phosphates and regulation of cytosolic free Ca2+ in a neural cell line. EMBO J. 1987 Jan;6(1):49–54. doi: 10.1002/j.1460-2075.1987.tb04717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Sandberg K., Baukal A. J., Catt K. J. Metabolism of inositol pentakisphosphate to inositol hexakisphosphate in Xenopus laevis oocytes. J Biol Chem. 1989 Dec 5;264(34):20185–20188. [PubMed] [Google Scholar]
- Kay R. R. Gene expression in Dictyostelium discoidium: mutually antagonistic roles of cyclic-AMP and ammonia. J Embryol Exp Morphol. 1979 Aug;52:171–182. [PubMed] [Google Scholar]
- Kay R. R., Trevan D. J. Dictyostelium amoebae can differentiate into spores without cell-to-cell contact. J Embryol Exp Morphol. 1981 Apr;62:369–378. [PubMed] [Google Scholar]
- Koppitz B., Vogel F., Mayr G. W. Mammalian aldolases are isomer-selective high-affinity inositol polyphosphate binders. Eur J Biochem. 1986 Dec 1;161(2):421–433. doi: 10.1111/j.1432-1033.1986.tb10462.x. [DOI] [PubMed] [Google Scholar]
- Lim P. E., Tate M. E. The phytases. II. Properties of phytase fractions F 1 and F 2 from wheat bran and the myoinositol phosphates produced by fraction F 2 . Biochim Biophys Acta. 1973 Apr 12;302(2):316–328. doi: 10.1016/0005-2744(73)90160-5. [DOI] [PubMed] [Google Scholar]
- Mayr G. W. A novel metal-dye detection system permits picomolar-range h.p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988 Sep 1;254(2):585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr G. W., Dietrich W. The only inositol tetrakisphosphate detectable in avian erythrocytes is the isomer lacking phosphate at position 3: a NMR study. FEBS Lett. 1987 Mar 23;213(2):278–282. doi: 10.1016/0014-5793(87)81505-3. [DOI] [PubMed] [Google Scholar]
- Phillippy B. Q., Bland J. M. Gradient ion chromatography of inositol phosphates. Anal Biochem. 1988 Nov 15;175(1):162–166. doi: 10.1016/0003-2697(88)90374-0. [DOI] [PubMed] [Google Scholar]
- Phillippy B. Q., White K. D., Johnston M. R., Tao S. H., Fox M. R. Preparation of inositol phosphates from sodium phytate by enzymatic and nonenzymatic hydrolysis. Anal Biochem. 1987 Apr;162(1):115–121. doi: 10.1016/0003-2697(87)90015-7. [DOI] [PubMed] [Google Scholar]
- Radenberg T., Scholz P., Bergmann G., Mayr G. W. The quantitative spectrum of inositol phosphate metabolites in avian erythrocytes, analysed by proton n.m.r. and h.p.l.c. with direct isomer detection. Biochem J. 1989 Dec 1;264(2):323–333. doi: 10.1042/bj2640323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Downes C. P. Product-precursor relationships amongst inositol polyphosphates. Incorporation of [32P]Pi into myo-inositol 1,3,4,6-tetrakisphosphate, myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol 3,4,5,6-tetrakisphosphate and myo-inositol 1,3,4,5,6-pentakisphosphate in intact avian erythrocytes. Biochem J. 1990 Jan 15;265(2):435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Downes C. P. An analysis of myo-[3H]inositol trisphosphates found in myo-[3H]inositol prelabelled avian erythrocytes. Biochem J. 1989 Sep 15;262(3):727–737. doi: 10.1042/bj2620727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Irvine R. F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990 Aug 9;346(6284):580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Kay R. R., Irvine R. F. A myo-inositol D-3 hydroxykinase activity in Dictyostelium. Biochem J. 1990 Nov 15;272(1):201–210. doi: 10.1042/bj2720201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON R. V., BALLOU C. E. Myoinositol polyphosphate intermediates in the dephosphorylation of phytic acid by phytase. Biochemistry. 1962 Jan;1:166–171. doi: 10.1021/bi00907a025. [DOI] [PubMed] [Google Scholar]
- Vallejo M., Jackson T., Lightman S., Hanley M. R. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987 Dec 17;330(6149):656–658. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. G. The role of phytic Acid in the wheat grain. Plant Physiol. 1970 Apr;45(4):376–381. doi: 10.1104/pp.45.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuiderweg E. R., van Beek G. G., de Bruin S. H. The influence of electrostatic interaction on the proton-binding behaviour of myo-inositol hexakisphosphate. Eur J Biochem. 1979 Feb 15;94(1):297–306. doi: 10.1111/j.1432-1033.1979.tb12895.x. [DOI] [PubMed] [Google Scholar]


