Abstract
Human papillomavirus capsid assembly requires intercapsomeric disulfide bonds between molecules of the major capsid protein L1. Virions isolated from naturally occurring lesions have a higher degree of cross-linking than virus-like particles (VLPs), which have been generated in eukaryotic expression systems. Here we show that DNA encapsidation into VLPs leads to increased cross-linking between L1 molecules comparable to that seen in virions. A higher trypsin resistance, indicating a tighter association of capsomeres through DNA interaction, accompanies this structural change.
Human papillomaviruses (HPV) are nonenveloped DNA viruses harboring a double-stranded DNA genome of approximately 8,000 bp. They exclusively infect epithelial cells of skin and mucosa, inducing benign and malignant lesions (32). The spherical viral capsid with T=7 icosahedral symmetry (5) is composed of 72 pentameric capsomers containing 360 copies of the major capsid protein L1 (1). Sixty of these capsomeres are hexavalent, i.e., have six nearest neighbors, whereas 12 capsomeres are pentavalent. It is believed that in addition to L1, 12 copies of the minor capsid protein L2 are associated with the pentavalent capsomeres (27).
Since the productive life cycle of HPV requires differentiated tissue, it is difficult to produce significant amounts of virions in vitro. Therefore DNA-free virus-like particles (VLPs) were generated for the study of structural and immunological aspects of the capsid and for the study of virus-cell interactions using eukaryotic expression systems (10, 14, 21, 29, 31). L1 alone is sufficient for VLP formation, but L2 is incorporated at the expected molar ratio when present. Electron microscopic analyses revealed that VLPs are structurally indistinguishable from virions isolated from naturally occurring lesions (11). In addition, they induce neutralizing antibodies (2, 3, 6, 19, 20, 25) and compete with virions for binding to the cellular receptor (18), suggestive of a high structural similarity. Recently, systems were developed that allowed incorporation of marker plasmids into VLPs in vitro (12, 26) and in vivo (24, 28), yielding pseudovirions. Pseudovirions are helpful tools for the detection of neutralizing antibodies (6, 9, 13, 19, 28) as well as for the study of very early events in infection, such as binding and uptake of virions (8).
Disulfide bonds between adjacent capsomeres stabilize HPV capsids (23). Recently, we showed that papillomavirus assembly requires two conserved cysteines to connect capsomeres, resulting in the formation of L1 trimers (22). In VLPs, about 50% of L1 proteins are cross-linked by disulfide bonds, whereas the L1 proteins of virions are completely cross-linked. To investigate which structural differences between VLPs and virions underlie this observation, we compared DNA-free VLPs and pseudovirions with regard to disulfide bonding and trypsin sensitivity.
HPV-33 VLPs encapsidate DNA upon long-term infection of insect cells.
VLPs of HPV type 33 (HPV-33) found in supernatants of insect cells infected with baculoviruses recombinant for HPV-33L1 (bac33L1) and HPV-33L2 (bac33L2) and cultivated for 2 to 3 weeks in serum-free Sf900II medium (Life Technologies) were subjected to cesium chloride density gradient centrifugation. Interestingly, they banded in two broad peaks corresponding to buoyant densities of 1.33 and 1.30 g/cm3 (Fig. 1A). Two peaks with similar densities were also observed when nuclear extracts from HPV-induced hand warts were analyzed (Fig. 1B). The corresponding peak fractions of HPV-33L1/L2 obtained from insect cell supernatants were further characterized by electron microscopy. As expected, VLPs were found in the light fraction but interestingly also in the heavy fractions (Fig. 1C). We therefore designated these fractions light VLPs (L-VLPs) and heavy VLPs (H-VLPs). Assembly of L1 into particles with these characteristic buoyant densities has also been reported during generation of pseudovirions in COS-7 cells, and it was shown previously that the packaged marker plasmid was exclusively present in H-VLP fractions (28). These observations suggested that the H-VLPs obtained from long-term expression of recombinant HPV-33 L1 and L2 proteins in insect cells contained DNA. To verify this assumption, we isolated DNA from L-VLPs and H-VLPs. Pooled fractions were extensively dialyzed against phosphate-buffered saline (PBS). Magnesium chloride (10 mM) and DNase I (250 μg/ml) were added to digest contaminating unpackaged DNA for 2.5 h at 37°C. The reaction was stopped with EDTA (200 mM), and VLPs were digested with proteinase K for 12 h. Subsequently, encapsidated DNA was extracted by phenol-chloroform, precipitated with ethanol, and labeled with [32P]dATP, using the Klenow fragment of Escherichia coli DNA polymerase I. The resulting products were analyzed by agarose gel electrophoresis and visualized by autoradiography (Fig. 1D). A DNA smear ranging from 1.5 to 8 kb was exclusively found in the H-VLP fraction. In addition to the DNA encapsidated by H-VLPs, a high-molecular-weight DNA larger than 20 kb, most likely DNA extracted from copurifying baculoviruses, was present in both L-VLPs and H-VLPs.
FIG. 1.
Analysis of VLPs from long-term infections. Supernatants of long-term cultures of insect cell infected by baculoviruses bac33L1 and bac33L2 (A) and a nuclear extract from an HPV-induced hand wart (B) were subjected to cesium chloride density gradient centrifugation. Fractions were analyzed by SDS-PAGE, and L1 proteins were detected by immunoblotting using MAb 33L1-7. The apparent molecular masses of marker proteins are indicated in kilodaltons. The buoyant densities of the peak fractions, as determined by refractometry, are indicated by arrows. (C) Electron micrograph of 1.33-g/cm3 H-VLPs from insect cell supernatants. (D) Autoradiography of 32P-labeled DNA extracted from L-VLPs (L) and H-VLPs (H) and subjected to agarose gel electrophoresis. Sizes of marker DNA fragments are indicated in kilobases.
Our results demonstrate that DNA encapsidation into papillomavirus-like particles occurred after long-term infection of insect cells with baculovirus-expressed HPV-33 L1 and L2. This could be observed in long-term infections only, and H-VLPs were detected exclusively in the supernatants of infected cells. We therefore assume that DNA packaging occurs late in infection, since DNA degradation induced by the baculovirus infection is required to generate DNA fragments small enough to be incorporated into VLPs (7, 16). It is highly likely that H-VLPs harbor chromatin since eukaryotic cells do not contain naked DNA. In H-VLPs, the size of encapsidated DNA is rather heterogenous, with an upper size limit in the range of 8 kb, which is also the size of the viral genome. The fact that the capsid does not discriminate against smaller DNA molecules argues against a minimal size requirement for incorporation into the viral capsid. This is in accordance with DNA incorporation into pseudovirions containing plasmids of variable lengths in the range from 5.4 to 8 kb (12, 24, 26, 28, 30).
DNA packaging into VLPs induces a high degree of disulfide cross-linking.
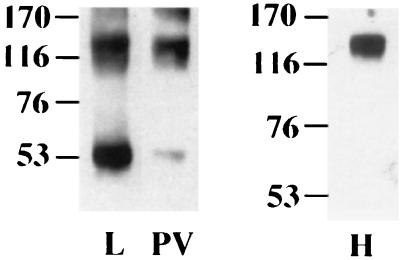
In virions, L1 molecules are completely cross-linked by intercapsomeric disulfide bonds (4, 22), whereas only 50% of the L1 protein found in VLPs is covalently connected (22, 29). The differences in disulfide bonding may possibly be due to different redox potentials in the differentiated keratinocytes, where HPV virions assemble, and in the cell lines used for VLP production. Alternatively, DNA encapsidation might induce a tighter packaging of capsid proteins and thus closer contacts between cysteines. If DNA encapsidation induces a higher degree of cross-linking, L-VLPs and H-VLPs prepared from the same cell line should display differences in disulfide cross-linking. As shown in Fig. 2, about 50% of the L1 molecules found in L-VLPs from COS-7 cells were disulfide bonded, forming trimers of 150 to 160 kDa as observed previously (22, 29). In H-VLPs, more than 90% of L1 molecules formed trimers. In addition, when H-VLPs from the supernatants of insect cells were analyzed under these conditions, complete cross-linking of L1 was observed. These observations support the hypothesis that DNA encapsidation allows the formation of additional disulfide bonds, possibly due to tighter packaging of capsid proteins.
FIG. 2.
Disulfide cross-linking of L1 proteins in VLPs and pseudovirions. L-VLPs (L) and pseudovirions (PV), prepared from COS-7 cells, and H-VLPs (H), prepared from supernatants of long-term-infected insect cells, were examined by SDS-PAGE under nonreducing conditions. L1 protein was visualized by immunoblotting using MAb 33L1-7. Apparent molecular masses of marker proteins are indicated in kilodaltons.
DNA encapsidation renders capsids less sensitive to trypsin digestion.
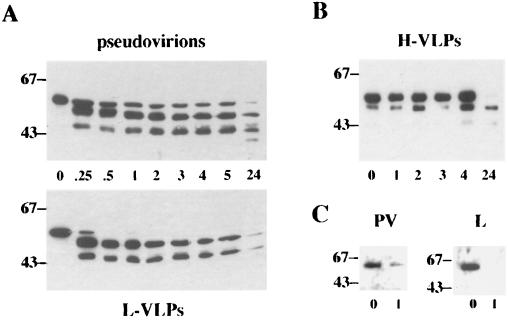
We noticed that the L1 protein present in high-density VLPs and pseudovirions was always less degraded than that in low-density VLP (Fig. 1A). Similar observations were made when wart extracts were analyzed in buoyant density gradients (Fig. 1B). This suggests that DNA encapsidation renders the L1 protein less sensitive to protease digestion. To further investigate this hypothesis, we carried out trypsin digestions of H-VLPs and pseudovirions encapsidating cellular and plasmid DNA, respectively, and DNA-free L-VLPs. Pseudovirions and L-VLPs generated in COS-7 cells were incubated at 37°C in a total volume of 50 μl of PBS–0.025% trypsin (Gibco BRL) for up to 24 h. Digestion was terminated by addition of trypsin inhibitor (Gibco BRL). Since H-VLPs prepared from supernatants of insect cells were less concentrated, digestion was carried out in a total volume of 500 μl of PBS–0.025% trypsin, followed by trichloroacetic acid precipitation. Samples were than resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis. Blots were stained using monoclonal antibody (MAb 33L1-7) or the polyclonal rabbit antiserum Rb890, respectively. Rb890 was raised against the carboxy-terminal 15 amino acids of HPV-33 L1 (8). As shown in Fig. 3A and B, pseudovirions and H-VLPs were less sensitive to trypsin than L-VLPs. After digestion with trypsin for more than 4 h, full-length L1 protein was still detected in Western blots of pseudovirions and H-VLPs. In contrast, no full-length L1 protein was present in L-VLP fractions treated with trypsin for only 30 min (Fig. 3A). The main degradation products were fragments with apparent molecular masses of about 53 and 45 kDa. As shown in Fig. 3C, using the polyclonal antiserum Rb890 directed toward the C terminus of L1, only full-length protein was detected. After 1 h of treatment with trypsin, full-length L1 protein was still present in preparations of pseudovirions but not in L-VLPs. Obviously, DNA packaging not only induces a higher degree of cross-linking of L1 proteins via disulfide bonds but also renders the carboxy terminus of L1 less sensitive to proteases.
FIG. 3.
Trypsin digestion of pseudovirions, L-VLPs, and H-VLPs. Pseudovirions (PV) and L-VLPs (L), purified from COS-7 cells infected with recombinant viruses vac33L1 and vac33L2, and H-VLPs, prepared from supernatants of long-term bac33L1- and bac33L2-infected insect cells, were digested with trypsin at 37°C for the indicated periods of time (hours). Samples were subjected to SDS-PAGE followed by immunoblotting using MAb 33L1-7 (A and B) or polyclonal rabbit antiserum Rb890 (C). The apparent molecular masses of marker proteins are indicated in kilodaltons.
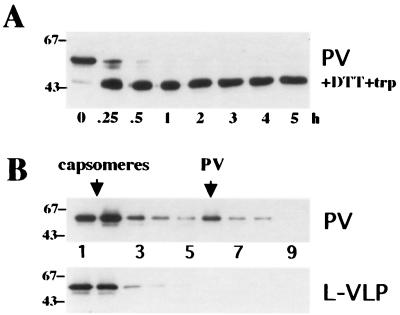
Li et al. (15) have shown that dithiothreitol (DTT) treatment of bovine papillomavirus type 1 virions renders the L1 carboxy terminus trypsin sensitive without complete disruption of the capsid structure. To investigate if similar observations can be made with pseudovirions, we carried out trypsin digestions and sucrose gradient sedimentations of pseudovirions treated with 20 mM DTT for 90 min at room temperature. As depicted in Fig. 4A, pseudovirions became sensitive to trypsin in a manner comparable to that seen for untreated L-VLPs (Fig. 3A). However, they partially retained their high sedimentation velocity, in contrast to L-VLPs, which completely dissociated into capsomeres under these conditions (Fig. 4B). Identical results were obtained for H- and L-VLPs generated in insect cells (data not shown). These data further demonstrate the similarity of pseudovirions and H-VLPs with authentic virions.
FIG. 4.
Trypsin digestion and sucrose gradient analysis of DTT-treated pseudovirions. (A) DTT-treated pseudovirions (PV) were digested with trypsin (trp) at 37°C for the indicated periods of time. Samples were subjected to SDS-PAGE followed by immunoblotting using MAb 33L1-7. (B) DTT-treated pseudovirions and L-VLPs were loaded onto a 20 to 40% sucrose gradient and spun for 2.5 h at 36,000 rpm and 4°C in a Beckman SW40 rotor. Eighteen fractions were collected from the top; proteins were precipitated with trichloroacetic acid and analyzed by immunoblotting. Analysis of the upper nine fractions is shown. The apparent molecular masses of marker proteins are indicated in kilodaltons.
Trypsin-treated pseudovirions remain infectious.
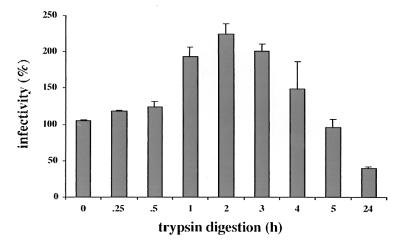
We were further interested to assay the infectivity of pseudovirions after trypsin digestion. Pseudovirions harboring a GFP expression cassette were further purified by a sucrose step gradient (28) and treated with trypsin as described above. After addition of trypsin inhibitor, 6.6 × 104 COS-7 cells (resuspended in PBS–100 μg of bovine serum albumin/ml [pH 6.8]) were added. Samples were incubated at 4°C under constant agitation, subsequently seeded into 24-well plates, and cultivated for 72 h at 37°C. To score the infection events, medium was removed and wells were screened for GFP-expressing cells in a fluorescence microscope. Surprisingly, trypsin treatment for 2 to 3 h resulted in a more than twofold increase in infectivity, as shown in Fig. 5, whereas longer digestions yielded a gradual decrease in infectivity as expected. Compared to untreated pseudovirions, a reduction in infectivity by trypsin treatment was observed only after overnight (24-h) incubations.
FIG. 5.
Infectivity assay of trypsin-digested pseudovirions. Digestions were carried out at 37°C for the indicated periods of time and terminated by addition of trypsin inhibitor. Subsequently COS-7 cells were added, and infected cells were counted after cultivation for 72 h.
The increase in infectivity after trypsin digestion may be due to the removal of noninfectious L-VLPs competing with intact pseudovirions for receptor binding. We have observed that only a fraction of pseudovirions present in our preparations bind to cells, suggesting that VLP binding sites on the cells are saturated (data not shown). Trypsin digestion may destroy the less stable particles, thus increasing the probability of specific uptake of pseudovirions. Alternatively, the increase of infectivity may be due to activation of pseudovirions after treatment with proteases. Similar observations have been made for poliovirus uptake (17). Activation might be achieved by a conformational change in the capsid structure resulting in facilitated binding, uptake, or uncoating. For bovine papillomavirus, Li et al. have shown that such an alteration in the capsid structure may be important for virus uncoating (15). According to their model, disulfide bridges in the capsid structure become cleaved in the reducing environment of the cytoplasm. This leads to a swelling of the capsid structure whereby the C terminus becomes accessible to proteolytic cleavage resulting in the release of DNA.
In this report we have demonstrated for the first time that DNA encapsidation into papillomavirus-like particles leads to the formation of additional disulfide bonds in addition to those shown previously to be essential for VLP assembly (22). The increase of disulfide bonding from about 50 to 100% may be due to a conformational change in L1 molecules, which allows a tighter association of capsomeres. The decrease in trypsin sensitivity of pseudovirions versus VLPs supports this hypothesis. These data are in line with the observation of Li et al. that reduction of disulfide bonds results in a relaxation of capsid structures (15). The increase of disulfide bonding between L1 molecules caused by DNA encapsidation does not significantly affect the overall outward capsid structure: no differences between VLPs and virions were detected by cryoelectron microscopy (11), and the antigenic properties of virions and VLPs are similar (2, 3, 20, 25). Since DNA-free particles can also be extracted from naturally occurring lesions in significant amounts (Fig. 1B) (5), the higher proteolytic sensitivity of empty capsids may be one way by which papillomaviruses avoid the competition between virions and VLPs for binding to the cellular receptor.
Acknowledgments
We are grateful to R. E. Streeck for critical reading of the manuscript and helpful suggestions throughout the work.
This work was supported by grants to M.S. from the Deutsche Forschungsgemeinschaft and the Stiftung Rheinland-Pfalz für Innovation. F.S. received a grant from the Graduiertenkolleg 194.
REFERENCES
- 1.Baker T S, Newcomb W W, Olson N H, Cowsert L M, Olson C, Brown J C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with virus-like particles from cotton tail rabbit papillomavirus (CRPV) can protect against experimentally CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen N D, Höpfl R, DiAngelo S L, Cladel N M, Patrick S D, Welsh P A, Budgeon L R, Reed C A, Kreider J W. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994;75:2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- 4.Doorbar J, Gallimore P H. Identification of proteins encoded by the L1 and L2 open reading frames of human papillomavirus 1a. J Virol. 1987;61:2793–2799. doi: 10.1128/jvi.61.9.2793-2799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch J T, Klug A. The structure of viruses of the papilloma-polyoma type. III. Structure of rabbit papillomavirus. J Mol Biol. 1965;13:1–12. doi: 10.1016/s0022-2836(65)80075-4. [DOI] [PubMed] [Google Scholar]
- 6.Fligge C, Giroglou T, Streeck R E, Sapp M. Induction of type-specific neutralizing antibodies by capsomeres of human papillomavirus type 33. Virology. 2001;283:353–357. doi: 10.1006/viro.2000.0875. [DOI] [PubMed] [Google Scholar]
- 7.Gillock E T, Rottinghaus S, Chang D, Cai X, Smiley S A, An K, Consigli R A. Polyomavirus major capsid protein VP1 is capable of packing cellular DNA when expressed in the baculovirus system. J Virol. 1997;71:2857–2865. doi: 10.1128/jvi.71.4.2857-2865.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giroglou T, Florin L, Schäfer F, Streeck R E, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giroglou T, Sapp M, Lane C, Fligge C, Christensen N D, Streeck R E, Rose R C. Immunological analysis of human papillomavirus capsids. Vaccine. 2001;19:1783–1793. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- 10.Hagensee M E, Yaegashi N, Galloway D A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagensee M E, Olson N H, Baker T S, Galloway D A. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J Virol. 1994;68:4503–4505. doi: 10.1128/jvi.68.7.4503-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. In vitro construction of pseudovirions of human papillomavirus type 16: incorporation of plasmid DNA into reassembled L1/L2 capsids. J Virol. 1998;72:10298–10300. doi: 10.1128/jvi.72.12.10298-10300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomaviruses 16 and 6. J Virol. 1999;73:6188–6190. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirnbauer R, Booy F, Cheng N, Lowy D R, Schiller J T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Beard P, Estes P A, Lyon M K, Garcea R L. Intercapsomeric disulfide bonds in papillomavirus assembly and disassembly. J Virol. 1998;72:2160–2167. doi: 10.1128/jvi.72.3.2160-2167.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlita M, Müller M, Oppenlander M, Zentgraf H, Herrmann M. DNA encapsidation by viruslike particles assembled in insect cells from the major capsid protein VP1 of B-lymphotropic papovavirus. J Virol. 1996;70:7517–7526. doi: 10.1128/jvi.70.11.7517-7526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piirainen L, Airaksinen A, Hovi T, Roivainen M. Selective cleavage by trypsin of the capsid protein VP1 of type 3 poliovirus results in improved sorting of cell bound virions. Arch Virol. 1996;141:1011–1020. doi: 10.1007/BF01718605. [DOI] [PubMed] [Google Scholar]
- 18.Roden R B S, Kirnbauer R, Jenson A B, Lowy D R, Schiller J T. Interaction of papillomaviruses with the cell surface. J Virol. 1994;68:7260–7266. doi: 10.1128/jvi.68.11.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roden R B, Greenstone H L, Kirnbauer R, Booy F P, Jessie J, Lowy D R, Schiller J T. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose R C, Reichman R C, Bonnez W. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J Gen Virol. 1994;75:2075–2079. doi: 10.1099/0022-1317-75-8-2075. [DOI] [PubMed] [Google Scholar]
- 21.Rose R C, Bonnez W, Reichman R C, Garcea R L. Expression of human papillomavirus type II L1 protein in insect cells: in vivo and in vitro assembly of virus-like particles. J Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapp M, Fligge C, Petzak I, Harris J R, Streeck R E. Papillomavirus assembly requires trimerization of the major capsid protein by disulfides between two highly conserved cysteines. J Virol. 1998;72:6186–6189. doi: 10.1128/jvi.72.7.6186-6189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapp M, Volpers C, Müller M, Streeck R E. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J Gen Virol. 1995;76:2407–2412. doi: 10.1099/0022-1317-76-9-2407. [DOI] [PubMed] [Google Scholar]
- 24.Stauffer Y, Raj K, Masternak K, Beard P J. Infectious human papillomavirus type 18 pseudovirions. Mol Biol. 1998;283:529–536. doi: 10.1006/jmbi.1998.2113. [DOI] [PubMed] [Google Scholar]
- 25.Suzich J A, Ghim S J, Palmer-Hill F J, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Touzé A, Coursaget P. In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res. 1998;26:1317–1323. doi: 10.1093/nar/26.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trus B L, Roden R B S, Greenstone H L, Vrhel M, Schiller J T, Booy F P. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9Å resolution. Nat Struct Biol. 1997;4:413–420. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- 28.Unckell F, Streeck R E, Sapp M. Generation and neutralization of pseudovirions of human papillomavirus type 33. J Virol. 1997;71:2934–2939. doi: 10.1128/jvi.71.4.2934-2939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpers C, Schirmacher P, Streeck R E, Sapp M. Assembly of the major and the minor capsid protein of human papillomavirus type 33 into virus-like particles and tubular structures in insect cells. Virology. 1994;200:504–512. doi: 10.1006/viro.1994.1213. [DOI] [PubMed] [Google Scholar]
- 30.Zhao K-N, Sun X-Y, Frazer I H, Zhou J. DNA packing by L1 and L2 capsid proteins of bovine papillomavirus type 1. Virology. 1998;243:482–491. doi: 10.1006/viro.1998.9091. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Sun X Y, Stenzel D J, Frazer I H. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185:251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 32.zur Hausen H, de Villiers E M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]