Abstract
Neutrophil-activating peptide 2 (NAP-2) is generated by cleavage of two inactive precursors, connective-tissue-activating peptide III (CTAP-III) and platelet basic protein (PBP), which are stored in the alpha-granules of blood platelets. Using highly purified CTAP-III as the substrate we studied the generation of NAP-2 by several neutral tissue proteinases. CTAP-III was rapidly cleaved by chymotrypsin, cathepsin G and trypsin, yielding products with neutrophil-stimulating activity. This activity remained unchanged for 24 h in the presence of chymotrypsin, decreased only slowly in the presence of cathepsin G, but was rapidly destroyed by trypsin. CTAP-III was also degraded by human neutrophil elastase and porcine pancreatic elastase, but no active fragments were obtained. By contrast, no degradation of CTAP-III was observed with thrombin, plasmin or 'granzymes' from cytolytic T-lymphocyte granules. Two active fragments of CTAP-III, generated by chymotrypsin or cathepsin G, were purified and partially sequenced, and were found to have the same N-terminal sequence as NAP-2. These results indicate that both proteinases cleave preferentially the bond between amino acids 15 (Tyr) and 16 (Ala) of CTAP-III. We conclude that chymotrypsin-like proteolytic activity in the vicinity of activated platelets may generate NAP-2 intravascularly. Due to its presence in the primary granules of neutrophils and monocytes cathepsin G is likely to be involved in this process.
Full text
PDF
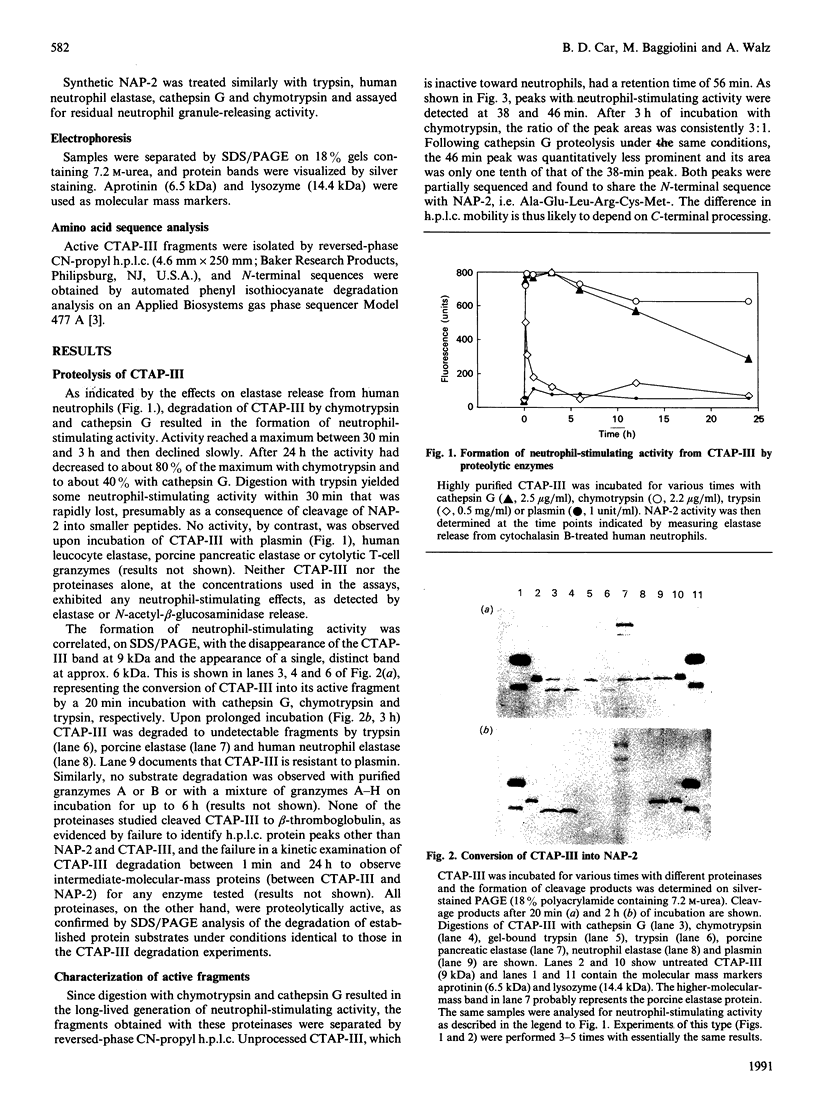
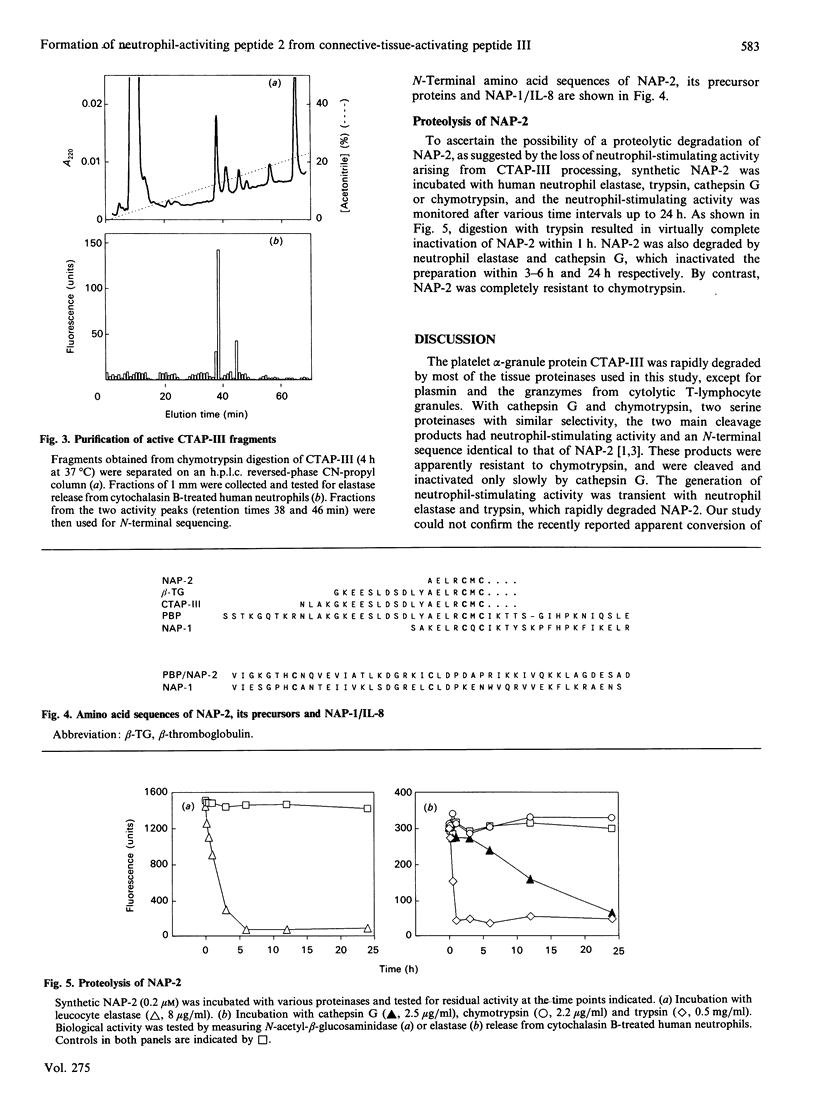

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B. Cellular models for the detection and evaluation of drugs that modulate human phagocyte activity. Experientia. 1988 Oct 15;44(10):841–848. doi: 10.1007/BF01941181. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow A. M., Barrett A. J. Action of human cathepsin G on the oxidized B chain of insulin. Biochem J. 1977 Jan 1;161(1):17–19. doi: 10.1042/bj1610017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., Silverman E. K., Campbell M. A. Elastase and cathepsin G of human monocytes. Quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. J Immunol. 1989 Nov 1;143(9):2961–2968. [PubMed] [Google Scholar]
- Castor C. W., Walz D. A., Ragsdale C. G., Hossler P. A., Smith E. M., Bignall M. C., Aaron B. P., Mountjoy K. Connective tissue activation. XXXIII. Biologically active cleavage products of CTAP-III from human platelets. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1071–1078. doi: 10.1016/0006-291x(89)92330-9. [DOI] [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Heiple J. M., Ossowski L. Human neutrophil plasminogen activator is localized in specific granules and is translocated to the cell surface by exocytosis. J Exp Med. 1986 Sep 1;164(3):826–840. doi: 10.1084/jem.164.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Granzymes: a family of serine proteases in granules of cytolytic T lymphocytes. Curr Top Microbiol Immunol. 1989;140:33–47. doi: 10.1007/978-3-642-73911-8_4. [DOI] [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Functional characteristics of receptor-bound urokinase on human monocytes: catalytic efficiency and susceptibility to inactivation by plasminogen activator inhibitors. Blood. 1989 Sep;74(4):1396–1402. [PubMed] [Google Scholar]
- Moser B., Clark-Lewis I., Zwahlen R., Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990 May 1;171(5):1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J. M., Persoon N. L., Christophers E. Lipopolysaccharide-stimulated human monocytes secrete, apart from neutrophil-activating peptide 1/interleukin 8, a second neutrophil-activating protein. NH2-terminal amino acid sequence identity with melanoma growth stimulatory activity. J Exp Med. 1990 Apr 1;171(4):1091–1100. doi: 10.1084/jem.171.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak M. A., Smith J. B. Cathepsin G binding to human platelets. Evidence for a specific receptor. Biochem J. 1990 Feb 15;266(1):55–62. doi: 10.1042/bj2660055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A. J., Strieter R. M., Lindley I., Baggiolini M., Kunkel S. L. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990 Apr 1;144(7):2609–2613. [PubMed] [Google Scholar]
- Walz A., Baggiolini M. A novel cleavage product of beta-thromboglobulin formed in cultures of stimulated mononuclear cells activates human neutrophils. Biochem Biophys Res Commun. 1989 Mar 31;159(3):969–975. doi: 10.1016/0006-291x(89)92203-1. [DOI] [PubMed] [Google Scholar]
- Walz A., Baggiolini M. Generation of the neutrophil-activating peptide NAP-2 from platelet basic protein or connective tissue-activating peptide III through monocyte proteases. J Exp Med. 1990 Feb 1;171(2):449–454. doi: 10.1084/jem.171.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Dewald B., von Tscharner V., Baggiolini M. Effects of the neutrophil-activating peptide NAP-2, platelet basic protein, connective tissue-activating peptide III and platelet factor 4 on human neutrophils. J Exp Med. 1989 Nov 1;170(5):1745–1750. doi: 10.1084/jem.170.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D. Z., Rowland A., Derynck R. Expression and secretion of gro/MGSA by stimulated human endothelial cells. EMBO J. 1989 Jun;8(6):1761–1766. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]