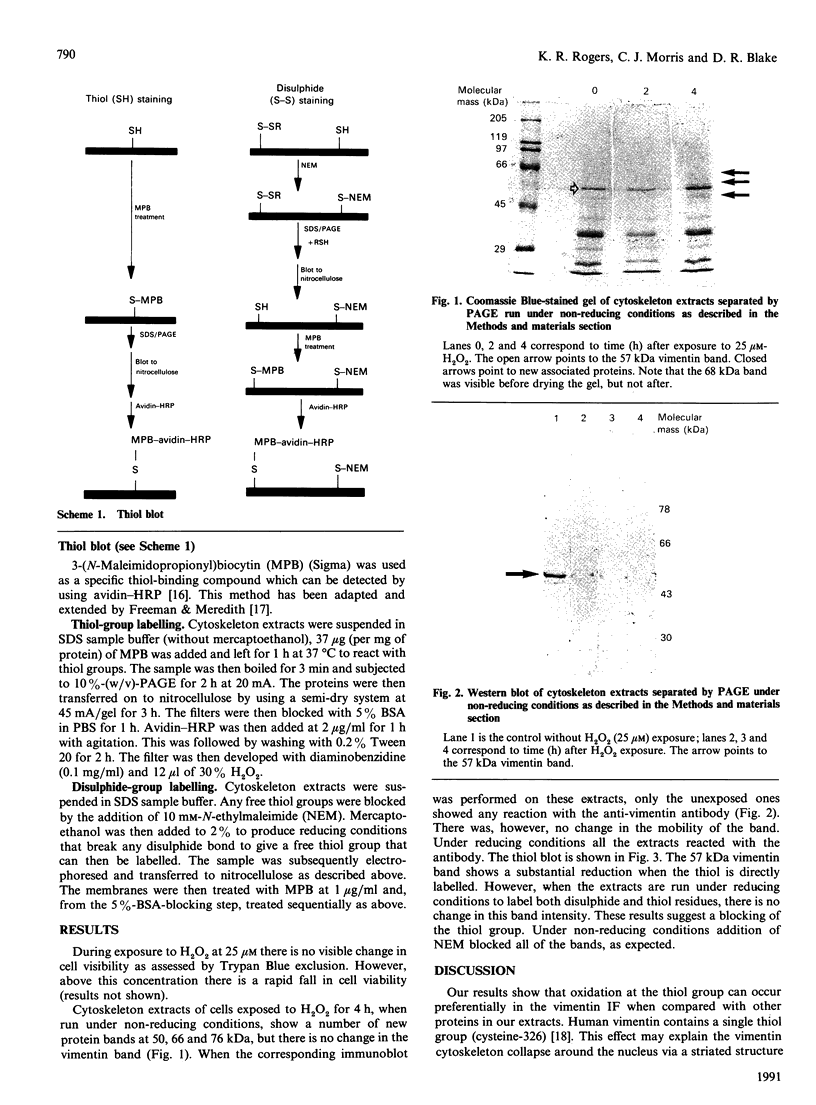
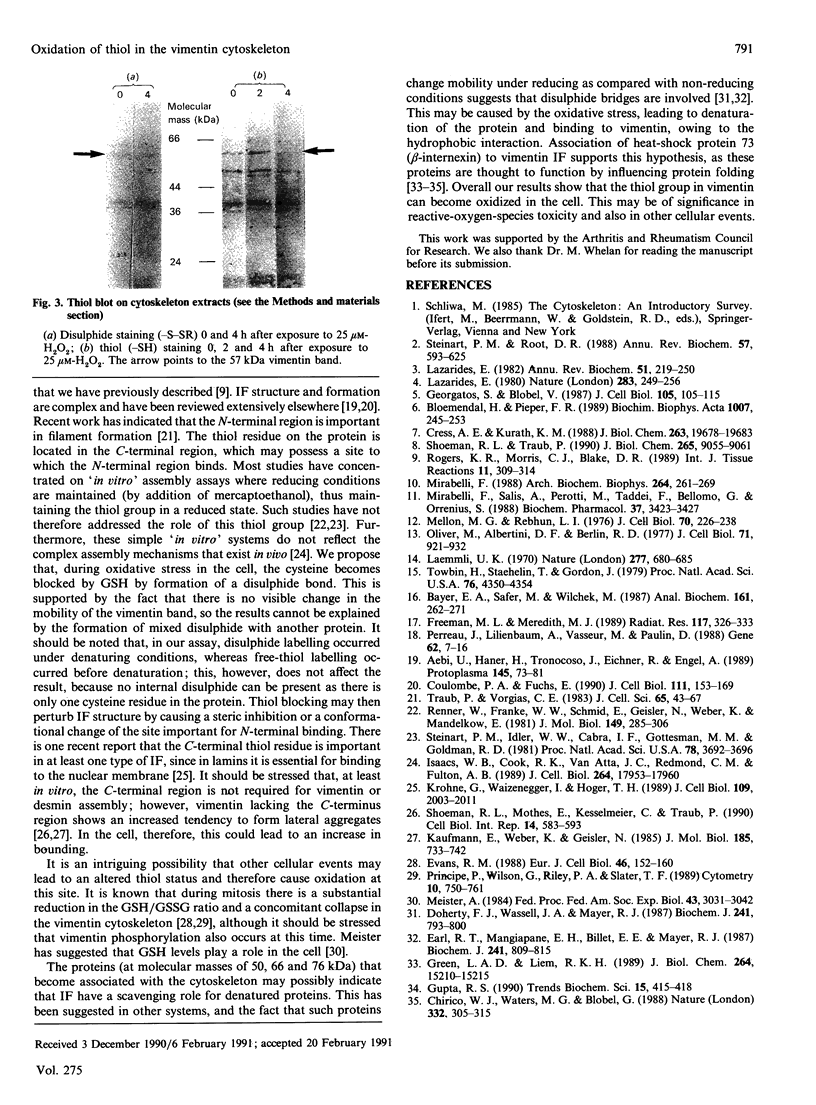
Abstract
Sublethal doses of H2O2, which induces oxidative stress, cause substantial alteration to the vimentin cytoskeleton in various cell types. We have used a thiol-blot assay to assess thiol status in individual proteins from cell extracts. Vimentin thiol is oxidized in preference to other cytoskeleton proteins. Immunoblot analysis also demonstrated a loss of reactivity to an anti-vimentin monoclonal antibody under non-reducing conditions, possibly due to thiol-group oxidation. During induced oxidative stress a number of proteins become associated with the cytoskeleton extracts.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Safars M., Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem. 1987 Mar;161(2):262–271. doi: 10.1016/0003-2697(87)90450-7. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Pieper F. R. Intermediate filaments: known structure, unknown function. Biochim Biophys Acta. 1989 Apr 12;1007(3):245–253. doi: 10.1016/0167-4781(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Fuchs E. Elucidating the early stages of keratin filament assembly. J Cell Biol. 1990 Jul;111(1):153–169. doi: 10.1083/jcb.111.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress A. E., Kurath K. M. Identification of attachment proteins for DNA in Chinese hamster ovary cells. J Biol Chem. 1988 Dec 25;263(36):19678–19683. [PubMed] [Google Scholar]
- Doherty F. J., Wassell J. A., Mayer R. J. A putative protein-sequestration site involving intermediate filaments for protein degradation by autophagy. Studies with microinjected purified glycolytic enzymes in 3T3-L1 cells. Biochem J. 1987 Feb 1;241(3):793–800. doi: 10.1042/bj2410793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl R. T., Mangiapane E. H., Billett E. E., Mayer R. J. A putative protein-sequestration site involving intermediate filaments for protein degradation by autophagy. Studies with transplanted Sendai-viral envelope proteins in HTC cells. Biochem J. 1987 Feb 1;241(3):809–815. doi: 10.1042/bj2410809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The intermediate-filament proteins vimentin and desmin are phosphorylated in specific domains. Eur J Cell Biol. 1988 Apr;46(1):152–160. [PubMed] [Google Scholar]
- Freeman M. L., Meredith M. J. Measurement of protein thiols after heat shock using 3-(-N-maleimido-propionyl) biocytin labeled proteins separated by SDS-PAGE and electroluted onto nitrocellulose: thiol blotting. Radiat Res. 1989 Feb;117(2):326–333. [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987 Jul;105(1):105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. A., Liem R. K. Beta-internexin is a microtubule-associated protein identical to the 70-kDa heat-shock cognate protein and the clathrin uncoating ATPase. J Biol Chem. 1989 Sep 15;264(26):15210–15215. [PubMed] [Google Scholar]
- Gupta R. S. Mitochondria, molecular chaperone proteins and the in vivo assembly of microtubules. Trends Biochem Sci. 1990 Nov;15(11):415–418. doi: 10.1016/0968-0004(90)90276-h. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Cook R. K., Van Atta J. C., Redmond C. M., Fulton A. B. Assembly of vimentin in cultured cells varies with cell type. J Biol Chem. 1989 Oct 25;264(30):17953–17960. [PubMed] [Google Scholar]
- Kaufmann E., Weber K., Geisler N. Intermediate filament forming ability of desmin derivatives lacking either the amino-terminal 67 or the carboxy-terminal 27 residues. J Mol Biol. 1985 Oct 20;185(4):733–742. doi: 10.1016/0022-2836(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Krohne G., Waizenegger I., Höger T. H. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol. 1989 Nov;109(5):2003–2011. doi: 10.1083/jcb.109.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Meister A. New aspects of glutathione biochemistry and transport: selective alteration of glutathione metabolism. Fed Proc. 1984 Dec;43(15):3031–3042. [PubMed] [Google Scholar]
- Mellon M. G., Rebhun L. I. Sulfhydryls and the in vitro polymerization of tubulin. J Cell Biol. 1976 Jul;70(1):226–238. doi: 10.1083/jcb.70.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli F., Salis A., Marinoni V., Finardi G., Bellomo G., Thor H., Orrenius S. Menadione-induced bleb formation in hepatocytes is associated with the oxidation of thiol groups in actin. Arch Biochem Biophys. 1988 Jul;264(1):261–269. doi: 10.1016/0003-9861(88)90593-0. [DOI] [PubMed] [Google Scholar]
- Mirabelli F., Salis A., Perotti M., Taddei F., Bellomo G., Orrenius S. Alterations of surface morphology caused by the metabolism of menadione in mammalian cells are associated with the oxidation of critical sulfhydryl groups in cytoskeletal proteins. Biochem Pharmacol. 1988 Sep 15;37(18):3423–3427. doi: 10.1016/0006-2952(88)90691-0. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau J., Lilienbaum A., Vasseur M., Paulin D. Nucleotide sequence of the human vimentin gene and regulation of its transcription in tissues and cultured cells. Gene. 1988;62(1):7–16. doi: 10.1016/0378-1119(88)90575-6. [DOI] [PubMed] [Google Scholar]
- Principe P., Wilson G. D., Riley P. A., Slater T. F. Flow cytometric analysis of protein thiol groups in relation to the cell cycle and the intracellular content of glutathione in rat hepatocytes. Cytometry. 1989 Nov;10(6):750–761. doi: 10.1002/cyto.990100613. [DOI] [PubMed] [Google Scholar]
- Renner W., Franke W. W., Schmid E., Geisler N., Weber K., Mandelkow E. Reconstitution of intermediate-sized filaments from denatured monomeric vimentin. J Mol Biol. 1981 Jun 25;149(2):285–306. doi: 10.1016/0022-2836(81)90303-x. [DOI] [PubMed] [Google Scholar]
- Rogers K. R., Morris C. J., Blake D. R. Cytoskeletal rearrangement by oxidative stress. Int J Tissue React. 1989;11(6):309–314. [PubMed] [Google Scholar]
- Shoeman R. L., Mothes E., Kesselmeier C., Traub P. Intermediate filament assembly and stability in vitro: effect and implications of the removal of head and tail domains of vimentin by the human immunodeficiency virus type 1 protease. Cell Biol Int Rep. 1990 Jul;14(7):583–594. doi: 10.1016/0309-1651(90)90038-z. [DOI] [PubMed] [Google Scholar]
- Shoeman R. L., Traub P. The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J Biol Chem. 1990 Jun 5;265(16):9055–9061. [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Cabral F., Gottesman M. M., Goldman R. D. In vitro assembly of homopolymer and copolymer filaments from intermediate filament subunits of muscle and fibroblastic cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3692–3696. doi: 10.1073/pnas.78.6.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Vorgias C. E. Involvement of the N-terminal polypeptide of vimentin in the formation of intermediate filaments. J Cell Sci. 1983 Sep;63:43–67. doi: 10.1242/jcs.63.1.43. [DOI] [PubMed] [Google Scholar]