Abstract
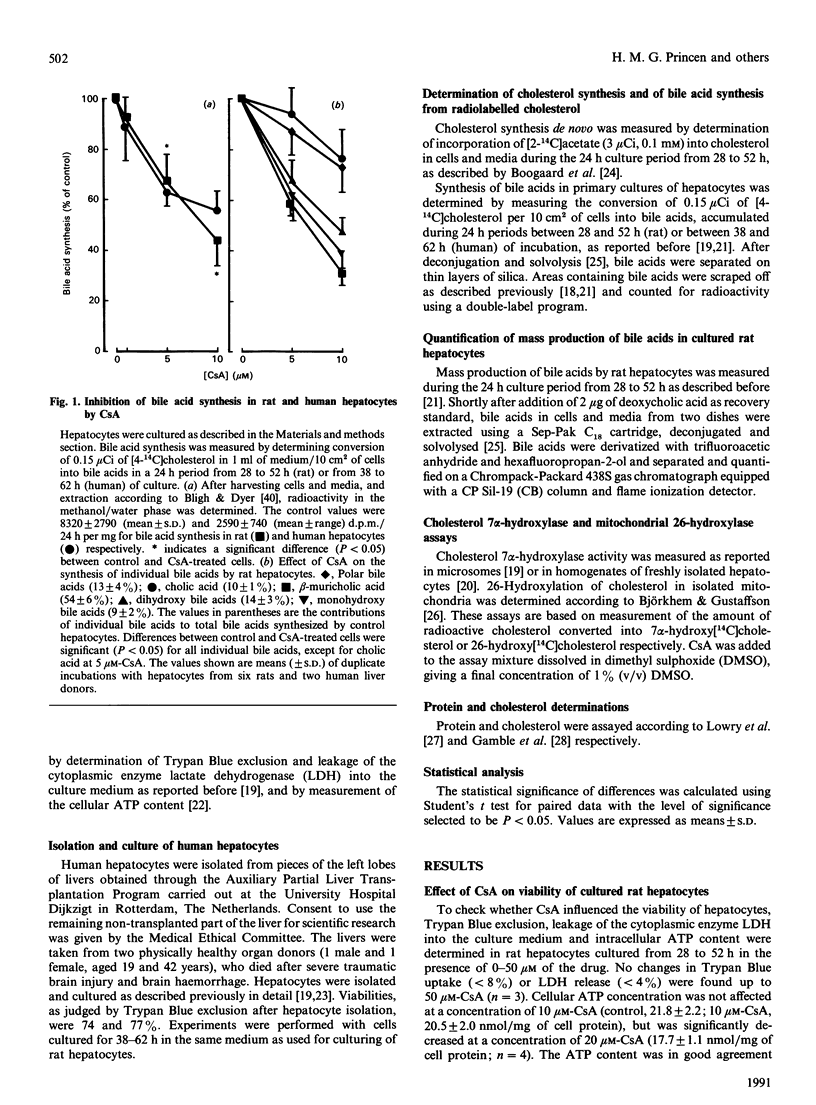
Bile acid synthesis, determined by conversion of [4-14C]cholesterol into bile acids in rat and human hepatocytes and by measurement of mass production of bile acids in rat hepatocytes, was dose-dependently decreased by cyclosporin A, with 52% (rat) and 45% (human) inhibition of 10 microM. The decreased bile acid production in rat hepatocytes was due only to a fall in the synthesis of beta-muricholic and chenodeoxycholic acids (-64% at 10 microM-cyclosporin A), with no change in the formation of cholic acid. In isolated rat liver mitochondria, 26-hydroxylation of cholesterol was potently inhibited by the drug (concn. giving half-maximal inhibition = 4 microM). These results suggest that cyclosporin A blocks the alternative pathway in bile acid synthesis, which leads preferentially to the formation of chenodeoxycholic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Kok E., Javitt N. B. Bile acid synthesis in man: metabolism of 7 -hydroxycholesterol- 14 C and 26-hydroxycholesterol- 3 H. J Clin Invest. 1972 Jan;51(1):112–117. doi: 10.1172/JCI106780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. A., Zemaitis M. A. The effects of cyclosporin A (CsA) on hepatic microsomal drug metabolism in the rat. Drug Metab Dispos. 1986 Jan-Feb;14(1):73–78. [PubMed] [Google Scholar]
- Axelson M., Sjövall J. Potential bile acid precursors in plasma--possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem. 1990 Aug 28;36(6):631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- Ayaki Y., Kok E., Javitt N. B. Cholic acid synthesis from 26-hydroxycholesterol and 3-hydroxy-5-cholestenoic acid in the rabbit. J Biol Chem. 1989 Mar 5;264(7):3818–3821. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bennett W. M., Norman D. J. Action and toxicity of cyclosporine. Annu Rev Med. 1986;37:215–224. doi: 10.1146/annurev.me.37.020186.001243. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Mitochondrial omega-hydroxylation of cholesterol side chain. J Biol Chem. 1974 Apr 25;249(8):2528–2535. [PubMed] [Google Scholar]
- Boogaard A., Griffioen M., Cohen L. H. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in human hepatoma cell line Hep G2. Effects of inhibitors of cholesterol synthesis on enzyme activity. Biochem J. 1987 Jan 15;241(2):345–351. doi: 10.1042/bj2410345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckart G. J., Starzl T. E., Venkataramanan R., Hashim H., Wong L., Wang P., Makowka L., Zeevi A., Ptachcinski R. J., Knapp J. E. Excretion of cyclosporine and its metabolites in human bile. Transplant Proc. 1986 Dec;18(6 Suppl 5):46–49. [PMC free article] [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Cronholm T., Einarsson K., Gustafsson J. A. Changes in in vivo metabolism of bile acids in rat after treatment with phenobarbital. Lipids. 1974 Nov;9(11):844–849. doi: 10.1007/BF02532607. [DOI] [PubMed] [Google Scholar]
- Einarsson K., Nilsell K., Leijd B., Angelin B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N Engl J Med. 1985 Aug 1;313(5):277–282. doi: 10.1056/NEJM198508013130501. [DOI] [PubMed] [Google Scholar]
- Frey F. J., Horber F. F., Frey B. M. Trough levels and concentration time curves of cyclosporine in patients undergoing renal transplantation. Clin Pharmacol Ther. 1988 Jan;43(1):55–62. doi: 10.1038/clpt.1988.11. [DOI] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Havekes L. M., Verboom H., de Wit E., Yap S. H., Princen H. M. Regulation of low density lipoprotein receptor activity in primary cultures of human hepatocytes by serum lipoproteins. Hepatology. 1986 Nov-Dec;6(6):1356–1360. doi: 10.1002/hep.1840060623. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Gurley E. C., Kubaska W. M., Whitehead T. R., Guzelian P. S., Vlahcevic Z. R. Suitability of primary monolayer cultures of adult rat hepatocytes for studies of cholesterol and bile acid metabolism. J Biol Chem. 1985 Jan 25;260(2):1015–1019. [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Ho J., Mehra R., Bruce-Robertson A. Hepatotrophic effects of insulin on glucose, glycogen, and adenine nucleotides in hepatocytes isolated from fasted adult rats. Gastroenterology. 1980 Mar;78(3):556–570. [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kempen H. J., Vos-Van Holstein M. P., de Lange J. Bile acids and lipids in isolated rat hepatocytes: content, synthesis, and release, as affected by cholestyramine treatment of the donor rats. J Lipid Res. 1982 Aug;23(6):823–830. [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Huijsmans C. M., Vonk R. J., Princen H. M. Inhibition and induction of bile acid synthesis by ketoconazole. Effects on bile formation in the rat. Lipids. 1989 Sep;24(9):759–764. doi: 10.1007/BF02544580. [DOI] [PubMed] [Google Scholar]
- Kwekkeboom J., Princen H. M., van Voorthuizen E. M., Kempen H. J. Cholesterol 7 alpha-hydroxylase activity and bile acid synthesis in hepatocytes of unweaned and weaned pigs in monolayer culture. Biochim Biophys Acta. 1990 Feb 23;1042(3):386–394. doi: 10.1016/0005-2760(90)90169-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li G., Treiber G., Meinshausen J., Wolf J., Werringloer J., Klotz U. Is cyclosporin A an inhibitor of drug metabolism? Br J Clin Pharmacol. 1990 Jul;30(1):71–77. doi: 10.1111/j.1365-2125.1990.tb03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer G., Loosli H. R., Schreier E., Keller B. Disposition of cyclosporine in several animal species and man. I. Structural elucidation of its metabolites. Drug Metab Dispos. 1984 Jan-Feb;12(1):120–126. [PubMed] [Google Scholar]
- Mitropoulos K. A., Avery M. D., Myant N. B., Gibbons G. F. The formation of cholest-5-ene-3 ,26-diol as an intermediate in the conversion of cholesterol into bile acids by liver mitochondria. Biochem J. 1972 Nov;130(2):363–371. doi: 10.1042/bj1300363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moochhala S. M., Renton K. W. Inhibition of hepatic microsomal drug metabolism by the immunosuppressive agent cyclosporin A. Biochem Pharmacol. 1986 May 1;35(9):1499–1503. doi: 10.1016/0006-2952(86)90115-2. [DOI] [PubMed] [Google Scholar]
- Myant N. B., Mitropoulos K. A. Cholesterol 7 alpha-hydroxylase. J Lipid Res. 1977 Mar;18(2):135–153. [PubMed] [Google Scholar]
- Princen H. M., Huijsmans C. M., Kuipers F., Vonk R. J., Kempen H. J. Ketoconazole blocks bile acid synthesis in hepatocyte monolayer cultures and in vivo in rat by inhibiting cholesterol 7 alpha-hydroxylase. J Clin Invest. 1986 Oct;78(4):1064–1071. doi: 10.1172/JCI112662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Hofstee B. Dexamethasone regulates bile acid synthesis in monolayer cultures of rat hepatocytes by induction of cholesterol 7 alpha-hydroxylase. Biochem J. 1989 Aug 15;262(1):341–348. doi: 10.1042/bj2620341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P. Hydroxylation, conjugation and sulfation of bile acids in primary monolayer cultures of rat hepatocytes. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1114–1121. doi: 10.1016/0006-291x(88)90256-2. [DOI] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Kuipers F. One-step solvolysis of 3-, 7- and 12-sulfated free and conjugated bile acids. Clin Chim Acta. 1990 Nov 15;192(1):77–83. doi: 10.1016/0009-8981(90)90274-v. [DOI] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Kwekkeboom J., Kempen H. J. Assay of cholesterol 7 alpha-hydroxylase activity in rat hepatocytes in primary monolayer culture. Anal Biochem. 1988 May 15;171(1):158–165. doi: 10.1016/0003-2697(88)90137-6. [DOI] [PubMed] [Google Scholar]
- Swell L., Gustafsson J., Schwartz C. C., Halloran L. G., Danielsson H., Vlahcevic Z. R. An in vivo evaluation of the quantitative significance of several potential pathways to cholic and chenodeoxycholic acids from cholesterol in man. J Lipid Res. 1980 May;21(4):455–466. [PubMed] [Google Scholar]
- Wachtel N., Emerman S., Javitt N. B. Metabolism of cholest-5-ene-3 beta, 26-diol in the rat and hamster. J Biol Chem. 1968 Oct 10;243(19):5207–5212. [PubMed] [Google Scholar]
- Whiting P. H., Burke M. D., Thomson A. W. Drug interactions with cyclosporine: implications from animal studies. Transplant Proc. 1986 Dec;18(6 Suppl 5):56–70. [PubMed] [Google Scholar]


