Abstract
Pregnant women are considered a high-risk group for COVID-19, and a priority for vaccination. Routine antenatal care (ANC) provides an opportunity to track trends and factors associated with vaccine uptake. We sought to evaluate COVID-19 vaccine uptake among pregnant women attending ANC and assess the factors associated with vaccine in Zambia. We conducted a repeated cross-sectional study in 39 public health facilities in four districts in Zambia from September 2021 to September 2022. Pregnant women who were aged 15–49 years were enrolled during their first ANC visit. Every month, ~20 women per facility were interviewed during individual HIV counseling and testing. We estimated vaccine uptake as the proportion of eligible participants who self-reported having received the COVID-19 vaccine. A total of 9,203 pregnant women were screened, of which 9,111 (99%) were eligible and had vaccination status. Of the 9,111 included in the analysis, 1,818 (20%) had received the COVID-19 vaccine during the study period, with a trend of increasing coverage with time (0.5% in September 2020, 27% in September 2022). Conversely, 3,789 (42%) reported not being offered a COVID-19 vaccine. We found that women aged 40–49 years, had no education or attained some primary school education, were not employed, and had prior COVID-19 infection were significantly associated with vaccine uptake. COVID-19 vaccine uptake among pregnant women was lower than estimates from the general population (27% across the four districts in September 2022), pointing to missed opportunities to protect this high-risk group. ANC visits were a viable point for conducting COVID-19 surveillance. Incorporating the vaccine as part of the routine ANC package might increase coverage in this group.
Introduction
The coronavirus disease 19 (COVID-19) pandemic has presented unprecedented challenges globally, affecting all aspects of life, including the health of vulnerable populations. As of October 2023, there were approximately 771,151,224 confirmed cases of COVID-19 and 6,960,783 deaths, globally [1]. Due to immunologic and physiologic changes, pregnant women are considered a high-risk group for COVID-19 [2–4]. Those with SARS-CoV-2 infection are more susceptible to severe disease and mortality as compared to non-pregnant women [5,6]. Although COVID-19 vaccines are recommended for pregnant women to reduce the risk of severe COVID-19 disease and prevent adverse obstetric outcomes, vaccine hesitancy–a delay or refusal of safe vaccines–is reported to be higher among pregnant women than in the general population [7,8]. While survey results from Iran show that more than two-fifths of participants accepted receiving any vaccine [9], fewer than one-third of pregnant women were interested in receiving the COVID-19 vaccine in Cameroon [10].
Understanding the factors influencing COVID-19 vaccine uptake among women attending antenatal care (ANC) is crucial for mitigating the impact of the virus on maternal and child health. Previous studies have shown variations in COVID-19 vaccine uptake among different populations, highlighting the influence of various factors such as age, education level, and socioeconomic status [11–13]. However, there is a limited understanding of these factors, particularly among pregnant women, who have distinct healthcare needs and considerations. Vaccination not only protects pregnant women from severe illness but also offers potential benefits to their unborn children through passive immunity [14,15]. By identifying risk factors and barriers to vaccine uptake, we can develop tailored interventions that address specific concerns and enhance vaccine acceptance, ultimately safeguarding the health and well-being of both expectant mothers and their babies.
In Zambia, COVID-19 vaccines were first made available in April 2021. The voluntary vaccination exercise initially targeted people above the age of 18 years and prioritized frontline health workers, people involved in the maintenance of core societal function, people with underlying conditions, and those in congregate settings. Despite initial uncertainties about the safety of COVID-19 vaccines in pregnancy, the Ministry of Health (MoH) updated guidelines in July 2021 to include vaccination for pregnant women [16]. Vaccine uptake among pregnant women in Zambia remains unknown as available evidence is based on results of vaccine acceptance among the general population [17,18].
This study aims to estimate COVID-19 vaccine uptake and associated risk factors among pregnant women attending their first ANC visits at public health facilities in Chadiza, Chipata, Chongwe, and Lusaka districts of Zambia during the height of the third (Delta variant) and fourth (Omicron variant) waves. We also sought to assess the feasibility of using routine ANC to track trends and factors associated with the uptake of vaccines. This study contributes to a broader knowledge base on COVID-19 vaccination by focusing on specific population subsets, which can provide useful guidance on developing targeted communication campaigns, healthcare policies, and interventions aimed at improving vaccine uptake and reducing disparities within this vulnerable group.
Materials and methods
Study design
We conducted repeated cross-sectional surveys on SARS-CoV-2 seroprevalence and vaccination coverage in pregnant women attending their first ANC visit in the maternal, newborn, and child health (MNCH) clinics. This analysis looks at interview data on COVID-19 vaccination among pregnant women enrolled into the broader seroprevalence study. Additional methodological details of the study are described elsewhere [19]. In Zambia, the health system is classified into three categories: 1) community-level health posts and health centers; 2) Level 1-provincial and district hospitals and 3) Level 2-central-level specialized hospitals [20]. Initially, 40 health facilities were drawn from a list of 131 health facilities with an average monthly total of 7, 530 first ANC visits. We selected 40 public and one private health facility in four districts (Chadiza, Chipata, Chongwe, and Lusaka). However, due to the disinclination of staff to initiate study activities at the private hospital, pregnant women were not enrolled. Participant recruitment was therefore restricted to 39 study sites (all public health facilities) were stratified according to location and selected using probability proportional to facility size based on each health facility’s monthly mean number of pregnant women attending their first ANC visit based on historic monthly ANC attendance during the 2020 fiscal year.
Study activities were incorporated into routine ANC services and leveraged infrastructure and human resources in the MNCH departments at public health facilities in the Centre for Infectious Disease Research in Zambia (CIDRZ) supported districts (i.e., Chipata, Chongwe and Lusaka districts). In Chadiza district, study activities were supported by PATH Zambia and incorporated into an ANC-based malaria surveillance pilot study. Health facility staff (CHWs, midwives, phlebotomists and research assistants were trained in the study protocol, laboratory procedures, and human subject protection. Participant recruitment was initiated on 4 September 2021 and was concluded on 30 September 2022 for the CIDRZ-supported study sites. Initiation of participant enrolment was delayed at study sites in Chadiza and only started on 23 September 2021 and ended on 31 July 2022.
Sample size
The sample size calculation for the main study was based on each health facility’s monthly ANC attendance and a confidence interval (CI) that reflected the bounds of the true seroprevalence of COVID-19 among pregnant women (and not the overall pregnant women or general populations). In rural areas where prevalence was expected to be low, a sample size of 200 women produced a 95% CI half-width of 2.4% for a low seroprevalence of 3%. Where prevalence was expected to be higher (i.e., urban areas), a sample size of 200 women produced a 95% CI half-width of 7.0% for 50% seroprevalence. An average of 6,378 pregnant women were expected to access ANC in the four selected districts. Therefore, a sample size of 200 women per district per month was estimated to be sufficient to detect a range of estimated proportions of SARS-CoV-2 seroprevalence if recruited evenly at each month every month. If monthly recruitment target was met, the study site paused participant recruitment and reconvened at the start of the following month.
Recruitment and enrollment of study participants
A CHW provided information on the ANC COVID-19 surveillance study during sensitization meetings in the community and group counseling sessions at the health facility. Once every week, on a day that each health facility provided routine ANC services for first-time attendees, CHWs conveniently identified pregnant women and screened them for eligibility to participate in the study. Pregnant women who were aged 15–49 years, confirmed to be pregnant, registered as a first ANC attendee, and provided consent, were enrolled in the study. Potential participants were screened for active SARS-CoV-2 infection using a paper-based checklist of symptoms. Those who showed one or more symptoms were excluded and referred to a SARS-CoV-2 testing center to be managed according to national guidelines if they had a positive test result. At each study site, trained midwives shared an information sheet with eligible participants and obtained consent from up to 20 pregnant women per month, cumulatively enrolling 200 pregnant women per district.
Data collection and management
At the time of providing routine ANC tasks such as performing physical examinations, administering antimalaria prophylaxis, prescribing prenatal vitamins and drawing blood samples for routine HIV and syphilis testing, a midwife administered an electronic questionnaire to consented participants. The study questionnaire was designed in Open Data Kit (ODK)–a free, open-source suite of software tools that facilitates the collection, management and use of data using portable mobile devices with Internet connectivity [21]. To ensure confidentiality and privacy, a unique identification number was assigned to individual participants during data collection. The questionnaire was uploaded onto a tablet which was handled by trained midwives and research assistants.
To estimate COVID-19 vaccine uptake and associated risk factors among pregnant women attending their first ANC visits, the study collected participants’ vaccination status, socio-demographic characteristics, self-reported past SARS-CoV-2 infection, and exposure. SARS-CoV-2 preventive measures and behavior such as the proper wearing of masks were observed and captured in the questionnaire. In addition, the study determined the acceptability and feasibility of monitoring COVID-19 vaccination uptake and hesitancy among pregnant women attending ANC. Acceptability was defined as a multi-faceted construct that reflects the extent to which the healthcare providers and pregnant women enrolled in the study considered the SARS-CoV-2 surveillance to be appropriate and was measured by its perceived effectiveness–the extent to which the SARS-CoV-2 surveillance was perceived as likely to achieve its purpose [22]. Feasibility was defined as the study’s appropriateness for further testing to determine relevance and sustainability [23] and was measured by recruitment rates, length of time from initiation to completion of the targeted number of participants, and number of eligible participants required to recruit sample size.
Initially, participants were asked only if they had been vaccinated or not. The questionnaire was updated in May 2021 to gather information about whether the participants had been offered a vaccine in addition to whether they had accepted it. To capture vaccination status, pregnant women were asked if they had received a COVID-19 vaccination before their ANC visit and enrolment into the study. For those who said they were unvaccinated, additional information was collected about the reasons for not being vaccinated. Response options included concerns about getting COVID-19, vaccine availability, efficacy and safety, religious beliefs, pregnancy, breastfeeding, and side effects on the unborn baby. Overall, 3,655 participants were recruited before the updated questionnaire was implemented, meaning they only had a binary vaccination status collected. COVID-19 vaccination status was self-reported, although study staff attempted to verify information from vaccination cards if participants had them available on the day of their ANC visit.
Data were reviewed on a scheduled basis and missing variables were communicated to health facility staff and research assistants to attempt follow-up and completion. All completed questionnaires were uploaded onto a central server individually or batched. Other data were documented in study-specific logs and registers. Additionally, COVID-19 vaccine coverage data in the general population in the four implementation districts were obtained from the Zambia MoH so that a comparison could be made to the estimates from this study. General population vaccination data were extracted from the national District Health Information System (DHIS) 2-COVAX tracker–a global digital health data toolkit that facilitated the capture of individual-level COVID-19 data of investigated cases; including vaccination status [24].
Statistical analysis
Background characteristics were summarized using frequency and proportions for categorical variables, and median and interquartile range for continuous variables. We estimated the prevalence of COVID-19 uptake as the proportion of pregnant women who received (i.e., reported or verified by vaccination card) COVID-19 vaccine and extracted monthly vaccine uptake data in the general population. The incremental vaccine uptake in the study population was calculated to estimate the overall change in vaccine uptake over time. The results were compared with the cumulative vaccine uptake in the general population. The corresponding 95% CIs were adjusted for the clustering of participants within a health facility. We used a mixed-effect generalized linear model to identify factors independently associated with uptake. To allow for variation in the secular trend across clusters (i.e., health facilities), we extended the random-effects components to allow a random interaction between time and health facilities (i.e., we generated a new variable by combining two dimensions of health facilities and time). P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using Stata 18 MP (StataCorp, College Station, TX, USA).
Ethics considerations
This study was reviewed and approved by the National Health Research Authority (NHRA), a statutory body under the Zambian MoH, and following the US Centers for Disease Control and Prevention (CDC) human research protection procedures as the CDC investigators were not engaged (i.e., did not interact with human subjects or have access to identifiable data or specimens for research purposes). It was also reviewed and approved, particularly for the Chadiza sites, by the University of Zambia Biomedical Research Ethics Committee (UNZABREC) and PATH’s Research Ethics Committee (PATH REC). Information sheets for participants were developed and translated into two local languages. Individual written consent and assent were obtained from adults and minors aged below 18 years, respectively, in a language of their choice and a copy of an information sheet was offered to the participant after consenting. Assent was obtained from minors only after consent was given by their parent(s) or guardian(s). Illiterate adults were required to provide consent in the presence of an impartial witness.
Results
Study enrollment cascade
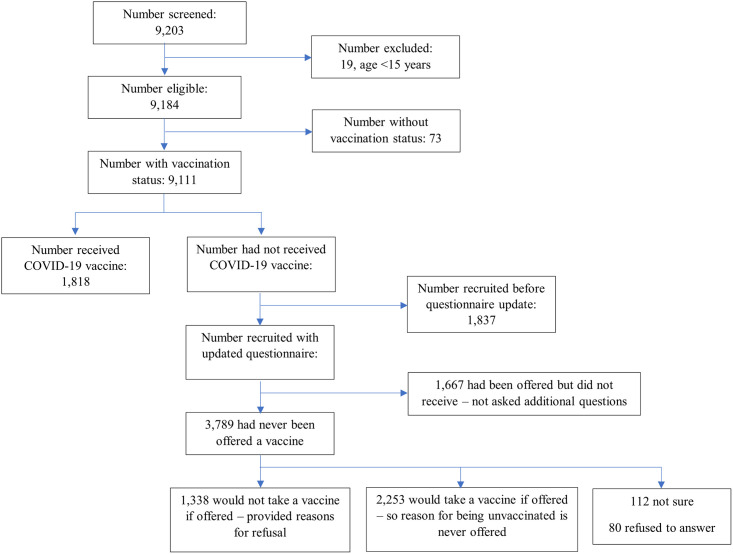
During the study period, a total of 9,203 pregnant women were approached to participate in the study. Of these, 19 (0.2%) were ineligible and excluded due to their age (Fig 1). A further 73 did not have vaccination status, giving a total of 9,111 included in the analysis. Overall, 1,818 (20.0%) pregnant women were vaccinated against COVID-19 by the time of their first ANC visit and, of 7,293 unvaccinated women, 5,456 had additional vaccination information collected (i.e., were administered the updated questionnaire). At least 1,667 (30.6%) had been offered a vaccine but refused (Fig 1) and provided reasons for refusal.
Fig 1. Flow chart of participant recruitment.
Background characteristics and COVID-19 uptake
The median age of participants was 24 years (interquartile range [IQR]: 20–30) and over half (4,944 [54.3%]) of participants were aged between 20–29 years (Table 1). Half of the participants (4,557) had attained some or completed primary school education while half were employed either formally or informally. Almost all, (8,917 [95%]) participants had never tested for COVID-19 before their ANC visit (Table 1). An estimated 4% of pregnant women reported ever being in contact with a COVID-19-infected individual, either within and/or outside their household. Proper use of masks was observed in only 3,492 (38.3%) of the participants. Seven hundred ninety-one (8.7%) of the pregnant women either tested positive for HIV or had a known positive HIV status on the day of their visit. Of the 9,111 participants with a vaccination status, 1,818 (20%) reported having received a COVID-19 vaccine while 3,789 (41.6%) of women said they had never been offered a vaccine (Fig 1).
Table 1. COVID-19 vaccine uptake among pregnant women attending 1st ANC visit by socio-demographic characteristics (n = 9111).
| Charecteristics | Total number of women N (% of total) |
|---|---|
| 9111 (100%) | |
| District | |
| Chadiza | 1915 (21.0) |
| Chipata | 2379 (26.1) |
| Chongwe | 2494 (27.4) |
| Lusaka | 2323 (25.5) |
| Age group | |
| Median (IQR) | 24 (20–30) |
| 15–19 | 1736 (19.1) |
| 20–29 | 4944 (54.3) |
| 30–39 | 2003 (22.0) |
| 40–49 | 232 (2.5) |
| Missing | 196 (2.2) |
| Education level | |
| Some or completed secondary education | 3783 (41.5) |
| Some or completed primary education | 4557 (50.0) |
| No education | 771 (8.5) |
| Employment status | |
| Employed | 4574 (50.2) |
| Unemployed | 4092 (44.9) |
| Other | 445 (4.9) |
| Ever tested positive for COVID-19 | |
| No | 8917 (97.9) |
| Household contact positive for COVID-19 | |
| No | 8800 (96.6) |
| Contact with anyone with COVID-19 | |
| No | 8590 (94.3) |
| Mask wearing frequency | |
| Everytime or most of the time | 3553 (39.0) |
| About half the time | 1009 (11.1) |
| Rarely | 1910 (21.0) |
| Never | 549 (6.0) |
| Missing | 2090 (22.9) |
| Currently wearing mask† | |
| Mask present and properly worn | 3492 (38.3) |
| Mask present but not properly worn | 2118 (23.2) |
| No mask | 1411 (15.5) |
| Missing | 2090 (22.9) |
| HIV status | |
| Negative | 8026 (88.1) |
| 1. Observations missing vaccine status were excluded, N = 73 (0.8%) | |
| † | |
Factors independently associated with vaccine uptake
In a multivariable mixed-effects generalized linear model, we found that age group, education, employment status, ever testing positive for COVID-19, and HIV infection were all significantly associated with COVID-19 vaccine uptake (Table 2). Increasing age was associated with increased COVID-19 vaccination uptake. We observed a lower proportion of COVID-19 vaccination among pregnant women who had no education ((aPR): 0.79, 95% CI: 0.62–1.00; P = 0.007) or had attained some or completed primary education (aPR): 0.86 95%CI: 0.75–0.97; P = 0.001) as compared to those who had some or completed secondary education. Unemployed pregnant women had lower vaccination prevalence ((aPR): 0.69, 95% CI: 0.58–0.82; P = <0.001) as compared to pregnant women who were employed. Additionally, pregnant women who had ever tested positive for COVID-19 had 1.70 times (95% CI: 1.31–2.21; P = <0.001) greater likelihood of receiving a vaccine compared to those who had not tested positive for COVID-19 in the past. Similarly, pregnant living with HIV were 1.18 times (95% CI:1.02–1.37; P = 0.026) more likely to be vaccinated compared to those who were not infected with HIV.
Table 2. Factors associated with vaccine coverage among pregnant women attending first ANC visit(n = 8,750).
| Characteristics | Total number of women (n) |
COVID-19 vaccine uptake n (row %) |
95% CI | Crude PR (95% CI) |
P-value | Overall P-value |
Adjusted PR (95% CI) |
P-value | Overall P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age group | |||||||||
| 15–19 | 1736 | 275 (15.8) | 12.8–19.5 | 0.72 (0.62–0.84) | ***<0.001 | <0.001 | 0.78 (0.66–0.92) | ***<0.001 | 0.019 |
| 20–29 | 4944 | 989 (20.0) | 15.7–25.1 | 1 | 1 | ||||
| 30–39 | 2003 | 467 (23.3) | 17.7–30.1 | 1.12 (1.01–1.25) | **0.028 | 1.06 (0.94–1.18) | 0.346 | ||
| 40–49 | 232 | 56 (24.1) | 17.4–32.4 | 1.11 (0.86–1.42) | 0.419 | 1.03 (0.81–1.31) | 0.797 | ||
| Education level | |||||||||
| Some or completed secondary education | 3783 | 796 (21.0) | 16–27.2 | 1 | <0.001 | 1 | 0.014 | ||
| Some or completed primary education | 4557 | 855 (18.8) | 14.5–23.9 | 0.85 (0.75–0.96) | ***0.009 | 0.86 (0.75–0.97) | ***0.001 | ||
| No education | 771 | 167 (21.7) | 14.4–31.3 | 0.87 (0.71–1.06) | 0.171 | 0.79 (0.62–1.00) | ***0.007 | ||
| Employment status | |||||||||
| Employed | 4574 | 1130 (24.7) | 19.8–30.4 | 1 | <0.001 | 1 | <0.001 | ||
| Unemployed | 4092 | 589 (14.4) | 10.5–19.4 | 0.65 (0.56–0.76) | ***<0.001 | 0.69 (0.58–0.82) | ***<0.001 | ||
| Other | 445 | 99 (22.2) | 14.4–32.7 | 0.85 (0.71–1.02) | *0.076 | 0.88 (0.74–1.05) | 0.127 | ||
| Ever tested positive for COVID-19 | |||||||||
| No | 8917 | 1725 (19.3) | 15.4–23.9 | 1 | <0.001 | 1 | <0.001 | ||
| Yes | 194 | 93 (47.9) | 38.5–57.5 | 2.01 (1.50–2.70) | ***<0.001 | 1.70 (1.31–2.21) | ***<0.001 | ||
| Household contact positive for COVID-19 | |||||||||
| No | 8800 | 1725 (19.6) | 15.6–24.3 | 1 | <0.001 | ||||
| Yes | 301 | 91 (30.2) | 20.9–41.5 | 1.46 (1.11–1.94) | ***0.007 | ||||
| Contact with anyone with COVID-19 | |||||||||
| No | 8590 | 1639 (19.1) | 15.1–23.8 | 1 | <0.001 | ||||
| Yes | 357 | 106 (29.7) | 20.2–41.3 | 1.47 (1.11–1.97) | ***0.008 | ||||
| Mask wearing frequency | |||||||||
| Everytime or most of the time | 3553 | 834 (23.5) | 17.8–30.3 | 1 | <0.001 | ||||
| About half the time | 1009 | 282 (27.9) | 20.8–36.5 | 0.96 (0.81–1.12) | 0.583 | ||||
| Rarely | 1910 | 437 (22.9) | 17.5–29.3 | 0.71 (0.57–0.88) | ***0.002 | ||||
| Never | 549 | 142 (25.9) | 18–35.6 | 0.80 (0.64–1.00) | **0.046 | ||||
| Currently wearing mask | |||||||||
| Mask present and properly worn | 3492 | 933 (26.7) | 20.8–33.6 | 1 | <0.001 | ||||
| Mask present but not properly worn | 2118 | 402 (19.0) | 14.3–24.7 | 0.71 (0.59–0.85) | ***<0.001 | ||||
| No mask | 1411 | 360 (25.5) | 20.5–31.3 | 0.83 (0.71–0.97) | **0.022 | ||||
| HIV status | |||||||||
| Negative | 8026 | 1617 (20.1) | 16–25 | 1 | <0.001 | 1 | 0.028 | ||
| Positive | 791 | 174 (22.0) | 17.9–26.8 | 1.27 (1.12–1.45) | ***<0.001 | 1.18 (1.02–1.37) | **0.026 |
Vaccine uptake over time
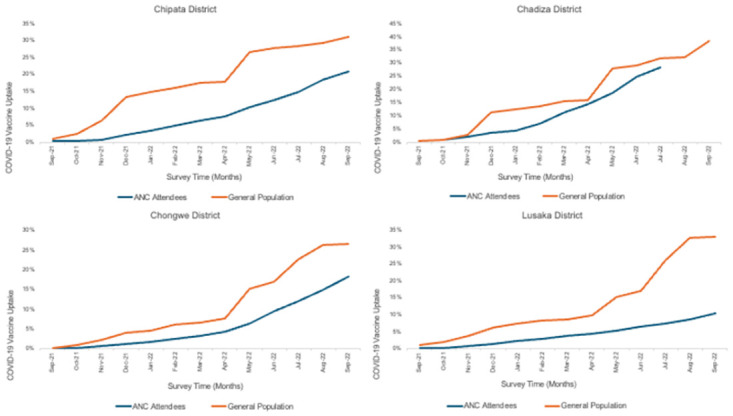
Overall, COVID-19 vaccination uptake among pregnant women was at 0.5% at the beginning of the study in September 2021 and had increased to 27% by September 2022 (Fig 2). When point estimates were compared by district, uptake varied between 2.8% and 17.4% in September 2021 and 21.8% to 29% in September 2022. Throughout implementation, a steady increase in vaccine uptake was observed among participants in Chadiza (Fig 2). COVID-19 vaccine coverage among women at first ANC visits was lower (p<0.001) than coverage in the general population in these districts (Fig 3).
Fig 2. Vaccine uptake among pregnant women by facility (September 2021 to September 2022 (n = 9,111).
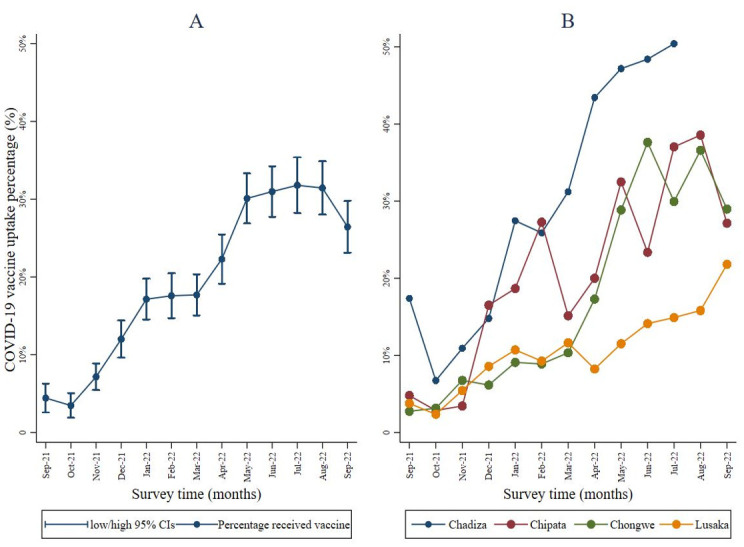
Fig 3. COVID-19 vaccine coverage estimates: Pregnant women attending first ANC visit versus co general population.
Reasons for not taking the COVID-19 vaccine if offered
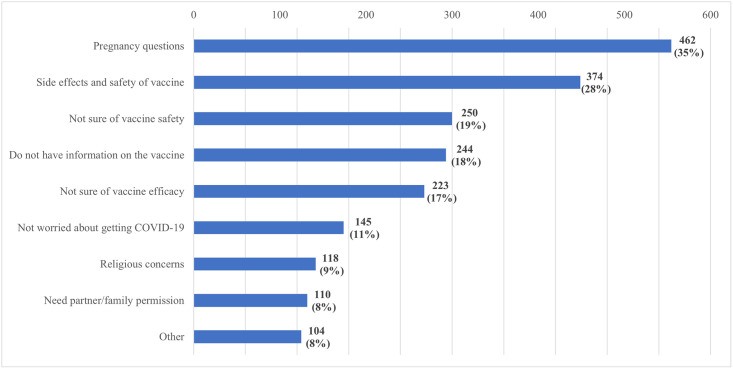
Of the 3,789 pregnant women who reported never having been offered a vaccine, 1,338 (35.3%) said that they would not take a COVID-19 vaccine even if they were offered and provided reasons for refusal (Fig 4). Pregnancy safety concerns and vaccine effects were the most common reasons for refusing to take the vaccine (462 [35%] and 374 [28%], respectively). An additional 250 (19%) would not take it because of uncertainties surrounding its safety, and 244 (18%) of the participants would not take a vaccine because they did not have sufficient information about the vaccine. The least cited reasons for refusal to take a COVID-19 vaccine were religious concerns and the need to seek permission from a partner or family (9% and 8%, respectively).
Fig 4. Reasons for not taking vaccine if offered.
Discussion
Most pregnant women in the four districts of Zambia were not vaccinated against COVID-19, despite being at high risk for severe outcomes. Vaccination coverage increased over the study period and was higher in all four districts compared to contemporaneous coverage estimates of the general population. Despite evidence that COVID-19 vaccines are safe in pregnancy, only ten of the 54 African countries were recommending COVID-19 vaccines for pregnant women as of February 2022 [20]. In Zambia, COVID-19 vaccines were first made available for the general population in April 2021 [25] but, vaccine coverage remains well below the World Health Organization’s (WHO) target to vaccinate 70% of the world’s population by mid-2022 [26]. Given initial uncertainty about vaccine safety in pregnancy, the MoH delayed recommending COVID-19 vaccination for pregnant women until July 2021, which might have contributed to vaccine hesitancy in this group. Our findings correlate with a study conducted in Ethiopia in which only 14.4% of the participants had received at least one dose of COVID-19 vaccines by March 2022 [27]. Similarly, vaccine uptake in Cameroon was low (31%) but, uptake did not differ between the pregnant and general population [28].
Low vaccination rates in low-and-middle-income (LMICs) African countries are partly due to the inequitable distribution of vaccines caused by constrained financial resources to support competing priorities in underdeveloped health systems and bottlenecks in the supply chain which may lead to delayed vaccine rollout [29,30]. Studies have also reported that pregnant women did not receive COVID-19 vaccines due to their limited availability in Africa, perhaps an indication of the inability of pharmaceutical companies to manufacture vaccines in Africa, and the compromised quality of the vaccines manufactured by international pharmaceutical companies for the African market [28,31]. COVID-19 vaccination uptake is also hampered by the low-risk perception of the pandemic, concern about adverse vaccine effects, low vaccination awareness, and low acceptance, intention and willingness to get vaccinated [32,33].
COVID-19 vaccine hesitancy occurs against a backdrop of social-cultural complexities, poor government response in demystifying social and traditional myths and theories, and poor community involvement in public health measures [34]. Pregnant women in our study reported not having adequate information about the COVID-19 vaccine and were therefore concerned about the side effects of the vaccine on their pregnancy, vaccine safety, and efficacy, findings which were similar to an earlier study by Dinga et al. [35] In our study, religious affiliation was not associated with vaccine hesitancy, and neither was the need to seek permission for vaccination from a family member or partner.
Most unvaccinated pregnant women recruited into this study were willing to accept a COVID-19 vaccine if it were offered to them. However, they reported that a vaccine had not been offered to them, potentially pointing to insufficient effectiveness of mass media campaigns (which were widespread during this period in Zambia) and individual outreach during routine health care visits in demand creation. Pregnant women who expressed willingness to receive a vaccine on the day of their first ANC visit were referred to a designated center to receive a vaccine even though their intake was not documented by the study.
Several factors associated with low vaccine uptake were identified, including; increasing age, low educational level, and HIV infection. In studies from other African countries, COVID-19 vaccination uptake has varied across age groups [33,36,37]. In our study, older pregnant women were more likely to have received a COVID-19 vaccine compared to younger women. This increase in uptake could be attributed to the government targeting older persons for receipt of the vaccine or increased understanding of risks and their prevailing knowledge of how the COVID-19 disease impacts older people given their own advancing age [27]. COVID-19 risk perception and behavior can be influenced by a higher level of education among populations [38]. While some studies have not observed any associations between educational level and COVID-19 vaccine uptake [39], our findings contradict those found in Ethiopia where vaccine uptake was lower among participants who had a higher level of education and had attended college or university possibly due to their increased access to information and awareness of the potential side effects and safety [27,40].
Risk perception is a critical factor in vaccine decisions [41]. In this study, pregnant women who were not employed were less likely to receive a COVID-19 vaccine compared to those who were engaged in formal and informal work. This reduced uptake among the unemployed could be attributed to their perceived lowered risk of infection as they had limited contact with individuals outside of their household, particularly when lockdown regulations were strictly enforced. Additionally, a history of ever testing positive for COVID-19 influenced pregnant women’s decision to get a COVID-19 vaccine. These results corroborate with findings from studies in Indonesia and the UK which found that a higher perceived risk of COVID-19 infection was associated with higher acceptance of the vaccine [42–44]. Moreover, in Indonesia and the UK, receiving the vaccine was perceived to be vital for protecting oneself and preventing disease transmission.
Similarly, a higher proportion of pregnant women who had contact with a known or suspected COVID-19 patient within or outside their household were more likely to receive a COVID-19 vaccine. However, a majority of the pregnant women in this study indicated that they did not know about being in contact with a positive COVID-19 case. This could be because many cases of COVID-19 are asymptomatic or, that symptoms are mild and nonspecific, and people did not get tested, or due to the limited availability of COVID-19 testing in Zambia. A household study in six districts in Zambia during the first wave estimated about one confirmed COVID-19 case for every 92 infections [45], and in this study there continued to be substantial case ascertainment gaps [19].
COVID-19 vaccine hesitancy has been investigated among people living with HIV (PLWHIV). In Zambia, the MOH targeted PLHIV for COVID-19 vaccinations given their elevated risk of severe disease and substantial investments and achievements in HIV care in the country [46]. In our study, we assessed vaccine hesitancy among HIV-infected pregnant women and found a high degree of COVID-19 vaccine uptake among HIV-infected pregnant women as compared to those who were not infected with HIV. These results suggest high levels of vaccine acceptability and accessibility among pregnant women living with HIV [47]. Our results are similar to those reported in Nigeria where one in five women living with and at risk of HIV had a lower likelihood of being vaccine-hesitant [48].
For this study, data collected over 13 months shows that there was a modest increase in vaccination uptake among study participants from 0.4% at the beginning of the study in September 2021 to 2.6% at the end of the study in September 2022. This is in contrast to results from a meta-analysis that showed a higher rise in pooled COVID-19 vaccine acceptance rates in Ethiopia from 14.1% in 2022 to 42.46% in 2023 [27,49]. Notably, in this study, a proportion of the pregnant women who reported receiving a vaccine may have been vaccinated before they conceived. The gradual increase in the proportion of vaccinated pregnant women in our study could be attributed to an increase in the number of vaccine doses received by the MoH over time and their embarking on an ambitious 10-day COVID-19 vaccination campaign to reach the 70% eligible population in May 2022 [50].
Strengths and limitations of the study
A major strength of this study was the leveraging of infrastructure and human resources in the MNCH departments at public health facilities to enroll pregnant women seeking routine ANC services. Healthcare providers and community-based volunteers already providing ANC services at the study sites were engaged to assist with carrying out study procedures, easing the study implementation process, reducing costs, and gaining participants’ confidence. The study was able to access a longitudinal selection of pregnant women over time and triangulate a limited amount of routine data collected from part of the national DHIS2-COVAX tracker with data collected from study participants from participants to validate the findings. A limitation of the study is that the analysis does not include vaccine data of almost 1,837 participants as they were recruited into the study before the vaccine follow-up questions were appended to the questionnaire. Additionally, the districts were purposefully selected so the findings might not be generalizable to all of Zambia.
Conclusion
Using pregnant women seeking routine ANC in public health facilities to assess COVID-19 vaccination status, uptake over time, intent, and acceptance of vaccine is acceptable and feasible in a low-resource setting. Overall, this study found a low uptake of COVID-19 vaccines among pregnant women attending first ANC in rural and urban settings, indicating a need to reinforce vaccine uptake efforts to prevent severe disease and adverse outcomes among these vulnerable populations. The leveraging of existing infrastructure and human resources provided an easy, sustainable platform for routinely monitoring the COVID-19 vaccine, and consequently helped to understand vaccine hesitancy among pregnant women. This is critical for devising key vaccination messages and can facilitate the design and adoption of mass vaccination strategies for minority groups, reduce vaccine hesitancy, and subsequently increase vaccine uptake. Demand generation for vaccines might be more effective if done during routine health services and targeted at individuals (i.e., inviting pregnant women to get vaccinated). This would be possible by incorporating the vaccine as part of the routine ANC package with healthcare providers offering the vaccine to increase coverage among pregnant women. Community health workers must strive to provide information about COVID-19 vaccinations during health education in ANC clinics and community sensitization meetings. The spread of the pandemic and the emergence of new COVID-19 variants can be minimized if vaccine development, equitable distribution, and timely access to the COVID-19 vaccine are a global priority.
Acknowledgments
The authors would like to acknowledge the Ministry of Health, Provincial Health Directors–Eastern and Lusaka provinces, District Health Directors–Chadiza, Chipata, Chongwe, and Lusaka Districts, and the National Health Research Authority for their collaboration at the preparatory stage of the study. We also wish to acknowledge the healthcare workers and community health workers (CHWs) at the health facilities for their participation and support during the implementation of study activities. Our special gratitude goes to the Research Assistants on the study–Beatrice Chibundi, Chikatizyo Mhango, Linda Phiri, and Patricia Z. Shabalu–for providing oversight for the implementation of study activities at each of the 39 health facilities.
Data Availability
The data that support the findings of this study are openly available in Figshare at https://doi.org/10.6084/m9.figshare.25974994.v3.
Funding Statement
Assessing SARS-CoV-2 Seroprevalence during Routine Antenatal Care Visits in Zambia” was supported by the US Centers for Disease Control and Prevention (CDC) under the terms of award number NU2GGH002251. The award was granted to IS. The terms of this arrangement have been reviewed and approved by the Centers for Disease Control and Prevention in accordance with its policy on objectivity in research. The sponsor’s website can be accessed via https://www.cdc.gov/index.html. The funder did not play any role in designing the study, data collection and analysis, decision to publish, and, preparation for the manuscript.
References
- 1.World Health Organization. Global Situation. 2023 [cited 2023 Jul 13]. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
- 2.Kolb JJ, Radin JM, Quer G, Rose AH, Pandit JA, Wiedermann M. Prevalence of Positive COVID-19 Test Results Collected by Digital Self-report in the US and Germany. JAMA Netw Open [Internet]. 2023. Jan 31 [cited 2023 May 28];6(1):e2253800. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2800848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S, Siddique R, Bai Q, Shabana, Liu Y, Xue M, et al. Coronaviruses disease 2019 (COVID-19): Causative agent, mental health concerns, and potential management options. Journal of Infection and Public Health [Internet]. 2020. Dec [cited 2023 May 28];13(12):1840–4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1876034120305761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sh. Nur MA, Dahie HA, Hassan NA, Garba B, Adam MH, Mohamoud JH, et al. Seroprevalence of SARS-CoV-2 virus antibodies and sociodemographic features of pregnant women in Mogadishu, Somalia: a cross-sectional survey study. BMJ Open [Internet]. 2022. Jun [cited 2023 May 28];12(6):e059617. Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2021-059617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. COVID Data Tracker [Internet]. Atlanta, GA: US Department of Health and Human Services; 2023 [cited 2023 May 7]. https://COVID.cdc.gov/COVID-data-tracker/#datatracker-home.
- 6.Dehingia N, Raj A. Sex differences in COVID-19 case fatality: do we know enough? The Lancet Global Health [Internet]. 2021. Jan [cited 2023 May 28];9(1):e14–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X20304642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanis P, Vraka I, Siskou O, Konstantakopoulou O, Katsiroumpa A, Kaitelidou D. Uptake of COVID-19 Vaccines among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines (Basel). 2022. May 12;10(5):766. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9145279/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald NE, SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015. Aug 14;33(34):4161–4. Available from: https://pubmed.ncbi.nlm.nih.gov/25896383/. [DOI] [PubMed] [Google Scholar]
- 9.Firouzbakht M, Sharif Nia H, Kazeminavaei F, Rashidian P. Hesitancy about COVID-19 vaccination among pregnant women: a cross-sectional study based on the health belief model. BMC Pregnancy Childbirth [Internet]. 2022. Dec [cited 2023 Aug 9];22(1):611. Available from: https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-022-04941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunawardhana N, Baecher K, Boutwell A, Pekwarake S, Kifem M, Ngong MG, et al. COVID-19 vaccine acceptance and perceived risk among pregnant and non-pregnant adults in Cameroon, Africa. Sallam M, editor. PLoS ONE [Internet]. 2022. Sep 13 [cited 2023 Jun 28];17(9):e0274541. Available from: https://dx.plos.org/10.1371/journal.pone.0274541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Huang Y. COVID-19 Vaccine Hesitancy: The Role of Socioeconomic Factors and Spatial Effects. Vaccines [Internet]. 2022. Feb 24 [cited 2023 Jul 13];10(3):352. Available from: https://www.mdpi.com/2076-393X/10/3/352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson L, Dziva Chikwari C, Simms V, Tembo M, Mahomva A, Mugurungi O, et al. Addressing sociodemographic disparities in COVID-19 vaccine uptake among youth in Zimbabwe. BMJ Glob Health [Internet]. 2023. Jul [cited 2023 Jul 13];8(7):e012268. Available from: https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2023-012268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adedeji-Adenola H, Olugbake OA, Adeosun SA. Factors influencing COVID-19 vaccine uptake among adults in Nigeria. Yunusa I, editor. PLoS ONE [Internet]. 2022. Feb 24 [cited 2023 Jul 13];17(2):e0264371. Available from: https://dx.plos.org/10.1371/journal.pone.0264371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centre for Disease Control and Prevention. Pregnancy and Breastfeeding: What You Need to Know. 2023. Pregnancy or Breastfeeding. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html#:~:text=Antibodies%20made%20after%20pregnant%20people,the%20mother%20to%20her%20baby.
- 15.Lamptey E, Senkyire EK, Banoya MT, Yaidoo S. COVID-19 vaccination in pregnancy: A review of maternal and infant benefits. Gynecology and Obstetrics Clinical Medicine [Internet]. 2022. Sep [cited 2023 Jul 13];2(3):124–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2667164622000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health. Statment on COVID-19 in Lusaka, Zambia, [Internet]. Facebook. 2021 [cited 2023 Nov 8]. https://www.facebook.com/mohzambia/photos/a.773733439467982/1949793125195335/?type=3.
- 17.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ [Internet]. 2020. Jun 8 [cited 2023 May 28];m2107. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S, Bose D, Alimohammadi S, et al. Clinical characteristics and outcomes of pregnant women with COVID‐19 and comparison with control patients: A systematic review and meta‐analysis. Rev Med Virol [Internet]. 2021. Sep [cited 2023 May 28];31(5):1–16. Available from: https://onlinelibrary.wiley.com/doi/10.1002/rmv.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilmann E, Tembo T, Fwoloshi S, Kabamba B, Chilambe F, Kalenga K, et al. Trends in SARS-CoV-2 seroprevalence among pregnant women attending first antenatal care visits in Zambia: A repeated cross-sectional survey, 2021-2022. Siqueira AM, editor. PLOS Glob Public Health [Internet]. 2024. Apr 3 [cited 2024 Apr 11];4(4):e0003073. Available from: https://dx.plos.org/10.1371/journal.pgph.0003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joi P. GAVI: The Vacicne Alliance. 2022 [cited 2023 Jun 28]. COVID-19 greatly increases risks to pregnant women: largest sub-Saharan study. https://www.gavi.org/vaccineswork/covid-19-greatly-increases-risks-pregnant-women-largest-sub-saharan-study.
- 21.Get ODK Inc. ODK Collect [Internet]. 2024. https://docs.getodk.org/collect-intro/.
- 22.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res [Internet]. 2017. Dec [cited 2023 Nov 20];17(1):88. Available from: http://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-017-2031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How We Design Feasibility Studies. American Journal of Preventive Medicine [Internet]. 2009. May [cited 2023 Dec 4];36(5):452–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0749379709000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DHIS2. DHIS2 COVID-19 Vaccine Delivery Toolkit Strengthen national immunization systems, introduce fit-for-purpose digital solutions and leverage local expertise for equitable COVID-19 vaccine delivery [Internet]. 2023 [cited 2023 Nov 7]. https://dhis2.org/covid-vaccine-delivery/.
- 25.World Health Organization. World Health Organization. 2021. Zambia launches the COVID-19 vaccination. https://www.afro.who.int/news/zambia-launches-covid-19-vaccination.
- 26.World Health Organization. WHO policy brief: Reaching COVID-19 vaccination targets [Internet]. Geneva: WHO; 2022 [cited 2023 Jun 28]. Report No.: 2022.1. https://www.who.int/publications/m/item/strategy-to-achieve-global-covid-19-vaccination-bymid-2022. 2.
- 27.Chekol Abebe E, Ayalew Tiruneh G, Asmare Adela G, Mengie Ayele T, Tilahun Muche Z, Behaile T/Mariam A, et al. COVID-19 vaccine uptake and associated factors among pregnant women attending antenatal care in Debre Tabor public health institutions: A cross-sectional study. Front Public Health [Internet]. 2022. Jul 19 [cited 2023 Jun 28];10:919494. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2022.919494/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunawardhana N, Baecher K, Boutwell A, Pekwarake S, Kifem M, Ngong MG, et al. COVID-19 vaccine acceptance and perceived risk among pregnant and non-pregnant adults in Cameroon, Africa. Sallam M, editor. PLoS ONE. 2022. Sep 13;17(9):e0274541. doi: 10.1371/journal.pone.0274541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee D, Mukhopadhyay S, Sahana Asmeen M, Javed A. COVID-19 Vaccination: crucial roles and opportunities for the mental health professionals. Glob Ment Health [Internet]. 2021. [cited 2023 Jun 30];8:e25. Available from: https://www.cambridge.org/core/product/identifier/S205442512100025X/type/journal_article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duroseau B, Kipshidze N, Limaye RJ. The impact of delayed access to COVID-19 vaccines in low- and lower-middle-income countries. Front Public Health [Internet]. 2023. Jan 12 [cited 2023 Jun 30];10:1087138. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1087138/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makoni M. The quest for more COVID-19 vaccinations in Africa. The Lancet Respiratory Medicine [Internet]. 2022. Jul [cited 2023 Jun 30];10(7):e70–1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S221326002200193X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawal L, Aminu Bello M, Murwira T, Avoka C, Yusuf Ma’aruf S, Harrison Omonhinmin I, et al. Low coverage of COVID-19 vaccines in Africa: current evidence and the way forward. Human Vaccines & Immunotherapeutics [Internet]. 2022. Jan 31 [cited 2023 Jun 28];18(1):2034457. Available from: https://www.tandfonline.com/doi/full/10.1080/21645515.2022.2034457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gudayu TW, Mengistie HT. COVID-19 vaccine acceptance in sub-Saharan African countries: A systematic review and meta-analysis. Heliyon. 2023. Feb;9(2):e13037. doi: 10.1016/j.heliyon.2023.e13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochola EA. Vaccine Hesitancy in Sub-Saharan Africa in the Context of COVID-19 Vaccination Exercise: A Systematic Review. Diseases [Internet]. 2023. Feb 9 [cited 2023 Jun 30];11(1):32. Available from: https://www.mdpi.com/2079-9721/11/1/32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinga JN, Sinda LK, Titanji VPK. Assessment of Vaccine Hesitancy to a COVID-19 Vaccine in Cameroonian Adults and Its Global Implication. Vaccines (Basel). 2021. Feb 19;9(2):175. doi: 10.3390/vaccines9020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worede DT, Kassahun M, Endalew B. COVID-19 vaccine acceptance and predictors among pregnant women in Ethiopia: Systematic Review and Meta-Analysis. Public Health in Practice [Internet]. 2023. Jun [cited 2023 Aug 10];5:100386. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666535223000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoque AM, Buckus S, Hoque M, Hoque ME, Van Hal G. COVID-19 Vaccine Acceptability Among Pregnant Women at a Primary Health Care Facility in Durban, South Africa. EJMED [Internet]. 2020. Oct 30 [cited 2023 Aug 10];2(5). Available from: https://www.ejmed.org/index.php/ejmed/article/view/493. [Google Scholar]
- 38.Zhang R, Wang C. Risk perception of COVID-19 and its related factors among centralized medical isolation groups in China. Front Psychol [Internet]. 2023. Feb 1 [cited 2023 Jun 29];14:1131076. Available from: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1131076/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rikitu Terefa D, Shama AT, Feyisa BR, Ewunetu Desisa A, Geta ET, Chego Cheme M, et al. COVID-19 Vaccine Uptake and Associated Factors Among Health Professionals in Ethiopia. IDR [Internet]. 2021. Dec [cited 2023 Jun 29];Volume 14:5531–41. Available from: https://www.dovepress.com/covid-19-vaccine-uptake-and-associated-factors-among-health-profession-peer-reviewed-fulltext-article-IDR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Marshoudi S, Al-Balushi H, Al-Wahaibi A, Al-Khalili S, Al-Maani A, Al-Farsi N, et al. Knowledge, Attitudes, and Practices (KAP) toward the COVID-19 Vaccine in Oman: A Pre-Campaign Cross-Sectional Study. Vaccines [Internet]. 2021. Jun 4 [cited 2023 Jun 29];9(6):602. Available from: https://www.mdpi.com/2076-393X/9/6/602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamal AHM, Sarkar T, Khan MM, Roy SK, Khan SH, Hasan SMM, et al. Factors Affecting Willingness to Receive COVID-19 Vaccine Among Adults: A Cross-sectional Study in Bangladesh. Journal of Health Management [Internet]. 2021. Oct 14 [cited 2023 Jun 30];097359842110506. Available from: http://journals.sagepub.com/doi/10.1177/09735984211050691. [Google Scholar]
- 42.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Acceptance of a COVID-19 Vaccine in Southeast Asia: A Cross-Sectional Study in Indonesia. Front Public Health [Internet]. 2020. Jul 14 [cited 2023 Jun 30];8:381. Available from: https://www.frontiersin.org/article/10.3389/fpubh.2020.00381/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Human Vaccines & Immunotherapeutics [Internet]. 2021. Jun 3 [cited 2023 Jun 30];17(6):1612–21. Available from: https://www.tandfonline.com/doi/full/10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerda AA, García LY. Hesitation and Refusal Factors in Individuals’ Decision-Making Processes Regarding a Coronavirus Disease 2019 Vaccination. Front Public Health [Internet]. 2021. Apr 21 [cited 2023 Jun 30];9:626852. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2021.626852/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulenga LB, Hines JZ, Fwoloshi S, Chirwa L, Siwingwa M, Yingst S, et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. The Lancet Global Health [Internet]. 2021. Jun [cited 2023 May 18];9(6):e773–81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X2100053X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bobo P, Hines JZ, Chilengi R, Auld AF, Agolory SG, Silumesii A, et al. Leveraging HIV Program and Civil Society to Accelerate COVID-19 Vaccine Uptake, Zambia. Emerg Infect Dis [Internet]. 2022. Oct [cited 2023 Oct 2];28(13). Available from: https://wwwnc.cdc.gov/eid/article/28/13/22-0743_article.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhindo R, Okoboi S, Kiragga A, King R, Arinaitwe WJ, Castelnuovo B. COVID-19 vaccine acceptability, and uptake among people living with HIV in Uganda. Amuzie CII, editor. PLoS ONE [Internet]. 2022. Dec 2 [cited 2023 Aug 31];17(12):e0278692. Available from: https://dx.plos.org/10.1371/journal.pone.0278692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folayan MO, Arije O, Enemo A, Sunday A, Muhammad A, Nyako HY, et al. Associations between COVID-19 vaccine hesitancy and the experience of violence among women and girls living with and at risk of HIV in Nigeria. African Journal of AIDS Research [Internet]. 2022. Oct 2 [cited 2023 Aug 25];21(4):306–16. Available from: https://www.tandfonline.com/doi/full/10.2989/16085906.2022.2118615. [DOI] [PubMed] [Google Scholar]
- 49.Worede DT, Kassahun M, Endalew B. COVID-19 vaccine acceptance and predictors among pregnant women in Ethiopia: Systematic Review and Meta-Analysis. Public Health in Practice. 2023. Jun;5:100386. doi: 10.1016/j.puhip.2023.100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ministry of Health. Nationwide 10-day COVID-19 Campaign [Internet]. Nationwide 10-day COVID-19 Campaign. 2022 [cited 2023 Jul 13]. https://twitter.com/mohzambia/status/1527013308305391619?lang=en.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Figshare at https://doi.org/10.6084/m9.figshare.25974994.v3.