Abstract
Background
Acoustic stimulation of the fetus has been suggested to improve the efficiency of antepartum fetal heart rate testing.
Objectives
To assess the advantages and disadvantages of the use of fetal vibroacoustic stimulation in conjunction with tests of fetal wellbeing.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2013).
Selection criteria
All published and unpublished randomised controlled trials assessing the merits of the use of fetal vibroacoustic stimulation in conjunction with tests of fetal wellbeing.
Data collection and analysis
All review authors independently extracted data and assessed trial quality. Authors of published and unpublished trials were contacted for further information.
Main results
Altogether 12 trials with a total of 6822 participants were included. Fetal vibroacoustic stimulation reduced the incidence of non‐reactive antenatal cardiotocography test (nine trials; average risk ratio (RR) 0.62, 95% confidence interval (CI) 0.48 to 0.81). Vibroacoustic stimulation compared with mock stimulation evoked significantly more fetal movements when used in conjunction with fetal heart rate testing (one trial, RR 0.23, 95% CI 0.18 to 0.29).
Authors' conclusions
Vibroacoustic stimulation offers benefits by decreasing the incidence of non‐reactive cardiotocography and reducing the testing time. Further randomised trials should be encouraged to determine not only the optimum intensity, frequency, duration and position of the vibroacoustic stimulation, but also to evaluate the efficacy, predictive reliability, safety and perinatal outcome of these stimuli with cardiotocography and other tests of fetal wellbeing.
Keywords: Humans; Vibration; Vibration/adverse effects; Acoustic Stimulation; Acoustic Stimulation/adverse effects; Acoustic Stimulation/methods; Cardiotocography; Cardiotocography/methods; Confidence Intervals; Fetal Monitoring; Fetal Monitoring/methods; Heart Rate, Fetal; Odds Ratio; Randomized Controlled Trials as Topic
Plain language summary
Fetal vibroacoustic stimulation for facilitation of tests of the wellbeing of the unborn baby
Acoustic stimulation of unborn babies may make tests on their wellbeing more effective.
Tests on unborn babies such as ultrasound, measuring the number of movements and the heart rate are carried out to check the baby’s wellbeing. As a baby's sleep periods can alter these results by making it non‐reactive, various methods are used to wake the baby so that it can respond to the stimulus. Fetal vibroacoustic stimulation uses a hand‐held electronic device placed just above the pregnant woman's abdomen. Brief sounds are sent through the mother’s abdomen to her baby. The vibroacoustic stimulation gives the opportunity to assess how the baby responds. Exposure of the baby to the vibroacoustic stimulation is generally considered safe but it can cause vigorous fetal movements and fetal distress.
This review of 12 randomised controlled trials involving 6822 mothers found that vibroacoustic stimulation improved the effectiveness of the baby's heart rate testing. However, the data on fetal distress and perinatal death were too few to draw any conclusions on safety. More research is needed to determine the optimal intensity, frequency, duration and position of the vibroacoustic stimulation and to evaluate the safety and perinatal outcomes when used with cardiotocography and other tests of fetal wellbeing.
Background
Antepartum fetal heart rate testing, fetal movement counts, fetal ultrasound examinations and biophysical profile of the fetus are methods of assessing fetal wellbeing. More recently vibroacoustic stimulation of the fetus is performed in conjunction with these tests.
Several studies (Ingemarsson 1989; Keegan 1987; Leader 1984; Smith 1985) have shown that fetal sleeping periods can lead to falsely non‐reactive tests, thereby increasing the risk of unnecessary obstetric intervention. Various methods of stimulation have been proposed to arouse the fetus from the sleep cycle or rest‐activity cycle. They include a change in maternal position, physical activity, maternal glucose ingestion, sound stimulation, light stimulation and manual fetal manipulation. If the fetus can be aroused sufficiently, such stimulations may be useful when used in conjunction with tests of fetal wellbeing.
Fetal vibroacoustic stimulation was first noted in 1947 by Bernard and Sontag (Bernard 1947), who observed that the fetal heart rate accelerated after acoustic stimulation. Sadovsky 1981 correlated fetal movements with fetal wellbeing. In modern obstetrics, vibroacoustic stimulation of the fetus is gained by using a hand‐held electronic device placed just above the pregnant woman's abdomen, which transmits brief sound stimuli through the abdominal wall to the fetus.
Antepartum fetal heart rate testing (cardiotocography test) has become a popular method of assessing fetal wellbeing. Acoustic stimulation of the fetus has been suggested to improve the efficiency of antepartum fetal heart rate testing (Serafini 1984; Trudinger 1980). By reducing the number of non‐reactive cardiotocography secondary to fetal sleep states, the vibroacoustic stimulation test may be expected to reduce maternal and provider anxiety, shorten overall testing time and allow perinatal resources to be better utilised.
Some authors of non‐randomised studies (Nyman 1992; Inglis 1993; Sarinoglu 1996) have reported success using fetal vibroacoustic stimulation to improve the efficiency of antepartum fetal heart rate testing without changing the predictive reliability of the tests.
Vibroacoustic stimulation of the human fetus profoundly alters fetal behaviour and heart rate. Fetal vibroacoustic stimulation is a stress in itself and there have been reports of severe fetal distress following vibroacoustic stimulation (Sherer 1988; Sherer 1991). Vigorous fetal movements evoked by the stimulus may result in tightening of a nuchal cord, bradycardia, and subsequent caesarean section for fetal distress. However, the available information from non‐randomised controlled trials suggests that exposure of the fetus to vibroacoustic stimulation is generally clinically safe (Arulkumaran 1991; Arulkumaran 1992).
Objectives
To assess the advantages and disadvantages of the use of fetal vibroacoustic stimulation in conjunction with tests of fetal wellbeing. In particular, to assess whether the adjunctive use of vibroacoustic stimulation to alter fetal behavioural states leads to less false positive non‐reactive tests.
To assess whether the use of fetal vibroacoustic stimulation improves perinatal outcome, leads to greater maternal satisfaction, and is associated with costs savings and a shorter testing time.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials assessing the merits of the use of fetal vibroacoustic stimulation in conjunction with tests of fetal wellbeing. Quasi‐randomised trials were excluded.
Types of participants
Pregnant women who have an antenatal non‐stress cardiotocography test or other tests of fetal wellbeing in conjunction with vibroacoustic stimulation.
Types of interventions
Vibroacoustic stimulation versus mock or no stimulation
Vibroacoustic stimulation versus mock stimulation
Vibroacoustic stimulation versus manual stimulation
Vibroacoustic stimulation and cardiotocography versus cardiotocography alone
Vibroacoustic stimulation and test of fetal wellbeing versus test of fetal wellbeing alone
Vibroacoustic stimulation versus light stimulation
Types of outcome measures
Primary outcomes
Reactive cardiotocography
Palpated or visualised movements
Secondary outcomes
Testing time for fetal wellbeing
Need for contraction stress test
Fetal distress
Gestation at delivery
Operative delivery
Perinatal mortality
Maternal anxiety
Maternal satisfaction
Fetal hearing impairment or loss
Impaired neurological development
Testing time for modified biophysical profile (not prespecified outcome)
Non‐reassuring biophysical profile (not prespecified outcome)
Search methods for identification of studies
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeTan 2001.
For this update we used the following methods when assessing the trials identified by the updated search (Berclaz 1991; Bolnick 2004; Bolnick 2006; Gonzalez 1995; Gonzalez 1998; Papadopoulos 2007; Pinette 2005; Sood 2007).
Selection of studies
Three review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, we extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
We independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel;
low risk, high risk or unclear risk for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to include missing data in the analyses which we undertook. We assessed methods as:
low risk;
high risk;
unclear risk.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias.
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion. In future updates, if identified and found to be eligible, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This is not a valid study design for this review.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if Tau² was greater than zero and either I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. No meta‐analysis included 10 or more studies in this update. In future updates, we will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not explore heterogeneity using subgroup analysis. If we identified substantial heterogeneity, we considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
Sensitivity analysis
We did not perform sensitivity analyses. In future updates, if more studies are included, we will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both, with poor‐quality (high risk or unclear risk of bias) studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
A total of 33 trials were identified from the search strategy.
Included studies
Twelve trials were eligible for inclusion. They were conducted in: California, USA (Smith 1986); Utah, USA (Sleutel 1990); New Mexico, USA (Bolnick 2006); Western Australia (Newnham 1990); Mexico (Marquez 1993); Thailand (Tongsong 1994); Greece (Salamalekis 1995;Papadopoulos 2007); Colorado, USA (Marden 1997); Turkey (Saracoglu 1999); New York, USA (Perez‐Delboy 2002); and India (Sood 2007).
In all the trials, participants in the intervention groups underwent transabdominal acoustic stimulation. In the trial by Marden et al (Marden 1997), the primary outcome was palpated or visualised fetal movements. For all the other trials, the primary outcome was fetal heart reactivity.
In Newnham 1990, the control group was subjected to manual fetal manipulation if the initial 20‐minute trace was not reactive and the test was continued for another 20 minutes. In the remainder of the trials, the control group did not have manual fetal manipulation.
In Sleutel 1990, in addition to the control group, there were two intervention groups. One intervention group underwent a single five‐second transabdominal acoustic stimulation while the other underwent four intermittent three‐second transabdominal acoustic stimulations, each stimulus separated by two minutes.
In Bolnick 2006, in addition to the control group (no stimulation) and the intervention group (vibroacoustic stimulation), a third group was assigned to receive transabdominal light stimulation.
For further details, seeCharacteristics of included studies.
Excluded studies
Twenty trials were excluded. For further details, seeCharacteristics of excluded studies. One trial is awaiting translation (Gonzalez 1995).
Risk of bias in included studies
SeeFigure 1 and Figure 2 for summaries of 'Risk of bias' assessments.
1.
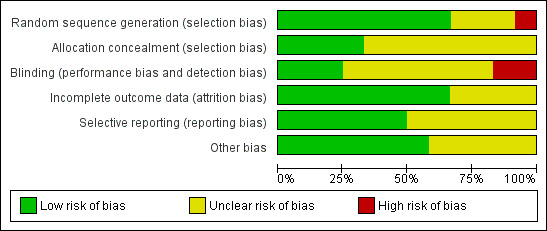
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
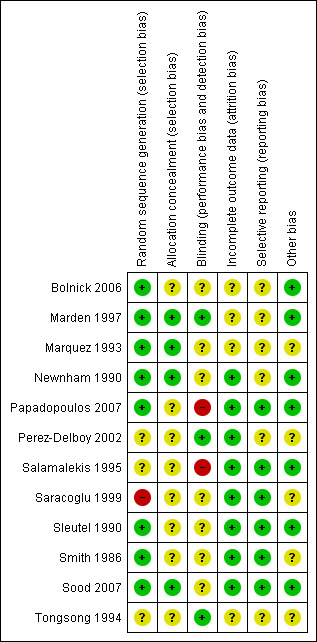
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All the included trials were randomised but the method of randomisation varied. Three trials (Bolnick 2006; Papadopoulos 2007; Sood 2007) mentioned the use of computer‐generated random numbers. Two trials (Marquez 1993; Smith 1986) mentioned the use of lottery and Sleutel 1990 used random‐number tables. In one trial (Saracoglu 1999), the study participants were randomly selected from patients applying to the unit. However, the assignment to acoustic stimulation or non‐stress test does not appear to be randomised but women were "divided equally" to interventions. In one trial randomisation was by draw of sealed envelopes (Newnham 1990) and in another randomisation was performed using a statistical package to generate the sequence of assignment (Marden 1997). The method of randomisation was unknown for the remaining three trials (Perez‐Delboy 2002; Salamalekis 1995; Tongsong 1994).
Four trials (Marden 1997; Marquez 1993; Newnham 1990; Sood 2007) specifically mentioned the use of sealed envelopes. In the remaining trials the method of allocation concealment was unclear.
Blinding
The procedure of vibroacoustic stimulation was only blinded in the Marden trial (Marden 1997) in which stimulation was performed. In the trial of Tongsong (Tongsong 1994) and of Perez‐Delboy (Perez‐Delboy 2002), all fetal heart rate tracings were interpreted blindly by one independent perinatologist, who did not have clinical information on the group of participants.
Incomplete outcome data
The risk of incomplete outcome data or attrition bias was low in all studies except Bolnick 2006, Marden 1997, Marquez 1993 and Tongsong 1994. In three studies, the outcome data was not available for some participants. In at least one study (Bolnick 2006) some women discontinued the trial before completion of non‐stress test.
Selective reporting
The reporting bias risk was low in most studies. In other cases it is difficult to assess because it was not clear whether there were any unreported findings.
Other potential sources of bias
In general the studies included in this review had low or unclear risk of other potential sources of bias.
Effects of interventions
A total of 12 trials with a total of 6822 participants were included.
Fetal vibroacoustic stimulation versus mock or no stimulation (nine trials involving 3757 participants)
Fetal acoustic stimulation reduced the incidence of non‐reactive cardiotocography in comparison with mock or no acoustic stimulation (nine trials; average risk ratio (RR) 0.62, 95% confidence interval (CI) 0.48 to 0.81; random‐effects analysis: Tau² = 0.04; I² = 32% ), Analysis 1.1.
1.1. Analysis.
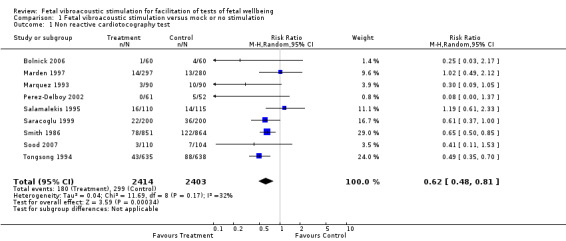
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 1 Non reactive cardiotocography test.
Fetal acoustic stimulation compared with mock or no stimulation reduced the overall mean cardiotocography testing time (three trials; average mean difference (MD) ‐6.93 minutes, 95% CI ‐12.09 minutes to ‐1.76 minutes; random‐effects analysis: Tau² = 19.54; I² = 97%), Analysis 1.2.
1.2. Analysis.
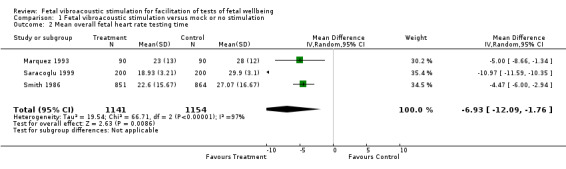
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 2 Mean overall fetal heart rate testing time.
Three studies suggested that fetal acoustic stimulation reduced the false positive rate (Analysis 1.5), but not the false negative rate (Analysis 1.6) in predicting perinatal morbidity.
1.5. Analysis.
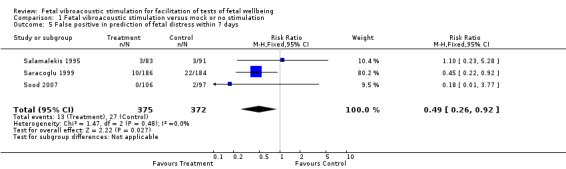
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 5 False positive in prediction of fetal distress within 7 days.
1.6. Analysis.
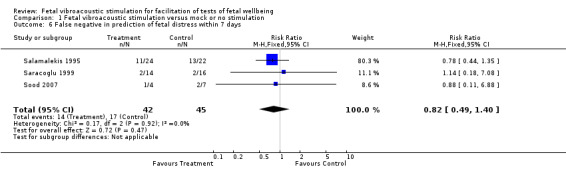
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 6 False negative in prediction of fetal distress within 7 days.
The data on fetal distress and perinatal death between the intervention and control groups were too few to draw any inferences. Twenty‐five deaths are reported in the five trials that mentioned perinatal mortality. Similarly the data on prediction of fetal distress between the intervention and control groups were too small for any meaningful inference.
Two studies separately reported on non‐prespecified outcomes such as mean testing time for modified biophysical profile and incidence of non‐reassuring biophysical profile (Analysis 1.12 and Analysis 1.13). Fetal vibroacoustic stimulation was found to reduce testing time and incidence of non‐reassuring biophysical profile.
1.12. Analysis.
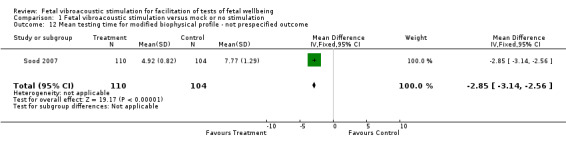
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 12 Mean testing time for modified biophysical profile ‐ not prespecified outcome.
1.13. Analysis.
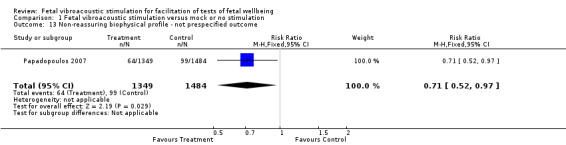
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 13 Non‐reassuring biophysical profile ‐ not prespecified outcome.
Fetal vibroacoustic stimulation versus mock stimulation (two trials involving 791 participants)
Two trials (Marden 1997; Sood 2007) compared fetal vibroacoustic stimulation with mock stimulation. However, the effect of vibroacoustic stimulation on non‐reactive cardiotocography was not statistically significant (two trials, RR 0.80, 95% CI 0.43 to 1.51), Analysis 2.1. Vibroacoustic stimulation compared with mock stimulation evoked significantly more fetal movements when used in conjunction with fetal heart rate testing (one trial, RR 0.23, 95% CI 0.18 to 0.29), Analysis 2.2.
2.1. Analysis.
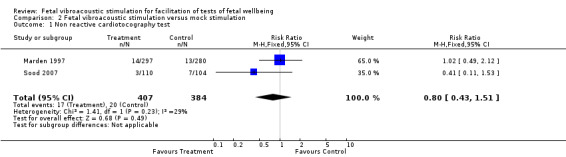
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 1 Non reactive cardiotocography test.
2.2. Analysis.
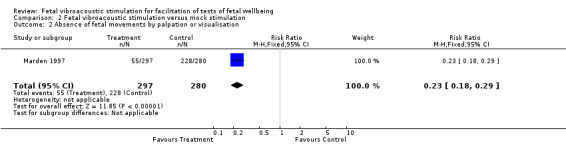
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 2 Absence of fetal movements by palpation or visualisation.
Fetal vibroacoustic stimulation versus manual stimulation (one trial involving 172 participants)
No differences were detected in the incidence of non‐reactive cardiotocography in comparison with manual stimulation of the fetus. Newnham 1990 showed no differences in the need for contraction stress test.
Intermittent vibroacoustic stimulation versus single vibroacoustic stimulation (one trial involving 60 participants)
The data comparing intermittent versus single stimulation were too small for any meaningful inferences.
Fetal vibroacoustic stimulation versus light stimulation (one trial involving 60 participants)
No differences were detected in the incidence of non‐reactive cardiotocography in comparison with light stimulation of the fetus.
Discussion
The benefits of using fetal vibroacoustic stimulation in conjunction with tests of fetal wellbeing must be weighed with respect to its effect on the predictive reliability of the tests and the safety of the procedure.
There is a void in the literature of randomised controlled trials relating to important outcomes such as fetal hearing impairment, impaired neurological development, gestation at delivery, maternal satisfaction and maternal anxiety. These are important safety considerations and aspects relating to hearing loss and possible cochlear damage, stress reaction and perinatal outcome should be further studied in the context of randomised trials before recommendations are made for routine use.
Vibroacoustic stimulation offers a unique opportunity to assess how the fetus responds to the external environment. Vibroacoustic stimulation has other potential advantages in the antepartum assessment of fetal wellbeing and in provoking fetal activity to improve ultrasonic visualisation and diagnosis. Additional prospective investigation is necessary to characterise further how this technique can be more useful clinically.
Authors' conclusions
Implications for practice.
By reducing the number of non‐reactive cardiotocography secondary to fetal sleep states and reducing the testing time, fetal vibroacoustic stimulation may help perinatal resources to be better utilised. By evoking fetal movements, fetal vibroacoustic stimulation may be useful in ultrasound examination and evaluation of fetal wellbeing.
However, due to the void in the literature of randomised controlled trials relating to important outcomes such as fetal hearing impairment, impairment of neurological development, maternal satisfaction and maternal anxiety, and perinatal mortality, there is still currently insufficient evidence within randomised controlled trials based upon which a firm recommendation regarding the routine use of fetal vibroacoustic stimulation can be made.
Implications for research.
More randomised studies are needed to define the role of fetal vibroacoustic stimulations in obstetrics. In particular, further randomised trials should be encouraged to determine not only the optimum intensity, frequency, duration and position of the vibroacoustic stimulation, but also to evaluate the efficacy, safety and perinatal outcome of these stimuli in conjunction with cardiotocography and other tests of fetal wellbeing.
Given the large number of excluded studies, future trials should not only be of high quality but need to report outcomes of clinical relevance. There was only one trial in which the comparison was with mock stimulation. It would be good to encourage more blinded studies with mock stimulation to ensure higher quality trials. Trials with outcomes such as fetal hearing impairment, impaired neurological development, maternal satisfaction and maternal anxiety should also be encouraged.
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2013 | New search has been performed | Search updated. Methods updated. |
| 30 September 2013 | New citation required but conclusions have not changed | Three new trials (Bolnick 2006; Papadopoulos 2007; Sood 2007) included. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | Amended | Search updated. Six reports added to Studies awaiting classification (Bolnick 2004; Bolnick 2006; Gonzalez 1998; Papadopoulos 2007; Pinette 2005; Sood 2007). |
| 3 November 2008 | Amended | Converted to new review format. |
| 25 November 2003 | New search has been performed | Two new trials (Saracoglu 1999 and Perez‐Delboy 2002) are included but they do not change the conclusions of the review. |
Acknowledgements
Thanks to Professor James Neilson who helped to initiate and conceive this review.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Fetal vibroacoustic stimulation versus mock or no stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non reactive cardiotocography test | 9 | 4817 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.81] |
| 2 Mean overall fetal heart rate testing time | 3 | 2295 | Mean Difference (IV, Random, 95% CI) | ‐6.93 [‐12.09, ‐1.76] |
| 3 Absence of fetal movements by palpation or visualisation | 1 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.18, 0.29] |
| 4 Fetal distress within 7 days | 2 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.62, 1.60] |
| 5 False positive in prediction of fetal distress within 7 days | 3 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.26, 0.92] |
| 6 False negative in prediction of fetal distress within 7 days | 3 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.40] |
| 7 Perinatal deaths | 5 | 4107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.46, 2.10] |
| 8 Impairment of fetal hearing | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Impairment of neurological development | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal anxiety | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal satisfaction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Mean testing time for modified biophysical profile ‐ not prespecified outcome | 1 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐2.85 [‐3.14, ‐2.56] |
| 13 Non‐reassuring biophysical profile ‐ not prespecified outcome | 1 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.97] |
1.3. Analysis.
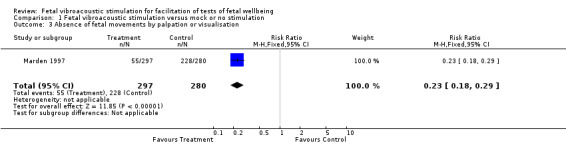
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 3 Absence of fetal movements by palpation or visualisation.
1.4. Analysis.
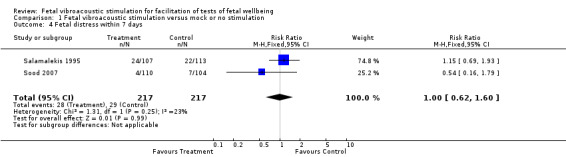
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 4 Fetal distress within 7 days.
1.7. Analysis.
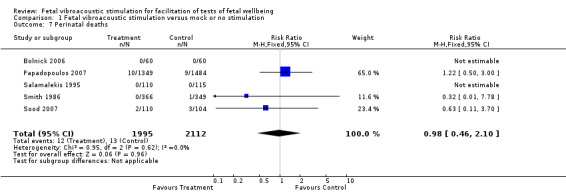
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 7 Perinatal deaths.
1.8. Analysis.
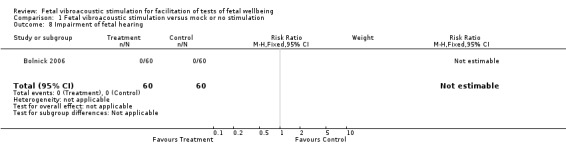
Comparison 1 Fetal vibroacoustic stimulation versus mock or no stimulation, Outcome 8 Impairment of fetal hearing.
Comparison 2. Fetal vibroacoustic stimulation versus mock stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non reactive cardiotocography test | 2 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.51] |
| 2 Absence of fetal movements by palpation or visualisation | 1 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.18, 0.29] |
| 3 Mean testing time for modified biophysical profile | 1 | 214 | Mean Difference (IV, Fixed, 95% CI) | ‐2.85 [‐3.14, ‐2.56] |
| 4 Fetal distress within 7 days | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.16, 1.79] |
| 5 False positive in prediction of fetal distress within 7 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.77] |
| 6 False negative in prediction of fetal distress within 7 days | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.11, 6.88] |
| 7 Perinatal deaths | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.11, 3.70] |
2.3. Analysis.
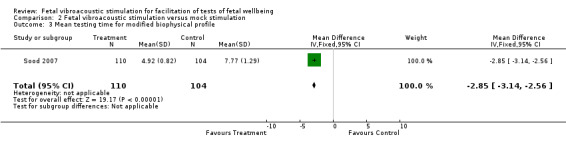
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 3 Mean testing time for modified biophysical profile.
2.4. Analysis.
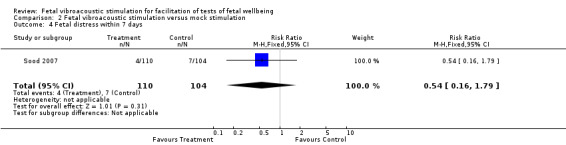
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 4 Fetal distress within 7 days.
2.5. Analysis.
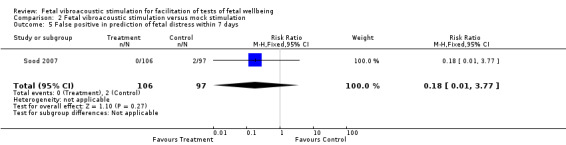
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 5 False positive in prediction of fetal distress within 7 days.
2.6. Analysis.
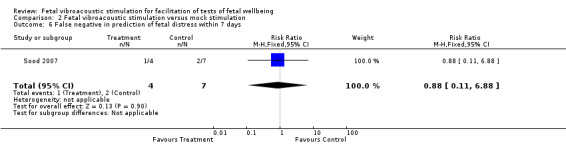
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 6 False negative in prediction of fetal distress within 7 days.
2.7. Analysis.
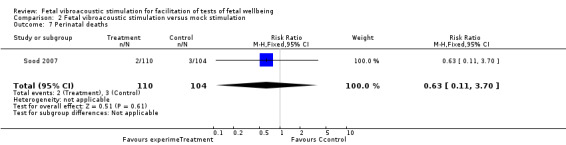
Comparison 2 Fetal vibroacoustic stimulation versus mock stimulation, Outcome 7 Perinatal deaths.
Comparison 3. Fetal vibroacoustic stimulation versus manual stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐reactive cardiotocography | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.38, 1.21] |
| 2 Need for contraction stress test | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.10, 1.39] |
| 3 Perinatal deaths | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
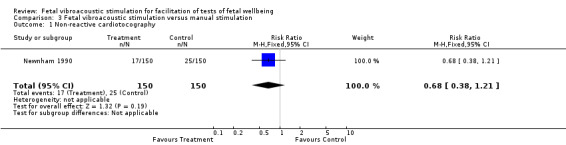
Comparison 3 Fetal vibroacoustic stimulation versus manual stimulation, Outcome 1 Non‐reactive cardiotocography.
3.2. Analysis.
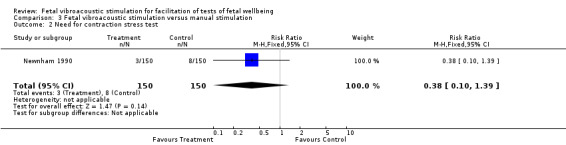
Comparison 3 Fetal vibroacoustic stimulation versus manual stimulation, Outcome 2 Need for contraction stress test.
3.3. Analysis.
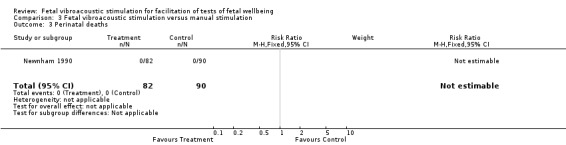
Comparison 3 Fetal vibroacoustic stimulation versus manual stimulation, Outcome 3 Perinatal deaths.
Comparison 4. Intermittent vibroacoustic stimulation versus single vibroacoustic stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean testing time for reactive tests | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.27, 1.87] |
4.1. Analysis.
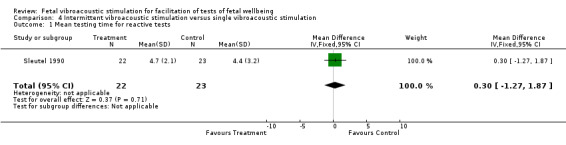
Comparison 4 Intermittent vibroacoustic stimulation versus single vibroacoustic stimulation, Outcome 1 Mean testing time for reactive tests.
Comparison 5. Fetal vibroacoustic stimulation versus light stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐reactive cardiotocography test | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.37] |
| 2 Perinatal deaths | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Impairment of fetal hearing | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
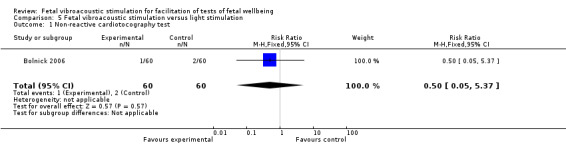
Comparison 5 Fetal vibroacoustic stimulation versus light stimulation, Outcome 1 Non‐reactive cardiotocography test.
5.2. Analysis.
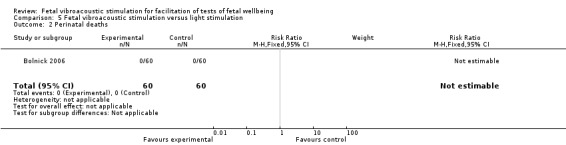
Comparison 5 Fetal vibroacoustic stimulation versus light stimulation, Outcome 2 Perinatal deaths.
5.3. Analysis.
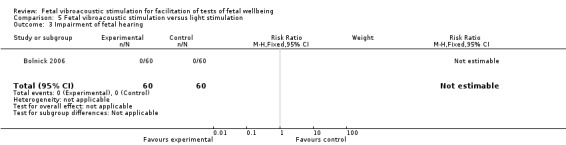
Comparison 5 Fetal vibroacoustic stimulation versus light stimulation, Outcome 3 Impairment of fetal hearing.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bolnick 2006.
| Methods | Computer‐generated randomisation schedule. | |
| Participants | The study population consisted of patients at 33 to 39 weeks of gestation who underwent an NST. Excluded were cases in which the fetus had a cardiac or central nervous system anomaly or had been exposed to a maternal drug that affected the central nervous system or FHR beat‐to‐beat variability. | |
| Interventions | Assigned to receive transabdominal light, vibroacoustic, or no stimulation. The order in which each pregnancy was assigned to receive transabdominal light, vibroacoustic, or no stimulation was determined before the first of the 3 tests according to a computer‐generated randomisation schedule. The minimum period between tests was 3 days.The 2 investigators who interpreted each tracing were blinded as to the type of stimulation. | |
| Outcomes | Primary outcome: FHR reactivity. An adequate FHR acceleration was defined as 15 bpm above baseline for 15 seconds. If it was absent, the stimulus was repeated 10 minutes later up to a maximum of 3 times during the 20‐minute NST. Primary endpoints for comparison were the time from the onset of stimulation until the first adequate FHR acceleration and the time before a reactive pattern (2 adequate accelerations). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented if participant or caregiver blinded. 2 outcome investigators who interpreted each tracing were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 cases discontinued the trial before completion of NST and data not analysed. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes documented in methods section. |
| Other bias | Low risk | No evidence of any other form of bias. |
Marden 1997.
| Methods | Randomisation: randomised by sealed envelopes with the use of a statistical package to generate the sequence of assignment. | |
| Participants | Women of at least 31 weeks' gestation. Inclusion criteria included singleton pregnancy, intact membranes, and no concurrent use of magnesium or narcotics. Country: USA, Colorado. 577 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were given acoustic stimulation for 3 seconds with the stimulator placed at the midpoint between the maternal pubic symphysis and umbilicus. Fetal movements were palpated with the other hand at the fundus. Women randomised to control group were given mock stimulation for 3 seconds with the stimulator placed at the midpoint between the maternal pubic symphysis and umbilicus. Fetal movements were palpated with the other hand at the fundus. | |
| Outcomes | Primary outcome: positive test as defined by palpation or visualisation of fetal movement only by the tester during a vibroacoustic stimulation. Other outcome: FHR reactivity. | |
| Notes | The vibratory acoustic stimulus was performed using a vibroacoustic stimulator (Corometrics 146 fetal acoustic stimulator). Randomisation was performed after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with the use of a statistical package to generate the sequence of assignment. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | By performing the fetal acoustic stimulation test before NST, the tester was blinded to the result of NST. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For 23 women, outcome data were not available. No further information provided. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes documented in methods section. |
| Other bias | Low risk | No evidence of any other form of bias. |
Marquez 1993.
| Methods | Randomisation: randomised by lottery. Effectiveness of randomisation was assessed by comparisons of several parameters within the two groups which included gestational ages and primary indications. | |
| Participants | Women of least 32 weeks' gestation. Country: Mexico. 180 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were given acoustic stimulation for 5 seconds. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 accelerations or more of greater than 15 bpm and of 15 seconds duration or more within a 10‐minute period. | |
| Notes | Stimulus had an audio frequency of 75 Hz and intensity of 74 db and a stimulation duration of 5 seconds. Randomisation was done after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Outcome assessor blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to assess. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Unclear risk | Unable to assess. |
Newnham 1990.
| Methods | Randomisation: randomised by draw of sealed envelopes. Effectiveness of randomisation was assessed by comparisons of several parameters within the 2 groups which included maternal ages, parities, gestational ages and primary indications. | |
| Participants | Women of least 34 weeks' gestation. Exclusion criteria ‐ no contraindications to contraction stress tests. Country: Western Australia. 172 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were monitored for an initial 5 minutes. If the pattern is non‐reactive, a 3‐second vibratory acoustic stimulation was applied to the maternal abdomen in the region of the fetal head. The stimulus was repeated a second and a third time, also at 1‐minute intervals, if satisfactory FHR accelerations had not occurred. Women randomised to non‐fetal acoustic stimulation group were monitored for an initial 20 minutes. If the trace was non‐reactive, the fetus was stimulated manually and the test was continued for a further 20 minutes. If satisfactory accelerations were not found, the women were then sent for a meal. On her return, a nipple stimulation contraction stress was performed if the subsequent test remained non‐reactive after a further 20 minutes. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 accelerations of greater than 15 bpm and of 15 seconds' duration or more within a 20‐minute period. In tests in which accelerations had been provoked by fetal acoustic stimulation, the definition of reactivity required 1 of the 2 accelerations to have been unprovoked. | |
| Notes | All tests were performed with Corometrics 115 monitors using Doppler FHR transducers. The tests were performed by specially trained fetal intensive care midwives with a nurse: patient ratio of 1:1. The vibratory acoustic stimulus had an audio frequency of 75 Hz, a sound intensity of 74 db at 1 m in air and a stimulation duration of 3 seconds. Randomisation was performed after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by draw of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No evidence of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 100%. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes documented in methods section. |
| Other bias | Low risk | No evidence of any other form of bias. |
Papadopoulos 2007.
| Methods | Randomisation was done with the use of a random number generator in the computer. | |
| Participants | The population of the study consisted of patients referred for fetal surveillance to the maternal‐fetal medicine department (either on an outpatient basis or after admission to the hospital for various reasons). Inclusion criteria were: singleton pregnancy, gestational age equal or more than 30 weeks + 0 day and BPPS ≤ 8/10 with a non‐reactive NST. Exclusion criteria were: gestational age before 30 weeks, multifetal pregnancy, premature rupture of membranes, known congenital anomalies of the fetus and maternal refuse to participate in the study. | |
| Interventions | The patients were followed according to department’s protocol, and biophysical profile was conducted for 30 minutes according to standard criteria. All participants with an abnormal or equivocal BPPS were assigned randomly to 1 of 2 groups. In group A (study group), VAS was applied and, in group B (control group), the observation time was extended. In group A, a 3‐second duration stimulus was applied with an artificial larynx placed on maternal abdomen over the fetal vertex. Following VAS, BPPS was reassessed for 30 minutes and if remained non‐reassuring a second 3‐second stimulus was applied. BPPS was assessed again for another 30 minutes. In group B, we followed the classical method of extended observation time for 60 minutes, divided into 2 periods of 30 minutes each to match the time periods of group A. Participants of both groups with a non‐reassuring BPPS at the end of the examination were managed accordingly. BPPS with or more than 8/10 with a reactive NST at any stage was considered indicative of a non‐compromised fetus. | |
| Outcomes | Intrauterine death, caesarean section for fetal distress, Apgar score at 5 minutes, meconium‐stained amniotic fluid, admission to NICU for whatever reason. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator in the computer. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 100%. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section have been reported on in the results section. |
| Other bias | Low risk | No evidence of any other form of bias. |
Perez‐Delboy 2002.
| Methods | Randomisation: randomisation method unknown. | |
| Participants | Women referred to antenatal testing unit for NST. Country: New York, USA. 113 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were given 1 second vibroacoustic stimulation at the maternal abdomen. The stimulus was repeated a second (for 2 seconds) and a third time (for 3 seconds), also at 10‐minute intervals, if still not reactive. Women randomised to non‐fetal acoustic stimulation group were given the traditional NST without the vibroacoustic stimulation. | |
| Outcomes | Primary outcome: FHR reactivity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method unknown. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 100%. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes documented in methods section. |
| Other bias | Unclear risk | Unable to assess. |
Salamalekis 1995.
| Methods | Randomisation: randomisation method unknown. Effectiveness of randomisation was assessed by comparisons of parities. There was no statistical significant difference. | |
| Participants | Women of least 37 weeks' gestation with singleton and high risk pregnancies. Country: Greece. 225 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were monitored for at least 5 minutes. A vibratory acoustic stimulation was applied to the maternal abdomen in the region of the fetal head and activated for 1 second 4 consecutive times, with 1 second intervals between stimulations. Women randomised to non‐fetal acoustic stimulation group were monitored for an initial 20 minutes. If the trace was non‐reactive, the test was continued for a further 20 minutes. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 accelerations of 15 bpm and of at least 15 seconds' duration or a FHR acceleration of 15 bpm over the baseline for 2 minutes within 5 minutes after acoustic stimulation. Non‐reactivity was defined as 40 minutes without a single FHR acceleration of 15 bpm or more, for 15 seconds or more. Other outcome: fetal distress within 7 days of test. This was defined by the presence of pathological FHR trace pattern, thick meconium or low Apgar at 5 minutes. | |
| Notes | All tests were performed with Corometrics 115 monitors. The tests were performed 1 hour after a meal in a low noise room with the mother in a semi‐recumbent position to minimise the risk of supine hypotension. The vibratory acoustic stimulus was performed using a vibroacoustic stimulator (Corometrics 146 fetal acoustic stimulator), with a sound intensity of 110 db in air. Randomisation after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 100%. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section have been reported on. |
| Other bias | Low risk | No evidence of any other form of bias. |
Saracoglu 1999.
| Methods | Randomisation: randomisation method unknown. | |
| Participants | Women seen at Perinatology Unit. Country: Turkey. 400 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were monitored for at least 5 minutes. A vibratory acoustic stimulation was applied to the maternal abdomen in the region of the fetal head and activated for 1 second up to 4 times. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 accelerations of 15 bpm and of at least 15 seconds' duration within a 20‐minute period. Non‐reactivity was defined as 40 minutes without the reactive criterion. | |
| Notes | A fetal acoustic stimulator (Model 146; Corometrics, Wallingford, CT;75 Hz, 74 db) was used, and FHR was recorded with a fetal heart monitor (Model 115; Corometrics). The report by Saracoglu 1998 only provided data in abstract form and these were inadequate. There was no reply despite various attempts to contact the authors. The abstract was subsequently published in full (Saracoglu 1999) and the published paper (Saracoglu 1999) is included in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study participants were randomly selected from patients applying to the unit. However, the assignment to acoustic stimulation or NST does not appear to be randomised ("divided equally"). |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 100%. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section have been reported on. |
| Other bias | Unclear risk | No information provided. |
Sleutel 1990.
| Methods | Randomisation: randomised by means of a random number table. | |
| Participants | 60 pregnant women at a university hospital who were scheduled for NST. Exclusion criteria were gestational age less than 30 weeks, abnormal FHR, non‐reactive NST or a positive contraction at the last antepartum evaluation, mothers who appeared sedated or had used narcotics, sedatives or street drugs within 8 hours and termination of testing on the mother or fetus before completion of NST. Country: USA, Utah. | |
| Interventions | They were randomised to 3 groups (control, single stimulation and intermittent stimulation). Women randomised to control group received the traditional NST. Women randomised to the single fetal acoustic stimulation group were given acoustic stimulation for a single 5‐second duration with the artificial larynx placed at the maternal abdomen over the fetal head. Women randomised to the intermittent fetal acoustic stimulation group were given acoustic stimulation for 4 3‐second duration with the artificial larynx placed at the maternal abdomen over the fetal head. Each stimulus was separated by 2 minutes. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 accelerations of 15 bpm and of at least 15 seconds' duration within a 20‐minute period. Non‐reactivity was defined as 90 minutes without the reactive criterion. | |
| Notes | The vibratory acoustic stimulus was performed using a hand held artificial larynx with a sound pressure level in air of 100 db at 1000 Hz. Randomisation was performed after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was used. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 100%. |
| Selective reporting (reporting bias) | Low risk | 2 hypotheses were stated in the methods section and both were tested. |
| Other bias | Low risk | No evidence of any other form of bias. |
Smith 1986.
| Methods | Randomisation: randomised by lottery. Effectiveness of randomisation was assessed by comparisons of several parameters within the 2 groups which included parities, gestational ages and primary indications. It was noted that there was a significantly greater number of postdates women in the non‐fetal acoustic stimulation group. | |
| Participants | Women of at least 28 weeks' gestation presenting to the Antepartum Fetal Testing Unit of the Hospital. Exclusion criteria ‐ none. Country: USA, California. 715 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were monitored for an initial 5 minutes. If the pattern is non‐reactive, a 3 second or less vibratory acoustic stimulation was applied to the maternal abdomen in the region of the fetal head. The stimulus was repeated for a maximum of 3 times, at 1‐minute intervals, if satisfactory FHR accelerations had not occurred. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 accelerations or more of greater than 15 bpm and of 15 seconds' duration or more within a 10‐minute period. Non‐reactivity was defined as 40 minutes without 2 qualifying accelerations. | |
| Notes | All acoustic tests were done with a Model 5C electronic artificial larynx (Western Electric, New York) and performed in the Antepartum Fetal Testing Unit by specially trained nurses with the woman in semi‐Fowler's position. Sound pressure levels of this device measured at 1 m in air averaged 82 db, with a fundamental frequency of approximately 80 Hz, and harmonics ranging from 20 to 9000 Hz. Randomisation was done after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by lottery. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were excluded. Those who refused participation served as a second control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section have been reported on. |
| Other bias | Unclear risk | Unclear. |
Sood 2007.
| Methods | Randomly allocated by computer‐generated random numbers kept in sealed envelopes to either vibroacoustic stimulated modified biophysical profile (VAS/mFBP) or mock stimulation (mFBP). | |
| Participants | 214 women with high risk singleton pregnancies detected amongst women attending antenatal clinic. Country: India. | |
| Interventions | Vibroacoustic stimulation was done with EMCO vibroacoustic stimulator (EMCO Health Care Pvt Ltd, Sion, Mumbai, India) with 75 db sound intensity at 1.0 meter and frequency of 75 Hz. | |
| Outcomes | Mean testing time for modified biophysical profile, caesarean section for fetal distress, 5 minute Apgar score < 7, admission to NICU for more than 24 hours. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Random numbers kept in sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 100%. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section have been reported on in the results section. |
| Other bias | Low risk | No evidence of any other form of bias. |
Tongsong 1994.
| Methods | Randomisation: randomisation method unknown. Effectiveness of randomisation was assessed by comparisons of several parameters within the 2 groups which include maternal ages, gestational ages and primary indications. All the FHR tracings were interpreted blindly by 1 independent perinatologist, who did not have any clinical information on the group of women. | |
| Participants | Women of least 28 weeks' gestation with indications including postterm pregnancy, intrauterine growth retardation, pregnancy‐induced hypertension, chronic hypertension, decreased fetal movement and diabetes mellitus. Country: Thailand. 1273 women randomised. | |
| Interventions | Women randomised to fetal acoustic stimulation group were given fetal acoustic stimulation for 1 second. If no qualifying acceleration was observed within 15 seconds, the stimulus were repeated up to 3 times. If reactive criteria were not achieved in 10 minutes, a new cycle of stimulation was begun. If both the women in the acoustic stimulation and standard non‐acoustic stimulation groups did not meet the reactive criteria within 20 minutes of the tests, the same technique was extended another 20 minutes. | |
| Outcomes | Primary outcome: FHR reactivity. This was defined by the presence of 2 FHR accelerations or more of greater than 15 bpm and of 15 seconds' duration within a 20‐minute period. 1 prolonged acceleration of the FHR of at least 15 bpm lasting more than 2 minutes was also interpreted as reactive. If these criteria were not met in 40 minutes of monitoring, the test was interpreted as non‐reactive. | |
| Notes | All acoustic tests were performed in the Maternal Fetal Medicine Unit by a specially trained physician with the woman in semi‐Fowler's position. Transabdominal acoustic stimulation overlying the fetal vertex was accomplished with an electronic fetal larynx of approximately 80 db and frequency of 80 Hz and a stimulation duration of 1 second. Randomisation was done after informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method unknown. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All the FHR tracings were interpreted blindly by one independent perinatologist, who did not have any clinical information on the group of women. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
bpm: beats per minute BPPS: biophysical profile score db: decibels FHR: fetal heart rate Hz: hertz NICU: neonatal intensive care unit NST: non‐stress test VAS: vibroacoustic stimulation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berclaz 1991 | There was a discrepancy between the number of participants presented in figure 3 (n = 18 for sham and n = 16 for real stimulation) and that stated in the text (n = 25). The definition of quiet and active fetuses was not clear. There were 2 types of bars representing tranquille (quiet) fetuses with different shades but no legend was given. |
| Devoe 1989 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Eller 1992 | Pseudo‐randomisation performed using hospital odd or even number. There was a large difference between those given vibroacoustic stimulation over the fetal vertex (n = 115) and over fetal breech (n = 90). There was also discrepancy between the total numbers presented for the results relating to reactive tests (the fetal vertex (n = 96) and over fetal breech (n = 55). |
| Gagnon 1986 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Gagnon 1987 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Gagnon 1988 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Gonzalez 1998 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Groome 1993 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Groome 1994 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Hamner 1988 | Data only available in abstract form and were inadequate. There was an unexplained discrepancy between the control (n = 286) and vibroacoustic stimulation (n = 135) groups. |
| Hasanpour 2013 | Not eligible as study compared acoustic stimulation and feeding mother stimulation. |
| Kisilevsky 1990 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Kisilevsky 1992 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Maesel 1994 | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Montan 1992a | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Montan 1992b | Data were not presented or available, or extractable as the specified clinical outcome measures for this review. |
| Petrovic 1998 | The control group (n = 326) was larger than the study group (n = 168). The control group had 2 distinct groups of which 1 group consisted of 158 women with evident fetal activity at the onset after the initial randomisation by schedule and the second group comprised 168 women after randomisation with no distinct fetal heart activity. These 2 distinct groups are analysed together as the control group. Analyses of the results of the 2 groups separately were not available. |
| Pinette 2005 | Quasi‐randomisation performed using hospital odd or even number. |
| Schiff 1992 | The results of the experimental group were compared to a big group (both experimental and control) and presented. Results relating to the control group alone were not available and not extractable. |
| Smith 1988 | The women were randomised but only women delivering within 7 days of a reactive cardiotocograph test were reported. There were 314 women in the control group and only 227 women in the study group. There is also a higher incidence of postdatism in the control group. |
Characteristics of studies awaiting assessment [ordered by study ID]
Gonzalez 1995.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Spanish ‐ awaiting translation |
Differences between protocol and review
Additional comparison added ‐ vibroacoustic stimulation versus light stimulation.
Two new outcomes were added in the 2013 update.
Testing time for modified biophysical profile (not prespecified outcome).
Non‐reassuring biophysical profile (not prespecified outcome).
Contributions of authors
In the update for September 2013, KH Tan, R Smyth and X Wei independently reviewed the new studies and discussed together.
Sources of support
Internal sources
Department of Maternal Fetal Medicine, KK Women's & Children's Hospital, Singapore.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bolnick 2006 {published data only}
- Bolnick J, Garcia G, Fletcher B, Rayburn W. Cross‐over trial of fetal heart rate response to external light and vibroacoustic stimulation [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S150. [Google Scholar]
- Bolnick JM, Garcia G, Fletcher BG, Rayburn WF. Cross‐over trial of fetal heart rate response to halogen light and vibroacoustic stimulation. Journal of Maternal‐Fetal & Neonatal Medicine 2006;19(4):215‐9. [DOI] [PubMed] [Google Scholar]
Marden 1997 {published data only}
- Marden D, McDuffie RS Jr, Allen R, Abitz D. A randomized controlled trial of a new fetal acoustic stimulation test for fetal wellbeing. American Journal of Obstetrics and Gynecology 1997;176:1386‐7. [DOI] [PubMed] [Google Scholar]
Marquez 1993 {published data only}
- Marquez TL, Andrade EH, Goldsmit DM, Huerta MIP, Garcia RBL. The value of antepartum fetal heart rate with vibroacoustic stimulation. Ginecologia y Obstetricia de Mexico 1993;61:356‐9. [PubMed] [Google Scholar]
Newnham 1990 {published data only}
- Newnham JP, Burns SE, Roberman BD. Effect of vibratory acoustic stimulation on the duration of fetal heart rate monitoring tests. American Journal of Perinatology 1990;7:232. [DOI] [PubMed] [Google Scholar]
Papadopoulos 2007 {published data only}
- Papadopoulos VG, Decavalas GO, Kondakis XG, Beratis NG. Vibroacoustic stimulation in abnormal biophysical profile: verification of facilitation of fetal well‐being. Early Human Development 2007;83(3):191‐7. [DOI] [PubMed] [Google Scholar]
Perez‐Delboy 2002 {published data only}
- Perez‐Delboy A, Weiss J, Michels A, Cleary J, Shevell T, Malone F. A randomized trial of vibroacoustic stimulation for antenatal fetal testing [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S146. [Google Scholar]
Salamalekis 1995 {published data only}
- Salamalekis E, Batalias L, Kassanos D, Loghis C, Pyrgiotis E, Zourlas PA. The acoustic stimulation test and antenatal cardiotocography as diagnostic tools in high risk pregnancies. Journal of Obstetrics and Gynaecology 1995;15:292‐4. [Google Scholar]
Saracoglu 1999 {published data only}
- Saracoglu F, Gol K, Sahin I, Turkkani B, Oztopcu C. The predictive value of fetal acoustic stimulation. Journal of Perinatology 1999;19:103‐5. [DOI] [PubMed] [Google Scholar]
- Saracoglu F, Gol K, Sahin I, Turkkani T, Atlay C. The predictive value of fetal acoustic stimulation. Prenatal and Neonatal Medicine 1998;3 Suppl 1:64. [Google Scholar]
Sleutel 1990 {published data only}
- Sleutel MR. Vibroacoustic stimulation and fetal heart rate in nonstress tests. Journal of Obstetric, Gynecologic and Neonatal Nursing 1990;19(3):199‐204. [DOI] [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith CV, Phelan JP, Platt LD, Broussard P, Paul RH. Fetal acoustic stimulation testing. II. A randomized clinical comparison with the nonstress test. American Journal of Obstetrics and Gynecology 1986;155:131‐4. [DOI] [PubMed] [Google Scholar]
Sood 2007 {published data only}
- Sood AK. Vibroacoustic stimulation and modified fetal biophysical profile in high risk pregnancy. Journal of Obstetrics and Gynaecology of India 2007;57(1):27‐36. [Google Scholar]
Tongsong 1994 {published data only}
- Tongsong T, Piyamongkol W. Comparison of the acoustic stimulation test with nonstress test. A randomized, controlled clinical trial. Journal of Reproductive Medicine 1994;39:17‐20. [PubMed] [Google Scholar]
References to studies excluded from this review
Berclaz 1991 {published data only}
- Berclaz G, Herrmann U. Does fetal reaction to vibro‐acoustic stimulation depend on its activity state?. Gynakologische Rundschau 1991;31(2):89‐97. [PubMed] [Google Scholar]
Devoe 1989 {published data only}
- Devoe LD, Searle NA, Ruedrich DA, Castillo RA, Metheny WP. The effects of vibroacoustic stimulation on baseline heart rate, breathing activity, and body movements of normal term fetuses. American Journal of Obstetrics and Gynecology 1989;161:524‐9. [DOI] [PubMed] [Google Scholar]
Eller 1992 {published data only}
- Eller DP, Robinson LJ, Newman RB. Position of the vibroacoustic stimulator does not affect fetal response. American Journal of Obstetrics and Gynecology 1992;167:1137‐9. [DOI] [PubMed] [Google Scholar]
Gagnon 1986 {published data only}
- Gagnon R, Hunse C, Carmichael L, Fellows F, Patrick J. Effects of vibratory acoustic stimulation on human fetal breathing and gross fetal body movements near term. American Journal of Obstetrics and Gynecology 1986;155:1227‐30. [DOI] [PubMed] [Google Scholar]
- Gagnon R, Hunse C, Carmichael L, Fellows F, Patrick J. External vibratory acoustic stimulation near term: fetal heart rate and heart rate variability responses. American Journal of Obstetrics and Gynecology 1987;156:323‐7. [DOI] [PubMed] [Google Scholar]
Gagnon 1987 {published data only}
- Gagnon R, Hunse C, Carmichael L, Fellows F, Patrick J. Human fetal responses to vibratory acoustic stimulation from twenty‐six weeks to term. American Journal of Obstetrics and Gynecology 1987;157:1375‐81. [DOI] [PubMed] [Google Scholar]
Gagnon 1988 {published data only}
- Gagnon R, Hunse C, Carmichael L, Fellows F, Patrick J. Fetal heart rate and fetal activity patterns after vibratory acoustic stimulation at thirty to thirty‐two weeks' gestational age. American Journal of Obstetrics and Gynecology 1988;158:75‐9. [DOI] [PubMed] [Google Scholar]
Gonzalez 1998 {published data only}
- Gonzalez Gonzalez NL, Martin JI, Marcos Y, Suarez MN, Laynez E, Jimenez A, et al. States of fetal behavior and vibroacoustic stiumulation with an artificial larynx [Estados de comportamiento fetal y estimulacion vibroacustica con laringe artificial]. Progresos de Obstetricia y Ginecologia 1998;41:403‐7. [Google Scholar]
Groome 1993 {published data only}
- Groome LJ, Bentz LS, Singh KP, Mooney DM. Behavioral state change following vibroacoustic stimulation: effect of the duration of quiet sleep prior to stimulation. American Journal of Obstetrics and Gynecology 1993;168:296. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Bentz LS, Singh KP, Mooney DM. Behavioural state change in normal human fetuses following a single vibroacoustic stimulus: effect of duration of quiet sleep prior to stimulation. Early Human Development 1993;33(1):21‐7. [DOI] [PubMed] [Google Scholar]
Groome 1994 {published data only}
- Groome LJ, Bentz LS, Singh KP. Behavioral state organization in human term fetuses: evidence of relatively tight control of state cycling. Journal of Maternal‐Fetal Medicine 1994;3:49‐55. [Google Scholar]
Hamner 1988 {published data only}
- Hamner LH, Latter DK, Mastroianni MA, Grossman JH. Efficacy and safety of fetal acoustic stimulation testing. Proceedings of 36th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1988 May 2‐5; Boston, Massachusetts, USA. 1988:2.
Hasanpour 2013 {published data only}
- Hasanpour S, Raouf S, Shamsalizadeh N, Bani S, Ghojazadeh M, Sheikhan F. Evaluation of the effects of acoustic stimulation and feeding mother stimulation on non‐reactive non‐stress test: a randomized clinical trial. Archives of Gynecology and Obstetrics 2013;287(6):1105‐10. [DOI] [PubMed] [Google Scholar]
Kisilevsky 1990 {published data only}
- Kisilevsky BS, Muir DW, Low JA. Maturation of responses elicited by a vibroacoustic stimulus in a group of high‐risk fetuses. Maternal‐Child Nursing Journal 1990;19:239‐50. [PubMed] [Google Scholar]
Kisilevsky 1992 {published data only}
- Kisilevsky BS, Muir DW, Low JA. Maturation of human fetal responses to vibroacoustic stimulation. Child Development 1992;63:1497‐508. [PubMed] [Google Scholar]
Maesel 1994 {published data only}
- Maesel A, Sladkevicius P, Valentin L, Marsal K. Effect of vibroacoustic stimulation on blood circulation in the middle cerebral and umbilical arteries in healthy term fetuses. Journal of Maternal Fetal Investigation 1994;4:69‐72. [Google Scholar]
Montan 1992a {published data only}
- Montan S, Arulkumaran S, Ratnam SS. Computerised cardiotocography following vibro‐acoustic stimulation. Journal of Perinatal Medicine 1992;20:471‐7. [DOI] [PubMed] [Google Scholar]
Montan 1992b {published data only}
- Montan S, Arulkumaran S, Nyman M, Ratnam SS. Vibro‐acoustic stimulation does not alter the duration of high and low fetal heart rate variability episodes. Journal of Perinatal Medicine 1992;20:331‐6. [DOI] [PubMed] [Google Scholar]
Petrovic 1998 {published data only}
- Petrovic O, Skunca E, Matejcic N. A simplified fetal biophysical profile. International Journal of Gynecology and Obstetrics 1998;61:9‐14. [DOI] [PubMed] [Google Scholar]
Pinette 2005 {published data only}
- Pinette MG, Blackstone J, Wax JR, Cartin A. Using fetal acoustic stimulation to shorten the biophysical profile. Journal of Clinical Ultrasound 2005;33(5):223‐5. [DOI] [PubMed] [Google Scholar]
Schiff 1992 {published data only}
- Schiff E, Lipitz S, Sivan E, Barkai G, Mashiach S. Acoustic stimulation as a diagnostic test: comparison with oxytocin challenge test. Journal of Perinatal Medicine 1992;20:275‐9. [DOI] [PubMed] [Google Scholar]
Smith 1988 {published data only}
- Smith CV, Phelan JP, Broussard P, Paul RH. Fetal acoustic stimulation testing. III. Predictive value of a reactive test. Journal of Reproductive Medicine 1988;33:217‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Gonzalez 1995 {published data only}
- Gonzalez GNL, Parache J, Armas H, Ormazabal JC, Rodriguez PP. Fetal response to vibroacoustic stimulation in basal conditions with low frequency sounds. Progresos de Obstetricia y Ginecologia 1995;38:374‐80. [Google Scholar]
Additional references
Arulkumaran 1991
- Arulkumaran S, Skurr B, Tong H, Kek LP, Yeoh KH, Ratnam SS. No evidence of hearing loss due to fetal acoustic stimulation test. Obstetrics and Gynecology 1991;78(2):283‐5. [PubMed] [Google Scholar]
Arulkumaran 1992
- Arulkumaran S, Talbert D, Hsu TS, Chua S, Anandakumar C, Ratnam SS. In‐utero sound levels when vibroacoustic stimulation is applied to the maternal abdomen: an assessment of the possibility of cochlea damage in the fetus. British Journal of Obstetrics and Gynaecology 1992;99(1):43‐5. [DOI] [PubMed] [Google Scholar]
Bernard 1947
- Bernard J, Sontag LW. Fetal reactivity to acoustic stimulation: a preliminary report. Journal of Genetic Psychology 1947;70:205‐10. [DOI] [PubMed] [Google Scholar]
Bolnick 2004
- Bolnick J, Garcia G, Fletcher B, Rayburn W. Cross‐over trial of fetal heart rate response to external light and vibroacoustic stimulation [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S150. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ingemarsson 1989
- Ingemarsson I, Arulkumaran S. Reactive fetal heart rate response to vibroacoustic stimulation in fetuses with low scalp blood pH. British Journal of Obstetrics and Gynecology 1989;96:562‐5. [DOI] [PubMed] [Google Scholar]
Inglis 1993
- Inglis SR, Druzin ML, Wagner WE, Kogut E. The use of vibroacoustic stimulation during the abnormal or equivocal biophysical profile. Obstetrics & Gynecology 1993;82(3):371‐4. [PubMed] [Google Scholar]
Keegan 1987
- Keegan RA. The nonstress test. Clinical Obstetrics and Gynecology 1987;30:931‐5. [DOI] [PubMed] [Google Scholar]
Leader 1984
- Leader LR, Baillie P, Martin B, Molteno C, Wynchank S. Fetal responses to vibroacoustic stimulation, a possible predictor of fetal and neonatal outcome. Australian and New Zealand Journal of Obstetrics and Gynaecology 1984;24:251. [DOI] [PubMed] [Google Scholar]
Nyman 1992
- Nyman M, Arulkumaran S, Jakobsson J, Westgren M. Vibro‐acoustic stimulation in high‐risk pregnancies; maternal perception of fetal movements, fetal heart rate and fetal outcome. Journal of Perinatal Medicine 1992;20(4):267‐74. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sadovsky 1981
- Sadovsky E. Fetal movements and fetal health. Seminars in Perinatology 1981;5:131‐43. [PubMed] [Google Scholar]
Sarinoglu 1996
- Sarinoglu C, Dell J, Mercer BM, Sibai BM. Fetal startle response observed under ultrasonography: a good predictor of a reassuring biophysical profile. Obstetrics & Gynecology 1996;88(4 Pt 1):599‐602. [DOI] [PubMed] [Google Scholar]
Serafini 1984
- Serafini P, Lindsay MBJ, Nagey DA, Pupkin MJ. Tseng P, Crenshaw C. Antepartum fetal heart rate response to sound stimulation: the acoustic stimulation test. American Journal of Obstetrics and Gynecology 1984;148:41. [DOI] [PubMed] [Google Scholar]
Sherer 1988
- Sherer DM, Menashe M, Sadovsky E. Severe fetal bradycardia caused by external vibratory acoustic stimulation. American Journal of Obstetrics and Gynecology 1988;159(2):334‐5. [DOI] [PubMed] [Google Scholar]
Sherer 1991
- Sherer DM, Abramowicz JS, Hearn‐Stebbins B, Woods JR Jr. Sonographic verification of a nuchal cord following a vibratory acoustic stimulation‐induced severe variable fetal heart rate deceleration with expedient abdominal delivery. American Journal of Perinatology 1991;8(5):345‐6. [DOI] [PubMed] [Google Scholar]
Smith 1985
- Smith CV, Phelan JP, Paul RH, Brossard P. Fetal acoustic stimulation testing: a retrospective experience with the fetal acoustic stimulation test. American Journal of Obstetrics and Gynaecology 1985;153:567‐8. [DOI] [PubMed] [Google Scholar]
Trudinger 1980
- Trudinger BJ, Boylan P. Antepartum fetal heart rate monitoring: value of sound stimulation. Obstetrics & Gynecology 1980;55:265. [PubMed] [Google Scholar]
References to other published versions of this review
Neilson 1995
- Neilson JP. Fetal vibroacoustic stimulation before labour. [revised 12 May 1994] In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Tan 2001
- Tan KH, Smyth RMD. Fetal vibroacoustic stimulation for facilitation of tests of fetal wellbeing. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002963] [DOI] [PubMed] [Google Scholar]


