Abstract
We show that inactivating the β2m gene increases the viral load of SJL/J mice persistently infected by Theiler's virus. Together with previous results, this shows that the characteristics of Tmevp1, a locus which controls the amount of viral RNA that persists in the central nervous system, are those of an H-2 class I gene.
The DA strain of Theiler's murine encephalomyelitis virus (TMEV), a picornavirus, is responsible for a biphasic neurological disease of mice. The first phase is an acute encephalomyelitis which takes place during the first 2 weeks that follow intracerebral inoculation. During the second phase, which occurs only in susceptible animals and is lifelong, persistent infection of the white matter of the spinal cord causes chronic inflammation and primary demyelination (14, 15). This natural disease is considered one of the best models for multiple sclerosis (13, 20).
The extent of demyelination and the amount of viral RNA that persists in the central nervous system (CNS) vary greatly among inbred mouse strains (8). A locus with a major effect on both phenotypes has been mapped by several groups to the H-2D region of the major histocompatibility complex (MHC) (10, 12, 17, 21, 23). This locus has been named Tmevp1 for Theiler's murine encephalomyelitis virus persistence locus 1. Three phenotypes have been defined for the amount of viral RNA that persists in the CNS. Resistant strains clear the infection, and their F1 crosses with the SJL/J strain clear the infection as well. Strains of intermediate susceptibility clear the infection, but their F1 crosses with the SJL/J strain do not and are infected at high levels. Finally, susceptible strains remain persistently infected at high levels. In 15 of 16 mouse strains examined in one study, the viral RNA load was determined by the Tmevp1 haplotype. The H-2b haplotype was associated with resistance, the H-2q haplotype was associated with susceptibility, and the H-2d, H-2k, and H-2s haplotypes were associated with intermediate levels of susceptibility (10). The SJL/J strain was the only strain which was more susceptible than predicted by its H-2s haplotype. In subsequent work, its susceptibility was explained by non-H-2 loci, which have been mapped, on the mouse genome (9). Two of these loci are located close to each other on the telomeric part of chromosome 10 (6). Interestingly, multiple susceptibility loci located in the same region have also been reported for other phenotypes induced by Theiler's virus infection and for other diseases.
Several observations suggest that the H-2D class I gene explains most of the characteristics of the Tmevp1 locus (3, 16, 22). The goal of the present work was to test if MHC class I genes control the load of viral RNA in the SJL/J strain, a prototypic susceptible strain with an H-2s haplotype normally associated with intermediate susceptibility. We examined this point using inbred SJL/J mice with an inactivated beta-microglobulin gene.
SJL/J β2m−/− mice obtained from the Jackson Laboratory (Bar Harbor, Maine) were crossed with wild-type SJL/J mice. SJL/J mice with the three genotypes (β2m−/−, β2m+/−, and β2m+/+) were inoculated intracerebrally with 104 PFU of the DA1 strain of TMEV and studied during the acute (6 days postinoculation [p.i.]) and the chronic phase (21 and 45 days p.i.) of the infection. DA1 is a molecularly cloned TMEV DA strain produced from the infectious pTMDA1 plasmid (18, 19). The amount of viral RNA in the CNS was measured by using a dot blot assay (1, 10). Briefly, for each mouse, fivefold dilutions of total RNA were dotted onto a filter and hybridized with either a viral cDNA probe or a control β-actin probe. The hybridized filters were analyzed with a phosphorimager. The highest dilution which gave a positive signal was used as a measure of viral RNA content (see Fig. 1 of reference 7). To normalize the results between experiments, dilutions of reference RNA samples were dotted onto each filter. The means of viral RNA content for mice with the three genotypes were compared by using analysis of variance, and two-tail comparisons were performed by using Scheffé's test. Viral antigens were detected in CNS sections by immunocytochemistry (2).
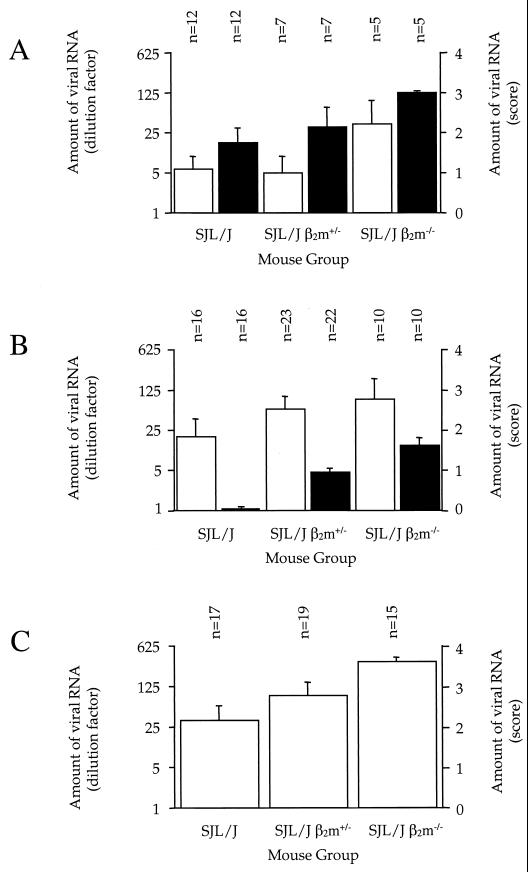
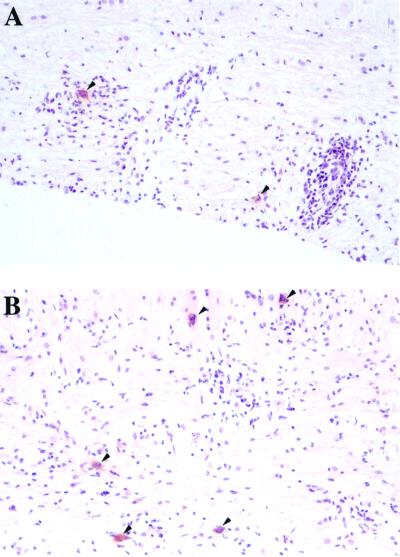
No clinical symptoms or mortality were observed in either group of mice. At 45 days p.i., in the spinal cord, the viral load was significantly higher in SJL/J β2m−/− mice (mβ2m−/− = 3.6 ± 0.1 [n = 15]) than in SJL/J β2m+/+ mice (mβ2m+/+ = 2.2 ± 0.4 [n = 17]) (P = 0.0099). SJL/J β2m+/− mice had an intermediate phenotype (mβ2m+/− = 2.8 ± 0.3 [n = 19]) (Fig. 1C). As determined by immunocytochemistry, the virus was localized in the white matter of spinal cord, often close to the border with the gray matter, in mice with the three genotypes (Fig. 2). A large number of infected cells were observed in the five SJL/J β2m−/− and five SJL/J β2m+/− mice examined. In contrast, only low numbers of infected cells were detected in SJL/J β2m+/+ mice and only in four of seven animals. The extent of inflammation (meningitis, perivascular cuffs, and diffuse parenchymal infiltration) correlated with the number of infected cells. In conclusion, the SJL/J β2m−/− mice are more susceptible to viral persistence than wild-type SJL/J mice. SJL/J β2m+/− mice have an intermediate phenotype. Viral localization within the CNS is the same for the three groups of mice.
FIG. 1.
Amount of viral RNA in the CNSs of mice with the wild-type SJL/J, SJL/J β2m+/− and SJL β2m−/− genotypes. Mice were examined at 6 days p.i. (A), 21 days p.i. (B), and 45 days p.i. (C). The amount of viral RNA is expressed as the highest RNA dilution which gave a hybridization signal in a dot blot assay. The left y axis shows the amount of viral DNA as a dilution factor, whereas the right y axis shows the amount of viral DNA as a score which corresponds to the dilution number in the series. Open bars, spinal cord; solid bars, brain. n, number of animals per group.
FIG. 2.
Detection of viral antigens at 45 days p.i. in longitudinal sections of white matter from the spinal cords of wild-type SJL/J (A) and SJL/J β2m−/− (B) mice. Viral antigens were detected by immunoperoxidase. Sections were counterstained with hematoxylin. Arrows point to cells containing viral antigens. Magnification, ×200.
To evaluate the effect of the β2m−/− mutation on the early phases of the infection, the load of viral RNA and histopathology were examined in brain and spinal cord at 6 and 21 days p.i. At 6 days p.i., no significant difference in the load of viral RNA was detected among the three groups of mice, in brain (P = 0.1476) as well as in spinal cord (P = 0.1622) (Fig. 1A). Histological studies showed viral antigens mainly in the cortex and hippocampus in three of six wild-type SJL/J mice, five of six SJL/J β2m+/ − mice, and both SJL/J β2m−/− mice examined. Infection was associated with perivascular cuffs and parenchymal inflammation (data not shown). At 21 days p.i., the amount of viral RNA in brain was significantly higher in SJL/J β2m+/− (mβ2m+/− = 1.0 ± 0.1 [n = 22], P < 0.001) and SJL/J β2m−/− mice (mβ2m−/− = 1.6 ± 0.2 [n = 10], P < 0.0001) than in SJL/J β2m+/+ mice (mβ2m+/+ = 0 ± 0 [n = 16] (Fig. 1B). No significant differences in the viral RNA load in the spinal cord were observed for mice of the different genotypes (P = 0.2976). Histological examination confirmed that by 21 days p.i., the virus had infected the white matter of the spinal cord for the three groups of mice. The viral RNA loads in the brains were significantly lower at 21 days p.i. than at 6 days p.i. regardless of the SJL/J genotypes (P < 0.0001). On the other hand, the amount of viral RNA in the spinal cord increased significantly between 6 and 21 days p.i. (P = 0.0151) but only slightly, and not significantly, between 21 and 45 days p.i. (P = 0.2152). This increase was independent of the genotype. In summary, these experiments show that SJL/J β2m−/− and SJL/J β2m+/− mice are more susceptible to persistent infection of the white matter of spinal cord than wild-type SJL/J mice. They also show that the differences in viral RNA load become significant only at the onset of the chronic phase of the disease.
In conclusion, our results show that MHC class I genes control the amount of viral RNA that persists in the CNS even in a highly susceptible strain, such as the SJL/J strain. The Tmevp1 locus has three major characteristics. First, it is located in the H-2D region of the MHC. Second, the resistant H-2b haplotype is dominant. Third, mice with Tmevp1 haplotypes of intermediate susceptibility control the viral RNA load to some extent. All these characteristics could be those of an H-2 class I gene. Indeed, the H-2Db gene, not the H-2Kb gene, confers resistance to persistent infection, which agrees with the H-2D localization of Tmevp1 (4, 5). Susceptible mice transgenic for the H-2Db gene become resistant, which is consistent with the resistant haplotype of Tmevp1 being dominant (3). Last, the present data show that mice with a Tmevp1 haplotype associated with intermediate susceptibility control the persisting viral RNA load through a class I-restricted mechanism. Therefore, TMEV persistence appears to be under the control of a single MHC-linked locus, Tmevp1, which has all the characteristics of an H-2 class I gene. Interestingly, for experimental autoimmune encephalomyelitis, which is another model of multiple sclerosis, several H-2 loci have been implicated in genetic control of the disease (11).
Acknowledgments
We thank M. Gau for secretarial assistance.
This work was supported in part by grants from the Institut Pasteur Fondation, the Centre National de la Recherche Scientifique, the Association pour la Recherche sur la Sclérose en Plaques, and the National Multiple Sclerosis Society. S.A. is a recipient of a scholarship from the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche.
REFERENCES
- 1.Aubagnac S, Brahic M, Bureau J-F. Viral load and a locus on chromosome 11 affect the late clinical disease caused by Theiler's virus. J Virol. 1999;73:7965–7971. doi: 10.1128/jvi.73.10.7965-7971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert C, Chamorro M, Brahic M. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay A, Brahic M, Bureau J-F. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler's virus. J Virol. 1994;68:4049–4052. doi: 10.1128/jvi.68.6.4049-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azoulay-Cayla A, Dethlefs S, Pérarnau B, Larsson-Sciard E-L, Lemonnier F A, Brahic M, Bureau J-F. H-2Db−/− mice are susceptible to persistent infection by Theiler's virus. J Virol. 2000;74:5470–5476. doi: 10.1128/jvi.74.12.5470-5476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azoulay-Cayla A, Syan S, Brahic M, Bureau J F. Roles of the H-2Db and H-2Kb genes in resistance to persistent Theiler's murine encephalomyelitis virus infection of the central nervous system. J Gen Virol. 2001;82:1043–1047. doi: 10.1099/0022-1317-82-5-1043. [DOI] [PubMed] [Google Scholar]
- 6.Bihl F, Brahic M, Bureau J-F. Two loci, Tmevp2 and Tmevp3, located on the telomeric region of chromosome 10, control the persistence of Theiler's virus in the central nervous system. Genetics. 1999;152:385–392. doi: 10.1093/genetics/152.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bihl F, Pena-Rossi C, Guénet J-L, Brahic M, Bureau J-F. The shiverer mutation affects the persistence of Theiler's virus in the central nervous system. J Virol. 1997;71:5025–5030. doi: 10.1128/jvi.71.7.5025-5030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahic M, Bureau J-F. Genetics of susceptibility to Theiler's virus infection. Bioessays. 1998;20:627–633. doi: 10.1002/(SICI)1521-1878(199808)20:8<627::AID-BIES5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Bureau J-F, Montagutelli X, Bihl F, Lefebvre S, Guénet J-L, Brahic M. Mapping loci influencing the persistence of Theiler's virus in the murine central nervous system. Nat Genet. 1993;5:87–91. doi: 10.1038/ng0993-87. [DOI] [PubMed] [Google Scholar]
- 10.Bureau J-F, Montagutelli X, Lefebvre S, Guénet J-L, Pla M, Brahic M. The interaction of two groups of murine genes determines the persistence of Theiler's virus in the central nervous system. J Virol. 1992;66:4698–4704. doi: 10.1128/jvi.66.8.4698-4704.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield R J, Sudweeks J D, Blankenhorn E P, Korngold R, Marini J C, Todd J A, Roper R J, Teuscher C. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 12.Clatch R J, Melvold R W, Miller S D, Lipton H L. Theiler's murine encephalomyelitis virus (TMEV) induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–1413. [PubMed] [Google Scholar]
- 13.Dal Canto M C, Lipton H L. Multiple sclerosis: animal model of human disease. Theiler's virus infection in mice. Am J Pathol. 1977;88:497–500. [PMC free article] [PubMed] [Google Scholar]
- 14.Lipton H L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipton H L, Kratochvil J, Sethi P, Dal Canto M C. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology. 1984;34:1117–1119. doi: 10.1212/wnl.34.8.1117. [DOI] [PubMed] [Google Scholar]
- 16.Lipton H L, Melvold R, Miller S D, Dal Canto M C. Mutation of a major histocompatibility class I locus, H-2D, leads to an increased virus burden and disease susceptibility in Theiler's virus-induced demyelinating disease. J Neurovirol. 1995;1:138–144. doi: 10.3109/13550289509113960. [DOI] [PubMed] [Google Scholar]
- 17.Lipton H L, Melvold R W. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- 18.McAllister A, Tangy F, Aubert C, Brahic M. Molecular cloning of the complete genome of Theiler's virus, strain DA, and production of infectious transcripts. Microb Pathog. 1989;7:381–388. doi: 10.1016/0882-4010(89)90041-7. [DOI] [PubMed] [Google Scholar]
- 19.Michiels T, Dejong V, Rodrigus R, Shaw-Jackson C. Protein 2A is not required for Theiler's virus replication. J Virol. 1997;71:9549–9556. doi: 10.1128/jvi.71.12.9549-9556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteyne P, Bureau J-F, Brahic M. The infection of mouse by Theiler's virus: from genetics to immunology. Immunol Rev. 1997;159:163–176. doi: 10.1111/j.1600-065x.1997.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez M, David C S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- 22.Rodriguez M, David C S. H-2Dd transgene suppresses Theiler's virus-induced demyelination in susceptible strains of mice. J Neurovirol. 1995;1:111–117. doi: 10.3109/13550289509111015. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez M, Leibowitz J L, David C S. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]