Abstract
Lysyl oxidase (protein-lysine 6-oxidase; EC 1.4.3.13) is a copper-containing enzyme that functions extracellularly and catalyses the oxidative deamination of peptidyl lysine. Lysyl oxidase was purified 150-175-fold from urea extracts of rat skin and uteri. Features of the enzyme were similar to those reported previously for lysyl oxidase obtained from rat aorta and bovine ligamenture. However, both approximately 40 and approximately 32 kDa polypeptide chains could be isolated from rat skin with apparent lysyl oxidase activity. Antibodies raised in chickens against the approximately 40 kDa form of lysyl oxidase detected the approximately 32 kDa form in immunoblots. Consequently it is inferred that the approximately 32 kDa form of lysyl oxidase is processed from the approximately 40 kDa form of the enzyme. The antibodies were also used to prepare anti(rat lysyl oxidase) affinity columns to facilitate the separation of lysyl oxidase from other proteins in studies to assess the extent to which lysyl oxidase serves as a reservoir for skin copper. At 16 h after an oral dose of copper, as 67Cu, about 6-8% of the total 67Cu incorporated into rat skin was found in association with lysyl oxidase. The lysyl oxidase concentration in rat skin was 2.5-7.5 nmol/g (determined by e.l.i.s.a.). Changing the copper status of rats by feeding a diet deficient in copper did not appear to influence lysyl oxidase accumulation in skin nor the percentage of incorporation of 67Cu in skin as lysyl oxidase. However, when rats were deprived of copper, the functional activity of lysyl oxidase in skin was one-third to one-half the normal values.
Full text
PDF

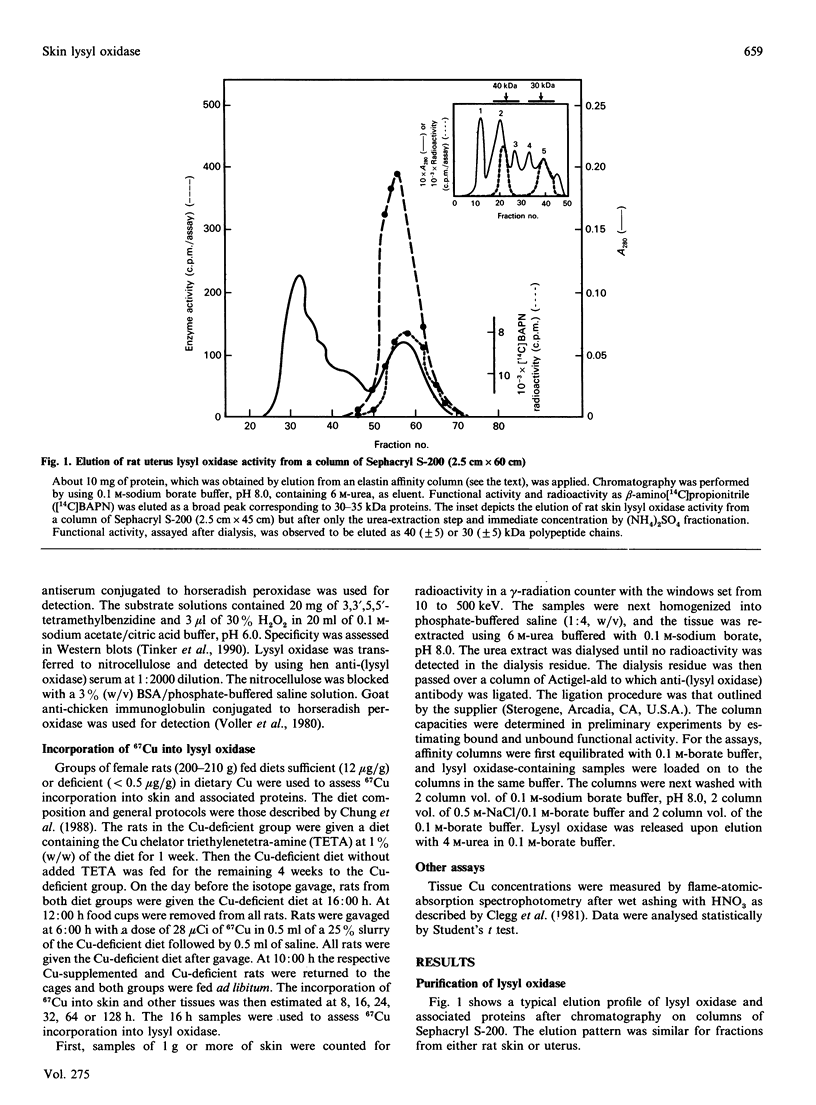
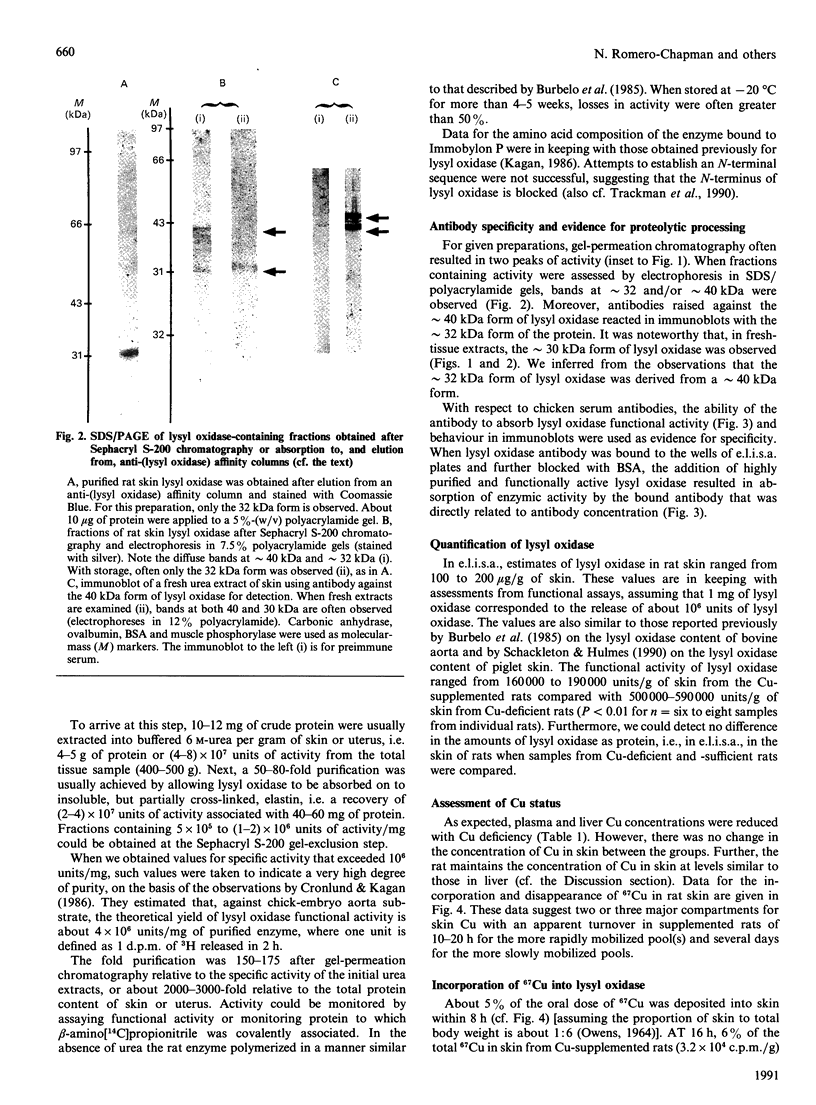
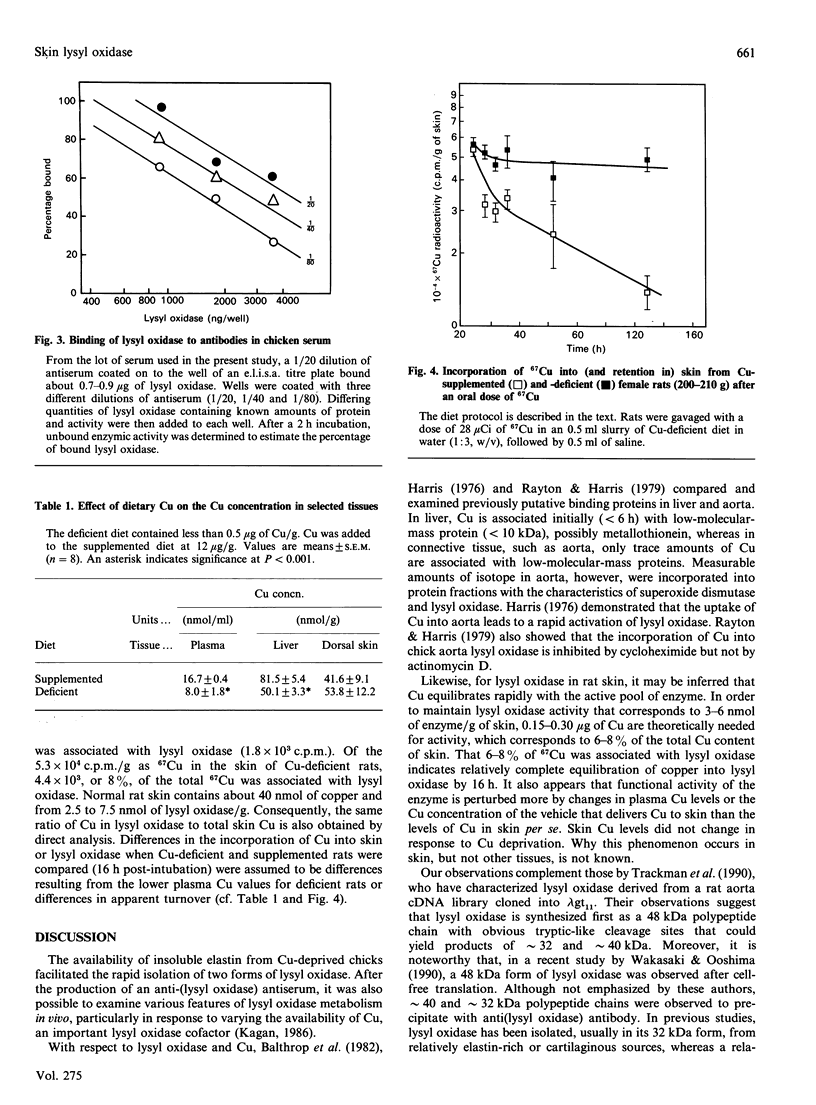

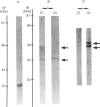
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bade H., Stegemann H. Rapid method of extraction of antibodies from hen egg yolk. J Immunol Methods. 1984 Sep 4;72(2):421–426. doi: 10.1016/0022-1759(84)90010-3. [DOI] [PubMed] [Google Scholar]
- Balthrop J. E., Dameron C. T., Harris E. D. Comparison of pathways of copper metabolism in aorta and liver. A functional test of metallothionein. Biochem J. 1982 May 15;204(2):541–548. doi: 10.1042/bj2040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R. E., Calaman S. D., Traish A. M., Kagan H. M. Stimulation of lysyl oxidase (EC 1.4.3.13) activity by testosterone and characterization of androgen receptors in cultured calf aorta smooth-muscle cells. Biochem J. 1987 Jun 1;244(2):317–323. doi: 10.1042/bj2440317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P. D., Kagan H. M., Chichester C. O. Immunological characterization of bovine lysyl oxidase. Comp Biochem Physiol B. 1985;81(4):845–849. doi: 10.1016/0305-0491(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Stollar B. D. Antibodies to calf thymus RNA polymerase II from egg yolks of immunized hens. J Biol Chem. 1983 Jan 10;258(1):24–26. [PubMed] [Google Scholar]
- Chung K., Romero N., Tinker D., Keen C. L., Amemiya K., Rucker R. Role of copper in the regulation and accumulation of superoxide dismutase and metallothionein in rat liver. J Nutr. 1988 Jul;118(7):859–864. doi: 10.1093/jn/118.7.859. [DOI] [PubMed] [Google Scholar]
- Cronlund A. L., Kagan H. M. Comparison of lysyl oxidase from bovine lung and aorta. Connect Tissue Res. 1986;15(3):173–185. doi: 10.3109/03008208609167141. [DOI] [PubMed] [Google Scholar]
- Dubick M. A., Keen C. L., Rucker R. B. Elastin metabolism during perinatal lung development in the copper-deficient rat. Exp Lung Res. 1985;8(4):227–241. doi: 10.3109/01902148509087806. [DOI] [PubMed] [Google Scholar]
- Gacheru S. N., Trackman P. C., Calaman S. D., Greenaway F. T., Kagan H. M. Vicinal diamines as pyrroloquinoline quinone-directed irreversible inhibitors of lysyl oxidase. J Biol Chem. 1989 Aug 5;264(22):12963–12969. [PubMed] [Google Scholar]
- Gavriel P., Kagan H. M. Inhibition by heparin of the oxidation of lysine in collagen by lysyl oxidase. Biochemistry. 1988 Apr 19;27(8):2811–2815. doi: 10.1021/bi00408a022. [DOI] [PubMed] [Google Scholar]
- Harris E. D. Copper-induced activation of aortic lysyl oxidase in vivo. Proc Natl Acad Sci U S A. 1976 Feb;73(2):371–374. doi: 10.1073/pnas.73.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., DiSilvestro R. A. Correlation of lysyl oxidase activation with the p-phenylenediamine oxidase activity (ceruloplasmin) in serum. Proc Soc Exp Biol Med. 1981 Apr;166(4):528–531. doi: 10.3181/00379727-166-41102. [DOI] [PubMed] [Google Scholar]
- Heng-Khoo C. S., Rucker R. B., Buckingham K. W. Additional evidence for a proform to tropoelastin from chick aorta. Biochem J. 1979 Feb 1;177(2):559–567. doi: 10.1042/bj1770559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes S. M., Mu D., Wemmer D., Smith A. J., Kaur S., Maltby D., Burlingame A. L., Klinman J. P. A new redox cofactor in eukaryotic enzymes: 6-hydroxydopa at the active site of bovine serum amine oxidase. Science. 1990 May 25;248(4958):981–987. doi: 10.1126/science.2111581. [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Vaccaro C. A., Bronson R. E., Tang S. S., Brody J. S. Ultrastructural immunolocalization of lysyl oxidase in vascular connective tissue. J Cell Biol. 1986 Sep;103(3):1121–1128. doi: 10.1083/jcb.103.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore J., Smidt C., Duich L., Romero-Chapman N., Tinker D., Reiser K., Melko M., Hyde D., Rucker R. B. Nutritional importance of pyrroloquinoline quinone. Science. 1989 Aug 25;245(4920):850–852. doi: 10.1126/science.2549636. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Savolainen E. R., Kivirikko K. I. Human placental lysyl oxidase. Purification, partial characterization, and preparation of two specific antisera to the enzyme. J Biol Chem. 1984 Jun 10;259(11):6996–7002. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Myers B. A., Dubick M. A., Reynolds R. D., Rucker R. B. Effect of vitamin B-6 (pyridoxine) deficiency on lung elastin cross-linking in perinatal and weanling rat pups. Biochem J. 1985 Jul 1;229(1):153–160. doi: 10.1042/bj2290153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A. S., Sandberg L. B., Jones K., Coleman S. S., Bagley R. A. Lysyloxidase activities of male and female turkey aortae. Exp Mol Pathol. 1982 Feb;36(1):107–117. doi: 10.1016/0014-4800(82)90083-1. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Siegel R. C., Martin G. R. Stability and purification of lysyl oxidase. Arch Biochem Biophys. 1974 May;162(1):231–237. doi: 10.1016/0003-9861(74)90123-4. [DOI] [PubMed] [Google Scholar]
- Opsahl W., Zeronian H., Ellison M., Lewis D., Rucker R. B., Riggins R. S. Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J Nutr. 1982 Apr;112(4):708–716. doi: 10.1093/jn/112.4.708. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayton J. K., Harris E. D. Induction of lysyl oxidase with copper. Properties of an in vitro system. J Biol Chem. 1979 Feb 10;254(3):621–626. [PubMed] [Google Scholar]
- Reiser K. M., Hennessy S. M., Last J. A. Analysis of age-associated changes in collagen crosslinking in the skin and lung in monkeys and rats. Biochim Biophys Acta. 1987 Dec 7;926(3):339–348. doi: 10.1016/0304-4165(87)90220-0. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S., Ville G. Collagen cross-linking. Int J Biochem. 1989;21(11):1185–1189. doi: 10.1016/0020-711x(89)90001-3. [DOI] [PubMed] [Google Scholar]
- Romero N., Tinker D., Hyde D., Rucker R. B. Role of plasma and serum proteases in the degradation of elastin. Arch Biochem Biophys. 1986 Jan;244(1):161–168. doi: 10.1016/0003-9861(86)90105-0. [DOI] [PubMed] [Google Scholar]
- Rucker R. B., Dubick M. A. Elastin metabolism and chemistry: potential roles in lung development and structure. Environ Health Perspect. 1984 Apr;55:179–191. doi: 10.1289/ehp.8455179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker R. B. Isolation of soluble elastin from copper-deficient chick aorta. Methods Enzymol. 1982;82(Pt A):650–657. doi: 10.1016/0076-6879(82)82093-4. [DOI] [PubMed] [Google Scholar]
- Shackleton D. R., Hulmes D. J. Purification of lysyl oxidase from piglet skin by selective interaction with Sephacryl S-200. Biochem J. 1990 Mar 15;266(3):917–919. [PMC free article] [PubMed] [Google Scholar]
- Stassen F. L. Properties of highly purified lysyl oxidase from embryonic chick cartilage. Biochim Biophys Acta. 1976 Jun 7;438(1):49–60. doi: 10.1016/0005-2744(76)90222-9. [DOI] [PubMed] [Google Scholar]
- Tang S. S., Chichester C. O., Kagan H. M. Comparative sensitivities of purified preparations of lysyl oxidase and other amine oxidases to active site-directed enzyme inhibitors. Connect Tissue Res. 1989;19(1):93–103. doi: 10.3109/03008208909016817. [DOI] [PubMed] [Google Scholar]
- Tang S. S., Trackman P. C., Kagan H. M. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem. 1983 Apr 10;258(7):4331–4338. [PubMed] [Google Scholar]
- Tinker D., Geller J., Romero N., Cross C. E., Rucker R. B. Tropoelastin production and tropoelastin messenger RNA activity. Relationship to copper and elastin cross-linking in chick aorta. Biochem J. 1986 Jul 1;237(1):17–23. doi: 10.1042/bj2370017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker D., Romero-Chapman N., Reiser K., Hyde D., Rucker R. Elastin metabolism during recovery from impaired crosslink formation. Arch Biochem Biophys. 1990 May 1;278(2):326–332. doi: 10.1016/0003-9861(90)90267-3. [DOI] [PubMed] [Google Scholar]
- Tinker D., Rucker R. B. Role of selected nutrients in synthesis, accumulation, and chemical modification of connective tissue proteins. Physiol Rev. 1985 Jul;65(3):607–657. doi: 10.1152/physrev.1985.65.3.607. [DOI] [PubMed] [Google Scholar]
- Trackman P. C., Pratt A. M., Wolanski A., Tang S. S., Offner G. D., Troxler R. F., Kagan H. M. Cloning of rat aorta lysyl oxidase cDNA: complete codons and predicted amino acid sequence. Biochemistry. 1990 May 22;29(20):4863–4870. doi: 10.1021/bi00472a016. [DOI] [PubMed] [Google Scholar]
- Wakasaki H., Ooshima A. Synthesis of lysyl oxidase in experimental hepatic fibrosis. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1201–1204. doi: 10.1016/0006-291x(90)90993-w. [DOI] [PubMed] [Google Scholar]
- Williamson P. R., Kagan H. M. Reaction pathway of bovine aortic lysyl oxidase. J Biol Chem. 1986 Jul 15;261(20):9477–9482. [PubMed] [Google Scholar]