Abstract
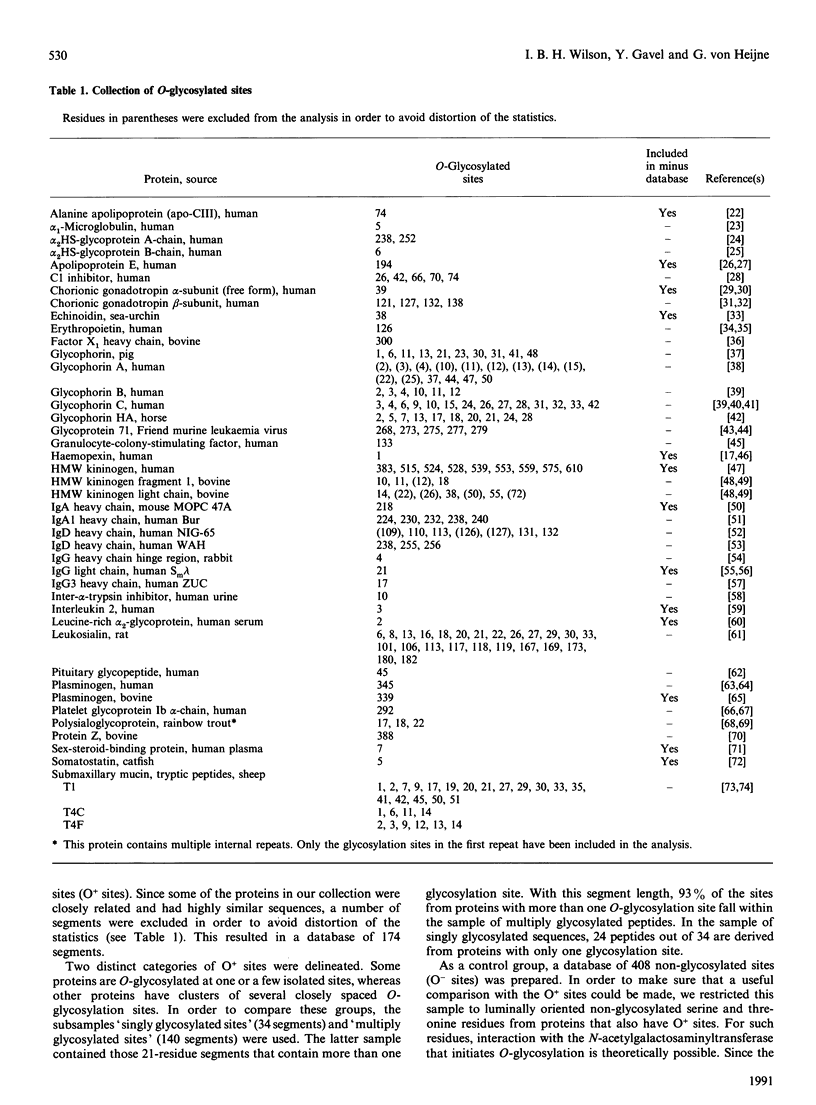
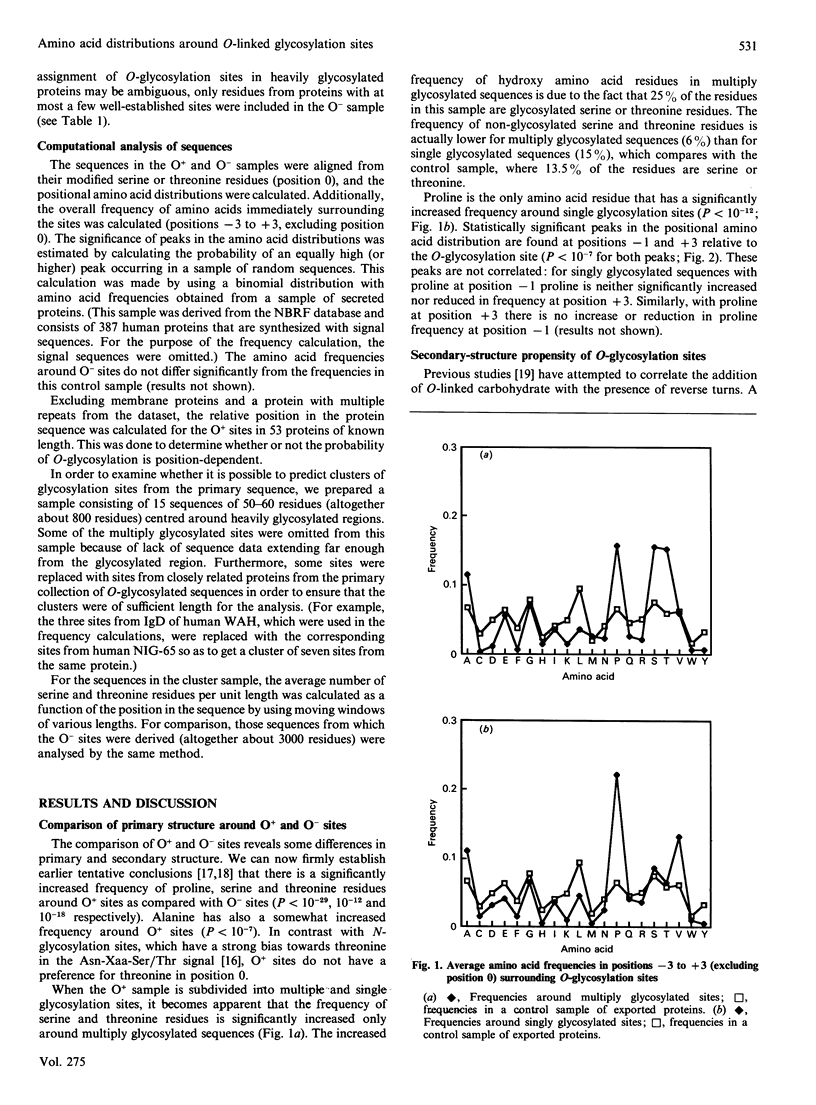
To study the sequence requirements for addition of O-linked N-acetylgalactosamine to proteins, amino acid distributions around 174 O-glycosylation sites were compared with distributions around non-glycosylated sites. In comparison with non-glycosylated serine and threonine residues, the most prominent feature in the vicinity of O-glycosylated sites is a significantly increased frequency of proline residues, especially at positions -1 and +3 relative to the glycosylated residues. Alanine, serine and threonine are also significantly increased. The high serine and threonine content of O-glycosylated regions is due to the presence of clusters of several closely spaced glycosylated hydroxy amino acids in many O-glycosylated proteins. Such clusters can be predicted from the primary sequence in some cases, but there is no apparent possibility of predicting isolated O-glycosylation sites from primary sequence data.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A., Creeth J. M. Properties of potato lectin and the nature of its glycoprotein linkages. Biochem J. 1978 Jun 1;171(3):665–674. doi: 10.1042/bj1710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. C., Pubols M. H., Hermodson M. A., Sheares B. T., Dixon J. E. Structure of the 22-residue somatostatin from catfish. An O-glycosylated peptide having multiple forms. J Biol Chem. 1984 Nov 10;259(21):13267–13272. [PubMed] [Google Scholar]
- Aubert J. P., Biserte G., Loucheux-Lefebvre M. H. Carbohydrate-peptide linkage in glycoproteins. Arch Biochem Biophys. 1976 Aug;175(2):410–418. doi: 10.1016/0003-9861(76)90528-2. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Blanchard D., Dahr W., Hummel M., Latron F., Beyreuther K., Cartron J. P. Glycophorins B and C from human erythrocyte membranes. Purification and sequence analysis. J Biol Chem. 1987 Apr 25;262(12):5808–5811. [PubMed] [Google Scholar]
- Blochberger T. C., Sabatine J. M., Lee Y. C., Hughey R. P. O-linked glycosylation of rat renal gamma-glutamyltranspeptidase adjacent to its membrane anchor domain. J Biol Chem. 1989 Dec 5;264(34):20718–20722. [PubMed] [Google Scholar]
- Bock S. C., Skriver K., Nielsen E., Thøgersen H. C., Wiman B., Donaldson V. H., Eddy R. L., Marrinan J., Radziejewska E., Huber R. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986 Jul 29;25(15):4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Shulman R., Herbert P., Ronan R., Wehrly K. The complete amino acid sequence of alanine apolipoprotein (apoC-3), and apolipoprotein from human plasma very low density lipoproteins. J Biol Chem. 1974 Aug 10;249(15):4975–4984. [PubMed] [Google Scholar]
- Briand J. P., Andrews S. P., Jr, Cahill E., Conway N. A., Young J. D. Investigation of the requirements for O-glycosylation by bovine submaxillary gland UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosamine transferase using synthetic peptide substrates. J Biol Chem. 1981 Dec 10;256(23):12205–12207. [PubMed] [Google Scholar]
- Broudy V. C., Tait J. F., Powell J. S. Recombinant human erythropoietin: purification and analysis of carbohydrate linkage. Arch Biochem Biophys. 1988 Sep;265(2):329–336. doi: 10.1016/0003-9861(88)90135-x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran E. V., Mendicino A., Garver F. A., Mendicino J. Structures of sialylated O-glycosidically and N-glycosidically linked oligosaccharides in a monoclonal immunoglobulin light chain. J Biol Chem. 1981 Feb 25;256(4):1549–1555. [PubMed] [Google Scholar]
- Chen R. Complete amino acid sequence and glycosylation sites of glycoprotein gp71A of Friend murine leukemia virus. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5788–5792. doi: 10.1073/pnas.79.19.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cole L. A., Perini F., Birken S., Ruddon R. W. An oligosaccharide of the O-linked type distinguishes the free from the combined form of hCG alpha subunit. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1260–1267. doi: 10.1016/0006-291x(84)91228-2. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio M., Starr C. M., Park M. K., Holt G. D., Haltiwanger R. S., Hart G. W., Hanover J. A. Partial cDNA sequence encoding a nuclear pore protein modified by O-linked N-acetylglucosamine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9595–9599. doi: 10.1073/pnas.85.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahr W., Beyreuther K., Kordowicz M., Krüger J. N-terminal amino acid sequence of sialoglycoprotein D (glycophorin C) from human erythrocyte membranes. Eur J Biochem. 1982 Jun 15;125(1):57–62. doi: 10.1111/j.1432-1033.1982.tb06650.x. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. Isolation, cloning and sequence analysis of the cDNA for the alpha-subunit of human chorionic gonadotropin. Nature. 1979 Oct 4;281(5730):351–356. doi: 10.1038/281351a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Garver F. A., Chang L., Mendicino J., Isobe T., Osserman E. F. Primary structure of a deleted human lambda type immunoglobulin light chain containing carbohydrate: protein Sm lambda. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4559–4563. doi: 10.1073/pnas.72.11.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejyo F., Chang J. L., Bürgi W., Schmid K., Offner G. D., Troxler R. F., Van Halbeek H., Dorland L., Gerwig G. J., Vliegenthart J. F. Characterization of the B-chain of human plasma alpha 2HS-glycoprotein. The complete amino acid sequence and primary structure of its heteroglycan. J Biol Chem. 1983 Apr 25;258(8):4966–4971. [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken T. A., Butenhof K. J., Shogren R. Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry. 1989 Jun 27;28(13):5536–5543. doi: 10.1021/bi00439a030. [DOI] [PubMed] [Google Scholar]
- Geyer R., Dabrowski J., Dabrowski U., Linder D., Schlüter M., Schott H. H., Stirm S. Oligosaccharides at individual glycosylation sites in glycoprotein 71 of Friend murine leukemia virus. Eur J Biochem. 1990 Jan 12;187(1):95–110. doi: 10.1111/j.1432-1033.1990.tb15281.x. [DOI] [PubMed] [Google Scholar]
- Giga Y., Ikai A., Takahashi K. The complete amino acid sequence of echinoidin, a lectin from the coelomic fluid of the sea urchin Anthocidaris crassispina. Homologies with mammalian and insect lectins. J Biol Chem. 1987 May 5;262(13):6197–6203. [PubMed] [Google Scholar]
- Hanover J. A., Elting J., Mintz G. R., Lennarz W. J. Temporal aspects of the N- and O-glycosylation of human chorionic gonadotropin. J Biol Chem. 1982 Sep 10;257(17):10172–10177. [PubMed] [Google Scholar]
- Hayes M. L., Castellino F. J. Carbohydrate of the human plasminogen variants. III. Structure of the O-glycosidically linked oligosaccharide unit. J Biol Chem. 1979 Sep 25;254(18):8777–8780. [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Hill H. D., Jr, Schwyzer M., Steinman H. M., Hill R. L. Ovine submaxillary mucin. Primary structure and peptide substrates of UDP-N-acetylgalactosamine:mucin transferase. J Biol Chem. 1977 Jun 10;252(11):3799–3804. [PubMed] [Google Scholar]
- Hochstrasser K., Schönberger O. L., Rossmanith I., Wachter E. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, V. Attachments of carbohydrates in the human urinary trypsin inhibitor isolated by affinity chromatography. Hoppe Seylers Z Physiol Chem. 1981 Oct;362(10):1357–1362. doi: 10.1515/bchm2.1981.362.2.1357. [DOI] [PubMed] [Google Scholar]
- Honma K., Tomita M., Hamada A. Amino acid sequence and attachment sites of oligosaccharide units of porcine erythrocyte glycophorin. J Biochem. 1980 Dec;88(6):1679–1691. doi: 10.1093/oxfordjournals.jbchem.a133143. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Bradbury A. F., Smyth D. G. Substrate recognition by UDP-N-acetyl-alpha-D-galactosamine: polypeptide n-acetyl-alpha-D-galactosaminyltransferase. Effects of chain length and disulphide bonding of synthetic peptide substrates. Carbohydr Res. 1988 Jul 15;178:259–269. doi: 10.1016/0008-6215(88)80117-4. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Højrup P., Jensen M. S., Petersen T. E. Amino acid sequence of bovine protein Z: a vitamin K-dependent serine protease homolog. FEBS Lett. 1985 May 20;184(2):333–338. doi: 10.1016/0014-5793(85)80633-5. [DOI] [PubMed] [Google Scholar]
- Kajinami K., Mabuchi H., Itoh H., Michishita I., Takeda M., Wakasugi T., Koizumi J., Takeda R. New variant of low density lipoprotein receptor gene. FH-Tonami. Arteriosclerosis. 1988 Mar-Apr;8(2):187–192. doi: 10.1161/01.atv.8.2.187. [DOI] [PubMed] [Google Scholar]
- Kellermann J., Lottspeich F., Henschen A., Müller-Esterl W. Completion of the primary structure of human high-molecular-mass kininogen. The amino acid sequence of the entire heavy chain and evidence for its evolution by gene triplication. Eur J Biochem. 1986 Jan 15;154(2):471–478. doi: 10.1111/j.1432-1033.1986.tb09421.x. [DOI] [PubMed] [Google Scholar]
- Kessler M. J., Mise T., Ghai R. D., Bahl O. P. Structure and location of the O-glycosidic carbohydrate units of human chorionic gonadotropin. J Biol Chem. 1979 Aug 25;254(16):7909–7914. [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima K., Inoue Y., Inoue S. Polysialoglycoproteins of Salmonidae fish eggs. Complete structure of 200-kDa polysialoglycoprotein from the unfertilized eggs of rainbow trout (Salmo gairdneri). J Biol Chem. 1986 Apr 25;261(12):5262–5269. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kubota N., Orita T., Hattori K., Oh-eda M., Ochi N., Yamazaki T. Structural characterization of natural and recombinant human granulocyte colony-stimulating factors. J Biochem. 1990 Mar;107(3):486–492. doi: 10.1093/oxfordjournals.jbchem.a123072. [DOI] [PubMed] [Google Scholar]
- Lai P. H., Everett R., Wang F. F., Arakawa T., Goldwasser E. Structural characterization of human erythropoietin. J Biol Chem. 1986 Mar 5;261(7):3116–3121. [PubMed] [Google Scholar]
- Levitt M. Conformational preferences of amino acids in globular proteins. Biochemistry. 1978 Oct 3;17(20):4277–4285. doi: 10.1021/bi00613a026. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottspeich F., Kellermann J., Henschen A., Foertsch B., Müller-Esterl W. The amino acid sequence of the light chain of human high-molecular-mass kininogen. Eur J Biochem. 1985 Oct 15;152(2):307–314. doi: 10.1111/j.1432-1033.1985.tb09199.x. [DOI] [PubMed] [Google Scholar]
- Malinowski D. P., Sadler J. E., Davie E. W. Characterization of a complementary deoxyribonucleic acid coding for human and bovine plasminogen. Biochemistry. 1984 Aug 28;23(18):4243–4250. doi: 10.1021/bi00313a035. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Meikle P., Richards G. N., Yellowlees D. Structural determination of the oligosaccharide side chains from a glycoprotein isolated from the mucus of the coral Acropora formosa. J Biol Chem. 1987 Dec 15;262(35):16941–16947. [PubMed] [Google Scholar]
- Morgan F. J., Birken S., Canfield R. E. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975 Jul 10;250(13):5247–5258. [PubMed] [Google Scholar]
- Morrow B., Rubin C. S. Biogenesis of glycophorin A in K562 human erythroleukemia cells. J Biol Chem. 1987 Oct 5;262(28):13812–13820. [PubMed] [Google Scholar]
- Murayama J. I., Tomita M., Hamada A. Primary structure of horse erythrocyte glycophorin HA. Its amino acid sequence has a unique homology with those of human and porcine erythrocyte glycophorins. J Membr Biol. 1982;64(3):205–215. doi: 10.1007/BF01870887. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kawabata S., Kisiel W., Hase S., Ikenaka T., Takao T., Shimonishi Y., Iwanaga S. Identification of a disaccharide (Xyl-Glc) and a trisaccharide (Xyl2-Glc) O-glycosidically linked to a serine residue in the first epidermal growth factor-like domain of human factors VII and IX and protein Z and bovine protein Z. J Biol Chem. 1989 Dec 5;264(34):20320–20325. [PubMed] [Google Scholar]
- Perkins S. J., Smith K. F., Amatayakul S., Ashford D., Rademacher T. W., Dwek R. A., Lachmann P. J., Harrison R. A. Two-domain structure of the native and reactive centre cleaved forms of C1 inhibitor of human complement by neutron scattering. J Mol Biol. 1990 Aug 5;214(3):751–763. doi: 10.1016/0022-2836(90)90290-3. [DOI] [PubMed] [Google Scholar]
- Piller V., Piller F., Klier F. G., Fukuda M. O-glycosylation of leukosialin in K562 cells. Evidence for initiation and elongation in early Golgi compartments. Eur J Biochem. 1989 Jul 15;183(1):123–135. doi: 10.1111/j.1432-1033.1989.tb14904.x. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Liu Y. S., Low T. L. Primary structure of a human IgA1 immunoglobulin. IV. Streptococcal IgA1 protease, digestion, Fab and Fc fragments, and the complete amino acid sequence of the alpha 1 heavy chain. J Biol Chem. 1979 Apr 25;254(8):2865–2874. [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Reid K. B. Complete amino acid sequences of the three collagen-like regions present in subcomponent C1q of the first component of human complement. Biochem J. 1979 May 1;179(2):367–371. doi: 10.1042/bj1790367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Kutny R. M., Panico M., Morris H. R., Chowdhry V. Amino acid sequence and post-translational modification of human interleukin 2. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6486–6490. doi: 10.1073/pnas.81.20.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. A., Appella E. Amino acid sequence of a mouse myeloma immunoglobin heavy chain (MOPC 47 A) with a 100-residue deletion. J Biol Chem. 1979 Nov 25;254(22):11418–11430. [PubMed] [Google Scholar]
- Roth J. Cytochemical localization of terminal N-acetyl-D-galactosamine residues in cellular compartments of intestinal goblet cells: implications for the topology of O-glycosylation. J Cell Biol. 1984 Feb;98(2):399–406. doi: 10.1083/jcb.98.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Schaller J., Moser P. W., Dannegger-Müller G. A., Rösselet S. J., Kämpfer U., Rickli E. E. Complete amino acid sequence of bovine plasminogen. Comparison with human plasminogen. Eur J Biochem. 1985 Jun 3;149(2):267–278. doi: 10.1111/j.1432-1033.1985.tb08921.x. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M. Complete amino acid sequence of a human pituitary glycopeptide: an important maturation product of pro-opiomelanocortin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4236–4240. doi: 10.1073/pnas.78.7.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]
- Sorimachi H., Emori Y., Kawasaki H., Kitajima K., Inoue S., Suzuki K., Inoue Y. Molecular cloning and characterization of cDNAs coding for apo-polysialoglycoprotein of rainbow trout eggs. Multiple mRNA species transcribed from multiple genes contain diverged numbers of exact 39-base (13-amino acid) repeats. J Biol Chem. 1988 Nov 25;263(33):17678–17684. [PubMed] [Google Scholar]
- Spielman J., Rockley N. L., Carraway K. L. Temporal aspects of O-glycosylation and cell surface expression of ascites sialoglycoprotein-1, the major cell surface sialomucin of 13762 mammary ascites tumor cells. J Biol Chem. 1987 Jan 5;262(1):269–275. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Strous G. J. Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2694–2698. doi: 10.1073/pnas.76.6.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Larsen K., Gunnarsson A. Characterization of a glucoamylase G2 from Aspergillus niger. Eur J Biochem. 1986 Feb 3;154(3):497–502. doi: 10.1111/j.1432-1033.1986.tb09425.x. [DOI] [PubMed] [Google Scholar]
- Takagi T., Takagi K., Kawai T. Complete amino acid sequence of human alpha 1-microglobulin. Biochem Biophys Res Commun. 1981 Feb 27;98(4):997–1001. doi: 10.1016/0006-291x(81)91209-2. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Complete amino acid sequence of human hemopexin, the heme-binding protein of serum. Proc Natl Acad Sci U S A. 1985 Jan;82(1):73–77. doi: 10.1073/pnas.82.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1906–1910. doi: 10.1073/pnas.82.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Structure of human hemopexin: O-glycosyl and N-glycosyl sites and unusual clustering of tryptophan residues. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2021–2025. doi: 10.1073/pnas.81.7.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Tetaert D., Debuire B., Lin L. C., Putnam F. W. Complete amino acid sequence of the delta heavy chain of human immunoglobulin D. Proc Natl Acad Sci U S A. 1982 May;79(9):2850–2854. doi: 10.1073/pnas.79.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu T., Suzuki S., Kametani F., Takahashi N., Shinoda T., Okuyama T., Munekata E. Amino acid sequence of galactosamine-containing glycopeptides in the hinge region of a human immunoglobulin D. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1066–1071. doi: 10.1016/0006-291x(82)91078-6. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Dwek R. A., Rademacher T. W. Structure, biosynthesis, and function of glycosylphosphatidylinositols. Biochemistry. 1990 Jun 12;29(23):5413–5422. doi: 10.1021/bi00475a001. [DOI] [PubMed] [Google Scholar]
- Titani K., Fujikawa K., Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H. Bovine factor X1 (Stuart factor): amino-acid sequence of heavey chain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3082–3086. doi: 10.1073/pnas.72.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. A., Titani K., Takio K., Kumar S., Hayes R., Petra P. H. Amino acid sequence of the sex steroid binding protein of human blood plasma. Biochemistry. 1986 Nov 18;25(23):7584–7590. doi: 10.1021/bi00371a048. [DOI] [PubMed] [Google Scholar]
- Wernette-Hammond M. E., Lauer S. J., Corsini A., Walker D., Taylor J. M., Rall S. C., Jr Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J Biol Chem. 1989 May 25;264(15):9094–9101. [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Gejyo F., Marti T., Rickli E. E., Bürgi W., Offner G. D., Troxler R. F., Schmid K. The complete amino acid sequence of the A-chain of human plasma alpha 2HS-glycoprotein. J Biol Chem. 1986 Feb 5;261(4):1665–1676. [PubMed] [Google Scholar]


