Abstract
Background
External counterpulsation (ECP) may improve cerebral blood flow, and it has been proposed as a potential therapy for patients with ischaemic stroke.
Objectives
To assess the efficacy and safety of ECP for acute ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (June 2011), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2011 Issue 2), MEDLINE (1948 to June 2011), EMBASE (1980 to June 2011), CINAHL (1982 to June 2011), AMED (Allied and Complementary Medicine) (1985 to June 2011), China Biological Medicine Database (CBM) (1978 to June 2011), Chinese National Knowledge Infrastructure (CNKI) (1979 to June 2011), Chinese Science and Technique Journals Database (VIP) (1989 to June 2011) and Wanfang Data (1984 to June 2011). We also searched ongoing trials registers, reference lists and relevant conference proceedings and contacted authors and manufacturers of external counterpulsation devices.
Selection criteria
Randomised controlled trials (RCTs) in which ECP (started within seven days of stroke onset) was compared with sham treatment or no treatment, or ECP plus routine treatment was compared with routine treatment alone, in patients with acute ischaemic stroke.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data, checked for adverse events data and contacted trialists for missing information.
Main results
We included two trials involving 160 patients. Numbers of death or dependent patients at the end of at least three months follow‐up were not reported in either of the included trials. The outcome measure used in the included trials was only the number of participants with improvement of neurological impairment after treatment according to the Modified Edinburgh‐Scandinavian Stroke Scale (MESSS) or self‐making criteria. ECP was associated with a significant increase in the number of participants whose neurological impairment improved (risk ratio (RR) 1.75, 95% confidence interval (CI) 1.37 to 2.23). Only one trial reported no adverse events.
Authors' conclusions
The methodological quality of the included studies was poor, and reliable conclusions could not be drawn from the present data. High‐quality and large‐scale RCTs are needed.
Keywords: Humans, Cerebrovascular Circulation, Brain Ischemia, Brain Ischemia/physiopathology, Brain Ischemia/therapy, Counterpulsation, Counterpulsation/methods, Randomized Controlled Trials as Topic, Randomized Controlled Trials as Topic/standards, Stroke, Stroke/physiopathology, Stroke/therapy, Treatment Outcome
Plain language summary
External counterpulsation for acute ischaemic stroke
Some studies have indicated that poor cerebral perfusion is related to unfavourable functional outcomes, precipitating strokes and other vascular events, which are often responsible for high mortality rates. External counterpulsation (ECP) is a non‐invasive and acceptable method which is known to improve perfusion of the brain. It provides pressure to the calves, thighs and buttocks by means of air‐filled cuffs. This helps to increase blood flow to the heart, brain and kidneys. This review identified two randomised controlled trials (RCTs) of ECP involving 160 participants with acute ischaemic stroke. There is no convincing evidence to support the routine use of ECP for the treatment of patients with acute ischaemic stroke. Further high‐quality and large‐scale RCTs are needed.
Background
Description of the condition
Stroke is the second most common cause of death and a major cause of disability worldwide (Donnan 2008). Ischaemic stroke, which accounts for 80% of all strokes (Thrift 2001), is caused by a blockage in an artery that supplies blood to the brain, resulting in a deficiency in blood flow which is also the cause of permanent brain damage and long‐term impairments. Despite the rapid progress in stroke prevention, an effective acute ischaemic stroke treatment with reliable evidence is still lacking, except for aspirin within 48 hours of stroke onset (Sandercock 2008), management in a stroke care unit (Stroke Unit Trialists' Collaboration 2007), thrombolysis with recombinant tissue plasminogen activator (in highly selected patients within 4.5 hours of stroke onset) (Adams 2007; Del Zoppo 2009; Wardlaw 2009) and decompressive surgery in patients with malignant middle cerebral artery (MCA) infarction (Vahedi 2007). Neuroprotection (Ginsberg 2009), Chinese herbal products (Wu 2007) and other therapies have been evaluated but their results have either been inconclusive or negative.
Description of the intervention
Some studies have indicated that poor cerebral perfusion is related to unfavourable functional outcomes, precipitating strokes and other vascular events, which are often responsible for high mortality rates (Ho 2006; Wong 2000; Wong 2003). As the main problem of ischaemic stroke is that the focal cerebral region cannot get enough blood, cerebral blood flow augmentation may be the first and most important target in acute ischaemic stroke management. External counterpulsation (ECP) is a non‐invasive and acceptable method which is known to improve the perfusion of vital organs.
ECP uses an ECG‐triggered diastolic pressure of approximately 250 mmHg to the calves, thighs and buttocks by means of air‐filled cuffs. The diastolic augmentation of the blood flow and the simultaneously decreasing systolic afterload therefore increase the blood flow to the heart, brain and kidneys (Han 2008a).
How the intervention might work
The possible factors responsible for its clinical improvement are summarised as follows.
ECP can significantly increase blood flow in carotid, renal, hepatic and coronary arteries. These haemodynamic effects result in a rise in blood flow in multiple vascular beds, including the brain, kidneys, liver and myocardium (Applebaum 1997; Werner 1999).
ECP may help open the preformed collateral vessels to augment collateral perfusion by releasing shear‐dependent vasomediators and augmenting arterial pressure. It has been verified that chronic exposure of the vascular bed to the augmented blood flow may increase vascular shear stress, and enhanced shear stress itself plays an important role in the maintenance of a functional endothelium (Niebauer 1996; Ozawa 2001; Soran 1999). Some studies have shown that increased shear stress can stimulate the release of a vasodilator, nitric oxide, and also inhibit the release of a vasoconstrictor, endothelial endothelin‐1 (ET‐1) (Akhtar 2006; Barsness 2001).
ECP may influence angiogenesis, which may also improve collateral perfusion. ECP may increase the endothelial shear stress, which is considered as a major stimulus for collateral development (Kersten 1999). Meanwhile, increased shear stress may upregulate the endothelial production of growth factors, such as vascular endothelial growth factor (VEGF), which plays a key role in angiogenesis (Gan 2000), thus angiogenesis is promoted by ECP.
Why it is important to do this review
ECP has been approved for use in angina, myocardial infarction, congestive heart failure and cardiogenic shock because it augments blood flow to cardiac and systemic circuits (Bonetti 2003; Lawson 2002). It may improve cerebral blood flow, so it has been proposed as a potential therapy for patients with brain ischaemia (Han 2008a). Although there are a few published studies about the clinical efficacy of ECP in patients with ischaemic stroke, the potential therapeutic effect is controversial. The aim of this review is to systematically analyse all the randomised controlled trials (RCTs) of ECP for acute ischaemic stroke in order to provide the best available evidence for clinical practice and further research planning for stroke treatment.
Objectives
To assess the efficacy and safety of ECP for acute ischaemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, irrespective of blinding, publication status or language, comparing ECP therapy with sham therapy or no therapy, were eligible for inclusion. We excluded trials in which the authors reported only laboratory parameters.
Types of participants
Trials that included patients of any age or sex with acute ischaemic stroke (within seven days of onset) were eligible. The clinical definition of ischaemic stroke is that of the World Health Organization criteria (Hatano 1976) with computerised tomography (CT) or magnetic resonance imaging (MRI) scanning to exclude haemorrhagic stroke.
Types of interventions
We included trials evaluating ECP in patients with ischaemic stroke, regardless of the frequency, intensity or the duration of treatment. The control interventions were sham treatment or no treatment. We also included trials where the addition of ECP to another treatment was compared with the other treatment alone.
Types of outcome measures
Primary outcomes
Death or dependency at the end of long‐term follow‐up (at least three months). Dependency is defined as a Barthel Index (BI) score less than or equal to 60, a modified Rankin Scale (mRS) of grade 3 to 5, or the trialists' own definition.
Secondary outcomes
Death from all causes within the first two weeks of treatment and by the end of the scheduled treatment period.
Improvement of neurological impairment (e.g. National Institute of Health Stroke Scale (NIHSS), Canadian Neurological Scale (CNS), European Stroke Scale (ESS), Scandinavian Stroke Scale (SSS) or Chinese Stroke Scale (CSS), etc) at the end of the scheduled treatment.
Improvement of activities of daily living (ADL) (e.g. BI, mRS or trialists' own definition) at the end of long‐term follow‐up.
Adverse effects of the intervention (e.g. skin abrasion, low back pain and muscle ache).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the following databases:
Cochrane Stroke Group Trials Register (which was last searched by the Managing Editor in June 2011);
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2011 Issue 2) (Appendix 1);
MEDLINE (1948 to June 2011) (Appendix 2);
EMBASE (1980 to June 2011) (Appendix 3);
CINAHL (1982 to June 2011) (Appendix 4);
AMED (Allied and Complementary Medicine) (1985 to June 2011) (Appendix 5);
The China Biological Medicine Database (CBM) (1978 to June 2011);
The Chinese National Knowledge Infrastructure (CNKI) (1979 to June 2011);
Chinese Science and Technique Journals Database (VIP) (1989 to June 2011); and
Wanfang Data (http://www.wanfangdata.com/) (1984 to June 2011).
The Cochrane Stroke Group Trials Search Co‐ordinator developed the search strategies for CENTRAL, MEDLINE, EMBASE, CINAHL and AMED and we adapted the MEDLINE search strategy for the other databases.
Searching other resources
In an effort to identify further published, unpublished and ongoing studies we:
-
searched the following ongoing trials and research registers (which was last searched in June 2011):
ClinicalTrials.gov (http://www.clinicaltrials.gov/);
Current Controlled Trials (http://www.controlled‐trials.com);
Stroke Trials Registry (http://www.strokecenter.org/trials/);
International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/)
Chinese Clinical Trials Registry (http://www.chictr.org/(S(chtp1eftsefc1afxrfrpdp55))/Default.aspx);
-
searched databases of conference abstracts:
Conference Proceedings Citation Index Science (CPCI‐S) (1990 to June 2011);
China Medical Academic Conferences (CMAC) in Chinese Medical Current Contents (CMCC) (1995 to June 2011);
searched reference lists from relevant articles and reviews;
contacted authors of relevant trials;
contacted manufacturers of external counterpulsation devices (Chongqing PSK‐Health Sci‐Tech Development Co Ltd, June 2011); and
used Science Citation Index Cited Reference Search for forward tracking of relevant references.
We searched for trials in all languages and arranged translation of relevant articles published in languages other than English and Chinese.
Data collection and analysis
Selection of studies
Two review authors independently scanned the titles, abstracts and keywords of records obtained from the electronic searches and excluded those that were obviously irrelevant. We obtained the full text of the remaining articles and selected those studies that met the selection criteria outlined previously. We resolved any disagreements through discussion and, when necessary, we consulted with a third review author (Ming Liu). If it had not been possible to resolve a disagreement, we planned to add the study to those awaiting assessment and to contact the study authors for clarification.
Data extraction and management
Two review authors independently extracted data on methods, patients, interventions, outcomes and results by using a data extraction form. The same two review authors cross‐checked all extracted data and resolved any disagreements through discussion. If consensus could not be reached, we asked a third review author (Ming Liu) to make a final decision. For dichotomous outcomes, we extracted the number of participants experiencing the event and the total number of participants in each arm of the trial. For continuous outcomes, we extracted the mean value and standard deviation for the changes in each arm of the trial along with the total number in each group. When necessary, we contacted the study authors for additional unpublished data.
Assessment of risk of bias in included studies
We assessed the methodological quality of selected studies as described in section 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We scored each of the following points as 'low risk', 'high risk' or 'unclear risk' of bias and reported them in the 'Risk of bias' tables.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Other bias.
On the basis of these criteria, we divided studies into the following three categories.
A ‐ all quality criteria met: low risk of bias.
B ‐ one or more of the quality criteria only partly met: moderate risk of bias.
C ‐ one or more criteria not met: high risk of bias.
Two review authors independently evaluated all the studies. They resolved any disagreements through discussion. If consensus could not be reached, they asked a third review author (Ming Liu) to make a final decision.
Measures of treatment effect
We expressed results for dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI), and expressed results for continuous outcomes as mean difference (MD) with 95% CI (if the same scale for each trial was available) or standardised mean difference (SMD) with 95% CI (if different scales were used).
Unit of analysis issues
For studies with non‐standard designs (e.g. cross‐over trials, cluster‐randomised trials), we planned to manage the data according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). For example, if we had found any cross‐over trials, we would only have analysed the data from the first period.
Dealing with missing data
If data were missing, we contacted the investigators for additional information. If some data remained unavailable, we considered both best‐case and worst‐case scenarios.
Assessment of heterogeneity
We determined heterogeneity according to the I2 statistic.
Assessment of reporting biases
We used the funnel plot method (Egger 1997).
Data synthesis
We performed statistical analysis using the Cochrane Review Manager software, RevMan 5.1 (RevMan 2011) and performed all analyses in accordance with the intention‐to‐treat method. We reported the results as RR with 95% CI for dichotomous data and as MD or SMD with 95% CI for continuous data. We used the random‐effects model to combine individual results. If there were no suitable studies, we provided a narrative summary of the study results.
Subgroup analysis and investigation of heterogeneity
If appropriate data were available, we intended to undertake subgroup analysis according to:
different times to the start of ECP therapy;
different intensity of ECP therapy; and
different duration of ECP therapy.
We quantified inconsistency across studies using the I2 statistic.
Sensitivity analysis
We re‐analysed the data excluding studies:
with inadequate allocation concealment;
not using sham therapy; and
not using a blind rater to evaluate outcomes.
Results
Description of studies
Results of the search
From a total of 1582 articles generated by the electronic searches and handsearches, we excluded 473 duplicates, 835 irrelevant references by reading the title and 258 references by reading the abstract or text. We identified 16 potentially eligible trials of which two completed trials (Han 2000; Wei 1995) and two ongoing trials (Guluma 2009; Wong 2007) met the inclusion criteria for this review. As for the remainder of the 16 trials identified, we excluded five trials (Han 2008b; Meng 2000; Sun 1989; Xu 1989; Zhang 2003) because they enrolled some patients with ischaemic stroke who were beyond seven days from onset, three trials (Cen 1994; Li 1997; Liu 2003) as they were not RCTs, another three trials (Li 2005; Yang 1996; Zhang 2001) because the comparison in each trial was inappropriate, and one trial (Gao 2009) on account of the inconsistent data in the text.
Included studies
Both included trials (Han 2000; Wei 1995) were conducted in China and published in Chinese journals. A total of 160 participants were enrolled in the two studies and the age of participants ranged from 46 to 80 years. One trial (Han 2000) only included males, the other (Wei 1995) included more males than females. Both of the included trials reported the inclusion criteria but did not report exclusion criteria. In one trial (Wei 1995), all patients were enrolled within the first 48 hours of stroke onset; in the other one (Han 2000), all patients were enrolled within the first 72 hours of stroke onset. The stroke severity of the participants were similar between treatment group and control group in each trial.
In the treatment groups of both trials, ECP was applied one hour once daily with a therapeutic pressure (about 290 to 330 mmHg). In both the treatment group and the control group of each trial, the same routine therapy (medicine or acupuncture, or both) was used. One trial reported the treatment period of ECP was 24 days (Wei 1995) while the other trial (Han 2000) did not mention the duration of treatment. Details of routine therapy are reported in the Characteristics of included studies table.
One trial (Wei 1995) evaluated the effect of ECP at the end of treatment on the number of participants with improvement of neurological impairment according to more than 45% decrease of Modified Edinburgh‐Scandinavian Stroke Scale (MESSS) score. The other trial (Han 2000) applied a measurement of neurological impairment that was similar to MESSS but defined by the trialists themselves. None of the participants in either of the trials were followed up after treatment was discontinued. No deaths were reported in either trial. Assessment of quality of life was not undertaken in either of the trials. Only one trial (Han 2000) reported no adverse events.
All information of included trials is presented in the Characteristics of included studies table.
As for the two ongoing trials, one (Wong 2007) was conducted in China, the other (Guluma 2009) in the USA. For details of the ongoing trials, see Characteristics of ongoing studies table.
Excluded studies
The reasons for excluding 12 trials are given in the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
The two included trials reported 'randomly allocating' participants but the method of randomisation was not described. Meanwhile, no trial stated the method of allocation concealment. Thus, we graded random sequence generation and allocation concealment for these trials as unclear risk of bias.
Blinding
No trial used sham ECP (placebo) as the control. None of the investigators stated the method of blinding, so we did not know what kind of blinding method for participants and outcome assessors was used in these included trials. We assessed blinding as unclear risk of bias.
Incomplete outcome data
No patients were lost to follow‐up. None of the included trials stated an intention‐to‐treat analysis. This we assessed as low risk of bias.
Selective reporting
No trial protocol was available, and we were not sure of any selective reporting of data. Therefore, there was insufficient information for us to make a judgement on selective reporting. We assessed this as unclear risk of bias.
Other potential sources of bias
We were not sure of any potential publication bias, however, this could not be investigated by using a funnel plot as only two trials were included.
Effects of interventions
Primary outcome measures
Death or dependency at the end of long‐term follow‐up (at least three months)
Neither trial observed the long‐term effect of ECP on death or dependency at the end of follow‐up.
Secondary outcomes measures
Death from all causes within the first two weeks of treatment and by the end of the scheduled treatment period
No death was reported within the first two weeks of treatment or by the end of the scheduled treatment period.
Improvement of neurological impairment at the end of the scheduled treatment
The outcomes measured after treatment were assessed according to MESSS or similar to MESSS in both trials. Meta‐analysis of the two trials, which used a dichotomous outcome variable, showed that a significantly higher proportion of participants treated with ECP had an improvement of their neurological impairment at the end of treatment compared with the control participants (RR 1.75, 95% CI 1.37 to 2.23) (See Analysis 1.1).
1.1. Analysis.
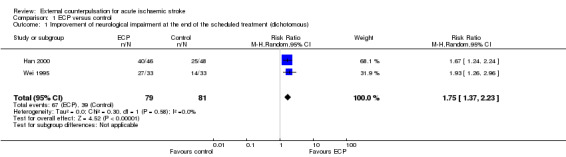
Comparison 1 ECP versus control, Outcome 1 Improvement of neurological impairment at the end of the scheduled treatment (dichotomous).
Improvement of ADL at the end of long‐term follow‐up
Assessment of improvement of ADL was not undertaken in either trial.
Adverse effects
Only one trial (Han 2000) reported no adverse effects.
Subgroup analysis
Effects in patients with different times to the start of ECP therapy
Neither of the two trials reported the accurate starting time of treatment. Therefore, we could not perform this subgroup analysis.
Effects in patients with different intensity of ECP therapy
The intensity of ECP therapy was similar (about 290 to 330 mmHg one hour once daily) in both included studies. Therefore, it was not possible to do a subgroup analysis for different intensity of ECP therapy.
Effects in patients with different duration of ECP therapy
Only one trial stated the duration of ECP therapy, thus it was not appropriate data to perform a subgroup analysis.
Sensitivity analysis
Excluding studies with inadequate allocation concealment
The method of allocation concealment was unclear in both included studies. Therefore, such sensitivity analyses could not be done. In future, investigators of RCTs should describe the methods of allocation concealment in detail when the trial results are published.
Excluding studies not using sham therapy
No study used sham therapy in the control group. To measure the effect of ECP for ischaemic stroke objectively, sham therapy should be used in the control group in future RCTs.
Excluding studies not using a blinded rater to evaluate outcomes
Neither trial stated whether a blinded rater was used to evaluate outcomes. Thus, there were no adequate data to conduct sensitivity analysis.
Discussion
Summary of main results
We included two RCTs (including 160 participants) of ECP for acute ischaemic stroke. The quality of these trials was generally poor. There is no convincing evidence to support the routine use of ECP for the treatment of patients with acute ischaemic stroke.
Neither of the trials reported the primary outcome (death or dependency at the end of long‐term follow‐up) for this review. Both trials only evaluated the effect of ECP at the end of treatment on the number of participants with improvement of neurological impairment. Meta‐analysis of the two trials, which used a dichotomous outcome variable, showed that a significantly higher proportion of participants treated with ECP had an improvement of their neurological impairment at the end of treatment period compared with the control participants (RR 1.75, 95% CI 1.37 to 2.23). Only one trial reported no adverse effects.
Overall completeness and applicability of evidence
The methodological quality of the included studies was poor, and reliable conclusions could not be drawn from the present data.
Several studies have certified that ECP was safe and feasible for patients with ischaemic stroke. Some key points of ECP treatment for patients with ischaemic stroke should be appreciated in future studies as follows.
Subtype of ischaemic stroke
Han et al (Han 2010) have indicated that it is important to identify the subtype of patients with ischaemic stroke who may benefit most from ECP treatment. In this review, we do not have sufficient data to analysis the effect of ECP for different subtypes of ischaemic stroke. Therefore, there is not enough evidence to prove which subtype of patients with ischaemic stroke might benefit most. More RCTs comparing ECP for different subtypes of ischaemic stroke are necessary to address this question.
Time window, intensity and duration of ECP treatment
We planned to carry out a subgroup analysis of ECP effects in patients with different starting times of treatment. However, no trial reported the accurate time of treatment being started. Some salvageable brain tissue may exist up to eight to 24 hours from symptom onset, while availability of a low‐risk non‐invasive intracranial blood flow augmentation device would provide a treatment option to a large number of patients with ischaemic stroke (Alexandrov 2008), so it is important to demonstrate whether there is a specific time window of ECP for treating patients.
We also planned to do some subgroup analyses of the effects on patients of different intensity or duration of ECP. However, the intensity of ECP was the same in both included trials, and only one trial stated the duration of ECP treatment. To the best of our knowledge, no clinical study or even animal model assessment has ever been carried out to explore whether ECP therapy has a different therapeutic effect under a different intensity or duration of treatment for patients with acute ischaemic.
As a result, more high‐quality, large‐scale RCTs are needed to address which time window, intensity and duration of ECP treatment are optimal for patients with ischaemic stroke.
Age
Most clinical trials of main therapies exclude patients over 80 years old (Wenger 2006), for example, most of the large trials on thrombolysis such as ECASS‐3 (Hacke 2008) and DIAS‐2 (Hacke 2009). But, some studies have suggested that age alone should not preclude very elderly patients from participating in clinical trials (Wang 2011). In this review, the age of the participants varied between 46 and 80 years and there is no information on ECP therapy on elderly patients with ischaemic stroke. Therefore, further RCTs should enrol patients over 80 years.
Quality of the evidence
The evidence summarised in this review comes from two small and low‐quality studies in which the total number of participants was 160. A robust conclusion cannot be drawn about the effectiveness of ECP for acute ischaemic stroke. Key methodological limitations of the included studies and recommendations for future trials are detailed below (see Implications for research).
Potential biases in the review process
We are sure that we have identified the existing large trials relevant to our question. However, we cannot deny the possibility that there are additional trials which are unpublished or published in sources not covered by our search. This may lead to some biases.
Agreements and disagreements with other studies or reviews
We did not find any other studies or reviews of ECP for acute ischaemic stroke.
Authors' conclusions
Implications for practice.
This review does not provide convincing evidence to support the routine use of ECP for the treatment of patients with acute ischaemic stroke.
Implications for research.
This review suggests that ECP may improve neurological impairment after acute ischaemic stroke. High‐quality, large‐scale RCTs are needed to confirm or refute these results. Future trials should overcome the limitations of the trials presented in this review.
The following features should be addressed in future studies:
adequate random sequence generation and allocation concealment;
sham therapy (placebo) control;
blinding of investigator, participants and outcome assessors;
use of internationally accepted scales and endpoint measurements of primary outcome measurements at long‐term follow‐up should be made;
adverse events should be critically assessed;
description of withdrawal and use of intention‐to‐treat analysis;
reports of the trials should conform to the recommendations of the CONSORT statement.
Acknowledgements
We would like to thank Hazel Fraser (Managing Editor of Cochrane Stroke Group), Peter Sandercock, Stefano Ricci, Brenda Thomas, Ashma Krishan and Will Whiteley (the Cochrane Stroke Group editors and peer reviewers for this protocol) for their valuable advice on writing this review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1.MeSH descriptor cerebrovascular disorders this term only #2.MeSH descriptor basal ganglia cerebrovascular disease this term only #3.MeSH descriptor brain ischemia explode all trees #4.MeSH descriptor carotid artery diseases this term only #5.MeSH descriptor carotid artery thrombosis this term only #6.MeSH descriptor intracranial arterial diseases this term only #7.MeSH descriptor cerebral arterial diseases this term only #8.MeSH descriptor intracranial embolism and thrombosis explode all trees #9.MeSH descriptor stroke explode all trees #10.(ischaemic in Title, Abstract or Keywords or ischemic in Title, Abstract or Keywords) #11.(stroke* in Title, Abstract or Keywords or apoplex* in Title, Abstract or Keywords or (cerebral in Title, Abstract or Keywords and vasc* in Title, Abstract or Keywords) or cerebrovasc* in Title, Abstract or Keywords or cva in Title, Abstract or Keywords or attack* in Title, Abstract or Keywords) #12.(#10 and #11) #13.(brain in Title, Abstract or Keywords or cerebr* in Title, Abstract or Keywords or cerebell* in Title, Abstract or Keywords or vertebrobasil* in Title, Abstract or Keywords or hemispher* in Title, Abstract or Keywords or intracran* in Title, Abstract or Keywords or intracerebral in Title, Abstract or Keywords or infratentorial in Title, Abstract or Keywords or supratentorial in Title, Abstract or Keywords or (middle in Title, Abstract or Keywords and cerebr* in Title, Abstract or Keywords) or mca* in Title, Abstract or Keywords or (anterior in Title, Abstract or Keywords and circulation in Title, Abstract or Keywords) ) #14.(ischemi* in Title, Abstract or Keywords or ischaemi* in Title, Abstract or Keywords or infarct* in Title, Abstract or Keywords or thrombo* in Title, Abstract or Keywords or emboli* in Title, Abstract or Keywords or occlus* in Title, Abstract or Keywords or hypoxi* in Title, Abstract or Keywords) #15.(#13 and #14) #16.(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #12 or #15) #17.MeSH descriptor counterpulsation this term only #18.MeSH descriptor assisted circulation this term only #19.(counterpulsation in Title, Abstract or Keywords or "counter pulsation" in Title, Abstract or Keywords or counter‐pulsation in Title, Abstract or Keywords or ECP in Title, Abstract or Keywords or EECP in Title, Abstract or Keywords or "diastolic augmentation" in Title, Abstract or Keywords or counterpressure in Title, Abstract or Keywords or "counter pressure" in Title, Abstract or Keywords or CardiAssist in Title, Abstract or Keywords) #20.(augment* in Title, Abstract or Keywords or assist* in Title, Abstract or Keywords) #21.(perfusion in Title, Abstract or Keywords or "blood flow" in Title, Abstract or Keywords or circulation in Title, Abstract or Keywords) #22.(#20 and #21) #23.(#17 or #18 or #19 or #22) #24.(#16 and #23)
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. assisted circulation/ or counterpulsation/ 6. (counterpulsation or counter pulsation or counter‐pulsation or ECP or EECP or diastolic augmentation or counterpressure or counter pressure or CardiAssist).tw. 7. ((augment$ or assist$) adj5 (perfusion or blood flow or circulation)).tw. 8. 5 or 6 or 7 9. 4 and 8
Appendix 3. EMBASE (Ovid) search strategy
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ 2. stroke patient/ or stroke unit/ 3. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 4. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 5. 1 or 2 or 3 or 4 6. assisted circulation/ or counterpulsation/ 7. (counterpulsation or counter pulsation or counter‐pulsation or ECP or EECP or diastolic augmentation or counterpressure or counter pressure or CardiAssist).tw. 8. ((augment$ or assist$) adj5 (perfusion or blood flow or circulation)).tw. 9. 6 or 7 or 8 10. 5 and 9
Appendix 4. CINAHL (Ebsco) search strategy
S16 .S9 and S15 S15 .S10 or S11 or S14 S14 .S12 and S13 S13 .TI ( perfusion or blood flow or circulation ) or AB ( perfusion or blood flow or circulation ) S12 .TI ( augment* or assist* ) or AB ( augment* or assist* ) S11 .TI ( counterpulsation or counter pulsation or counter‐pulsation or ECP or EECP or diastolic augmentation or counterpressure or counter pressure or CardiAssist ) or AB ( counterpulsation or counter pulsation or counter‐pulsation or ECP or EECP or diastolic augmentation or counterpressure or counter pressure or CardiAssist ) S10 .(MH "Assisted Circulation") OR (MH "Counterpulsation") S9 .S1 or S2 or S5 or S8 S8 .S6 and S7 S7 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) S6 .TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation ) S5 .S3 and S4 S4 .TI ( stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack* ) or AB ( stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack* ) S3 .TI ( ischemi* or ischaemi* ) or AB ( ischemi* or ischaemi* ) S2 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases") OR (MH "Carotid Artery Thrombosis") OR (MH "Cerebral Ischemia+") OR (MH "Intracranial Arterial Diseases") OR (MH "Cerebral Arterial Diseases") OR (MH "Intracranial Embolism and Thrombosis+") OR (MH "Stroke") S1 .(MH "stroke patients") or (MH "stroke units")
Appendix 5. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. 1 or 2 or 3 5. (counterpulsation or counter pulsation or counter‐pulsation or ECP or EECP or diastolic augmentation or counterpressure or counter pressure or CardiAssist).tw. 6. ((augment$ or assist$) adj5 (perfusion or blood flow or circulation)).tw. 7. 5 or 6 8. 4 and 7
Data and analyses
Comparison 1. ECP versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement of neurological impairment at the end of the scheduled treatment (dichotomous) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.37, 2.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Han 2000.
| Methods | RCT Method of randomisation: not stated Allocation concealment: not stated Blinding: not stated ITT analysis: not stated Losses to follow‐up: none |
|
| Participants | Country: China 94 participants with acute ischaemic stroke within 72 hours (46 treatment, 48 control) Sex: all males Age: 46 to 80 years Comparability: age and stroke severity similar |
|
| Interventions | Treatment: ECP 1 hour once daily with therapeutic pressure (about 290 mmHg) plus compound danshen injection 20 ml once daily plus hydroxyethyl starch 500 ml once daily plus acupuncture Control: compound danshen injection 20 ml once daily plus hydroxyethyl starch 500 ml once daily plus acupuncture |
|
| Outcomes | Number of participants with neurological improvement (as defined by the trialists; similar to MESSS) at the end of the treatment period | |
| Notes | Duration of ECP therapy: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation was reported but the method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, and we were not sure of any selective reporting of the results |
| Other bias | Unclear risk | There was Insufficient information to assess whether an important risk of bias exists |
Wei 1995.
| Methods | RCT Method of randomisation: not stated Allocation concealment: not stated Blinding: not stated ITT analysis: not stated Losses to follow‐up: none |
|
| Participants | Country: China 66 participants with acute ischaemic stroke within 48 hours (33 treatment, 33 control); all had brain CT scan proven Sex: treatment: 30 males, 3 females; control: all males Age: treatment: 55 to 75 years, 62.3 ± 4.6 years; control: 48 to 80 years, 63 ± 5.2 years Comparability: stroke severity similar |
|
| Interventions | Treatment: ECP 1 hour once daily with therapeutic pressure (about 290 to 330 mmHg) for 24 days plus dextran 40 and venoruton for 28 days Control: dextran 40 and venoruton for 28 days |
|
| Outcomes | Number of participants with neurological improvement (MESSS score decrease > 45%) at the end of the treatment period (28 days) | |
| Notes | Duration of ECP therapy: 24 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation was reported but the method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, and we were not sure of any selective reporting of the results |
| Other bias | Unclear risk | There was Insufficient information to assess whether an important risk of bias exists |
CT: computerised tomography ECP: external counterpulsation ITT: intention‐to‐treat MESSS: Modified Edinburgh‐Scandinavian Stroke Scale RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cen 1994 | Quasi‐RCT |
| Gao 2009 | The data of this trial was inconsistent in the text |
| Han 2008b | Some eligible patients over 7 days from onset |
| Li 1997 | Not RCT |
| Li 2005 | The comparison was inappropriate: ECP + ligustrazine injection + naoxing decoction versus nimodipine tablets + decoction placebo |
| Liu 2003 | Quasi‐RCT |
| Meng 2000 | Some eligible patients over 7 days from onset |
| Sun 1989 | Some eligible patients over 7 days from onset |
| Xu 1989 | Some eligible patients over 7 days from onset |
| Yang 1996 | The comparison was inappropriate: ECP versus danshen injection |
| Zhang 2001 | The comparison was inappropriate: ECP versus dextran 40 + danshen injection |
| Zhang 2003 | Some eligible patients over 7 days from onset |
ECP: external counterpulsation RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Guluma 2009.
| Trial name or title | A randomised, controlled phase 1 study of external counterpulsation as a treatment for acute ischaemic stroke |
| Methods | RCT Blinding: single blind (subject) Intervention model: parallel assignment Placebo control |
| Participants | Country: USA Estimated enrolment: 40 Ages eligible for study: 18 to 85 years Genders eligible for study: both Inclusion criteria:
Exclusion criteria:
|
| Interventions | Treatment: ECP at a full pressure: a 1‐hour treatment of ECP at full pressure, which will be applied in a tiered, dose‐escalating manner, starting at 200 mmHg and increasing up to 300 mmHg based on assessments made Control: ECP at sham‐pressure: a 1‐hour treatment of ECP at an inactive pressure, which will be applied at 75 mmHg and kept there for the hour while assessments are made |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2009 |
| Contact information | ClinicalTrials.gov identifier: NCT00983749 Principal Investigator: Kama Z Guluma MD, University of California, San Diego, USA |
| Notes | Estimated study completion date: December 2011 Duration of follow‐up: 30 days |
Wong 2007.
| Trial name or title | A multi‐centre, randomised, controlled study of external counterpulsation for patients with recent atherosclerotic stroke |
| Methods | RCT Blinding: open label Intervention model: parallel assignment |
| Participants | Country: China Estimated enrolment: 250 Ages eligible for study: not less than 18 years Genders eligible for study: both Inclusion criteria:
Exclusion criteria:
|
| Interventions | Treatment: external counterpulsation 35 x 1‐hour sessions over a 7‐week period |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 1 May 2007 |
| Contact information | Registration number: ChiCTR‐TRC‐09000706 Dr Ka Sing Wong, Department of Medicine and Therapeutics, Hong Kong, SAR, China Tel: 2632‐3471 Email: ks‐wong@cuhk.edu.hk |
| Notes | Duration of follow‐up: 12 weeks |
AICD: automated implanted defibrillator AVM: arteriovenous malformation BP: blood pressure CHF: congestive heart failure CT: computerised tomography DVT: deep venous thrombosis ECP: external counterpulsation EDH: extradural haemorrhage INR: International Normalised Ratio IPH: intraparenchymal haemorrhage LACI: lacunar infarction MCA: middle cerebral artery MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale PACI: partial anterior circulation infarct PVOD: peripheral vascular occlusive disease RCT: randomised controlled trial SAH: subarachnoid haemorrhage SDH: subdural haemorrhage TACI: total anterior circulation infarct TCD: transcranial doppler WHO: World Health Organization
Differences between protocol and review
None
Contributions of authors
Draft the protocol: Sen Lin, Ming Liu, Bo Wu, Zilong Hao
Develop a search strategy: Sen Lin, Ming Liu, Bo Wu, Zilong Hao
Search for trials: Sen Lin, Bo Wu
Obtain copies of relevant references: Sen Lin, Bo Wu, Zilong Hao
Trials selection: Sen Lin, Jie Yang, Ming Liu
Data extraction and data entry: Sen Lin, Wendan Tao
Analysis, writing the final review and interpreting the results: Sen Lin, Ming Liu, Bo Wu, Zilong Hao
The review will be updated by Sen Lin, Bo Wu
Declarations of interest
None known.
New
References
References to studies included in this review
Han 2000 {published data only}
- Han LH, Zhang MZ. Analysis of the efficacy of external counterpulsation for 46 cerebral infarction patients. Practical New Medcine 2000;2:893. [Google Scholar]
Wei 1995 {published data only}
- Wei B, Yang B. Curative effects of a combination of external counterpulsation and medicine on cerebral infarction. Journal of Chengde Medical College 1995;12:126‐8. [Google Scholar]
References to studies excluded from this review
Cen 1994 {published data only}
- Cen ZX, Liu HL, Tao GW. Effect of external counterpulsation in 74 patients with lacunar stroke and cerebral artery atherosclerosis‐an observational study. Journal of Guangdong Medical College 1994;12:231‐2. [Google Scholar]
Gao 2009 {unpublished data only}
- Gao JB. The clinical research of treating acute ischemic stroke with naoxingwan and enhanced external counterpulsation combination therapy. Master Thesis of Guangzhou University of Chinese Medcine.
Han 2008b {published data only}
- Han JH, Leung TW, Lam WW, Soo YO, Alexandrov AW, Mok V, et al. Preliminary findings of external counterpulsation for ischemic stroke patient with large artery occlusive disease. Stroke 2008;39:1340‐3. [DOI] [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li ZC, Luan MH, Li SY. Observation of the curative effect of external counterpulsation combined hyperbaric oxygen on cerebral infarction. Chinese Journal of Nautical Medicine 1997;4:181‐2. [Google Scholar]
Li 2005 {published data only}
- Li R, Wu W, Liu YD, Hong YD, Huang YS, Chen HG, et al. Observation of the curative effect of external counterpulsation combined Chinese herbal medicine on acute ischemic stroke. Chinese Journal Integrative Medicine of Cardio‐/Cerebrovascular Disease 2005;3:221‐3. [Google Scholar]
Liu 2003 {published data only}
- Liu MX, Lu H, Liu XM, Zhang XL. Observation of the curative effect of external counterpulsation combined early rehabilitation on hemiplegia after cerebral infarction. Chinese Journal of Physical Medicine and Rehabilitation 2003;25:160‐1. [Google Scholar]
Meng 2000 {published data only}
- Meng ZW, Hu Y, Jiang LW, Li XZ. Clinical recovery of ischemic stroke patients after external counterpulsation. Modern Rehabilitation 2000;4:894. [Google Scholar]
Sun 1989 {published data only}
- Sun ZL, Hu YS, Zhang GR, Hu XW, He HY. Comparison of the efficacy between using dextran 40 plus venoruton and their combination with external counterpulsation to treat cerebral thrombosis. Chengdu Medicine 1989;15:20. [Google Scholar]
Xu 1989 {published data only}
- Xu LX, Zong Q. External counterpulsation combined comprehensive therapy on cerebral infarction: report of 60 cases. Journal of Heze Medical College 1989;1:58‐60. [Google Scholar]
Yang 1996 {published data only}
- Yang SJ, Gu DX, Li F, Cao ZZ, Shi JM. Assessment of cerebral blood flow by TCD and gamma‐CBF in patients with ischemic stroke after external counterpulsation. Modern Medical Equipment and Application 1996;8:16‐8. [Google Scholar]
Zhang 2001 {published data only}
- Zhang RH. External counterpulsation in treatment of ischemic stroke. Chinese Journal of Rehabilitation 2001;16:172‐3. [Google Scholar]
Zhang 2003 {published data only}
- Zhang JL, Jiang LW, Li XZ. Effect of external counterpulsation on cerebral hemodynamics among patients with ischemic stroke. Chinese Journal of Cardiovascular Rehabilitation Medicine 2003;12:242‐3. [Google Scholar]
References to ongoing studies
Guluma 2009 {published data only}
- Guluma KZ. A randomized, controlled phase 1 study of external counterpulsation as a treatment for acute ischemic stroke. http://www.clinicaltrials.gov/ct2/show/NCT00983749.
Wong 2007 {published data only}
- Wong KS. A multi‐centre, randomized, controlled study of external counterpulsation for patients with recent atherosclerotic stroke. http://www.chictr.org/(S(dknwt13dftlbwt55oak2pkuq))/Site/TaskView.aspx?ID=abcf9eb8‐b5eb‐4f49‐ad22‐1ab6c47da671.
Additional references
Adams 2007
- Adams HP Jr, Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655‐711. [DOI] [PubMed] [Google Scholar]
Akhtar 2006
- Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin‐1 levels. American Journal of Cardiology 2006;98:28‐30. [DOI] [PubMed] [Google Scholar]
Alexandrov 2008
- Alexandrov AW, Ribo M, Wong KS, Sugg RM, Garami Z, Jesurum JT, et al. Perfusion augmentation in acute stroke using mechanical counter‐pulsation‐phase IIa: effect of external counterpulsation on middle cerebral artery mean flow velocity in five healthy subjects. Stroke 2008;39:2760‐4. [DOI] [PubMed] [Google Scholar]
Applebaum 1997
- Applebaum RM, Kasliwal R, Tunick PA, Konecky N, Katz ES, Trehan N, et al. Sequential external counterpulsation increases cerebral and renal blood flow. American Heart Journal 1997;133:611‐5. [DOI] [PubMed] [Google Scholar]
Barsness 2001
- Barsness GW. Enhanced external counterpulsation in unrevascularizable patients. Current Interventional Cardiology Reports 2001;3:37‐43. [PubMed] [Google Scholar]
Bonetti 2003
- Bonetti PO, Holmes DR Jr, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what's behind the curtain?. Journal of the American College of Cardiology 2003;41:1918‐25. [DOI] [PubMed] [Google Scholar]
Del Zoppo 2009
- Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009;40:2945‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donnan 2008
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008;371:1612‐23. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gan 2000
- Gan L, Miocic M, Doroudi R, Selin‐Sjogren L, Jern S. Distinct regulation of vascular endothelial growth factor in intact human conduit vessels exposed to laminar fluid shear stress and pressure. Biochemical and Biophysical Research Communications 2000;272:490‐6. [DOI] [PubMed] [Google Scholar]
Ginsberg 2009
- Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke 2009;40:S111‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacke 2008
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New England Journal of Medicine 2008;359:1317‐29. [DOI] [PubMed] [Google Scholar]
Hacke 2009
- Hacke W, Furlan AJ, Al‐Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion‐diffusion weighted imaging or perfusion CT (DIAS‐2): a prospective, randomised, double‐blind, placebo‐controlled study. Lancet Neurology 2009;8:141‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2008a
- Han JH, Wong KS. Is counterpulsation a potential therapy for ischemic stroke?. Cerebrovascular Diseases 2008;26:97‐105. [DOI] [PubMed] [Google Scholar]
Han 2010
- Han JH, Leung WH, Wong KS. Role of external counterpulsation in the treatment of ischemic stroke. Journal of Geriatric Cardiology 2010;7:88‐92. [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54:541‐53. [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Ho 2006
- Ho SS, Lam WW, Wong KS, Leung CS, Metreweli C. Potential value of post stroke extracranial arterial blood flow volume in the prediction of stroke functional outcome. Cerebrovascular Diseases 2006;21:54‐9. [DOI] [PubMed] [Google Scholar]
Kersten 1999
- Kersten JR, Pagel PS, Chilian WM, Warltier DC. Multifactorial basis for coronary collateralization: a complex adaptive response to ischemia. Cardiovascular Research 1999;43:44‐57. [DOI] [PubMed] [Google Scholar]
Lawson 2002
- Lawson WE. Current use of enhanced external counterpulsation and patient selection. Clinical Cardiology 2002;25:II16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niebauer 1996
- Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. Journal of the American College of Cardiology 1996;28:1652‐60. [DOI] [PubMed] [Google Scholar]
Ozawa 2001
- Ozawa ET, Bottom KE, Xiao X, Kamm RD. Numerical simulation of enhanced external counterpulsation. Annals of Biomedical Engineering 2001;29:284‐97. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sandercock 2008
- Sandercock PAG, Counsell C, Gubitz GJ, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD000029.pub2] [DOI] [PubMed] [Google Scholar]
Soran 1999
- Soran O, Crawford LE, Schneider VM, Feldman AM. Enhanced external counterpulsation in the management of patients with cardiovascular disease. Clinical Cardiology 1999;22:173‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroke Unit Trialists' Collaboration 2007
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000197.pub2] [DOI] [PubMed] [Google Scholar]
Thrift 2001
- Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne stroke incidence study (NEMESIS). Stroke 2001;32:1732‐8. [DOI] [PubMed] [Google Scholar]
Vahedi 2007
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurology 2007;6:215‐22. [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang D, Hao Z, Tao W, Kong F, Zhang S, Wu B, et al. Acute ischemic stroke in the very elderly Chinese: risk factors, hospital management and one‐year outcome. Clinical Neurology and Neurosurgery 2011;113:442‐6. [DOI] [PubMed] [Google Scholar]
Wardlaw 2009
- Wardlaw JM, Murray V, Berge E, Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000213.pub2] [DOI] [PubMed] [Google Scholar]
Wenger 2006
- Wenger NK. Enrollment and maintenance of elderly patients in cardiovascular clinical trials. American Journal of Geriatric Cardiology 2006;15:352‐6. [DOI] [PubMed] [Google Scholar]
Werner 1999
- Werner D, Schneider M, Weise M, Nonnast‐Daniel B, Daniel WG. Pneumatic external counterpulsation: a new noninvasive method to improve organ perfusion. American Journal of Cardiology 1999;84:950‐2, A957‐8. [DOI] [PubMed] [Google Scholar]
Wong 2000
- Wong KS, Li H, Chan YL, Ahuja A, Lam WW, Wong A, et al. Use of transcranial doppler ultrasound to predict outcome in patients with intracranial large‐artery occlusive disease. Stroke 2000;31:2641‐7. [DOI] [PubMed] [Google Scholar]
Wong 2003
- Wong KS, Li H. Long‐term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke 2003;34:2361‐6. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu B, Liu M, Liu H, Li W, Tan S, Zhang S, et al. Meta‐analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 2007;38:1973‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lin 2011
- Lin S, Liu M, Wu B, Hao Z, Yang J, Tao W. External counterpulsation for acute ischaemic stroke (Protocol). Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009264] [DOI] [PMC free article] [PubMed] [Google Scholar]


