Abstract
The proportion of ferritin light-chain and heavy-chain subunits (L and H) present in the ferritin multimeric shell varies between different tissues. To identify the regulatory mechanisms responsible for the greater amount of L in liver than in heart isoferritins, we analysed ferritin-gene expression at the RNA and protein levels in these two tissues of the rat. In the heart the ratio between the amount of L and H, at the level both of synthesis and accumulation, is about 1 and is the same as the ratio between their respective mRNAs. In contrast, in the liver, the ratio between the L- and H-mRNAs is approx. 2 and cannot entirely explain the large predominance of L in isoferritins in this tissue. Since in the liver the L-mRNA is neither preferentially associated with polyribosomes nor translated more efficiently than its H- counterpart, it seems that the liver-specific isoferritin profile is determined by a combination of pre- and post-translational mechanisms, whereas in heart the post-translational regulation does not seem to be relevant and the tissue-specific pattern is determined at the level of mRNA accumulation.
Full text
PDF

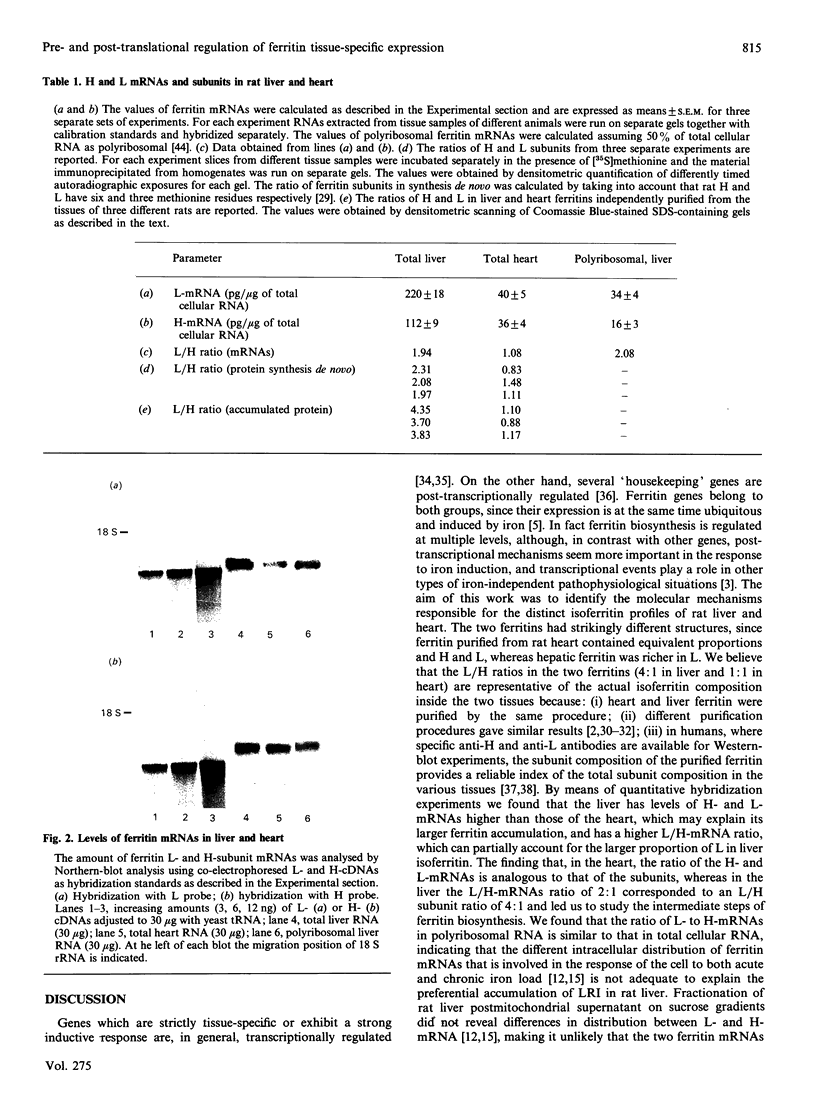

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Arosio P., Yokota M., Drysdale J. W. Structural and immunological relationships of isoferritins in normal and malignant cells. Cancer Res. 1976 May;36(5):1735–1739. [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C., Dugast I., Renaudie F., Souroujon M., Grandchamp B. Transcriptional regulation of ferritin H and L subunits in adult erythroid and liver cells from the mouse. Unambiguous identification of mouse ferritin subunits and in vitro formation of the ferritin shells. J Biol Chem. 1989 May 5;264(13):7498–7504. [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Bomford A., Lis Y., McFarlane I. G., Williams R. Variation in the distribution of two human heart ferritin species. Isoferritin profile and subunit composition in normal and iron-overloaded subjects. Biochem J. 1977 Oct 1;167(1):309–312. doi: 10.1042/bj1670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo G., Bardella L., Schiaffonati L., Arosio P., Levi S., Bernelli-Zazzera A. Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Commun. 1985 Nov 27;133(1):314–321. doi: 10.1016/0006-291x(85)91877-7. [DOI] [PubMed] [Google Scholar]
- Cairo G., Tacchini L., Schiaffonati L., Rappocciolo E., Ventura E., Pietrangelo A. Translational regulation of ferritin synthesis in rat liver. Effects of chronic dietary iron overload. Biochem J. 1989 Dec 15;264(3):925–928. doi: 10.1042/bj2640925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo G., Vezzoni P., Bardella L., Schiaffonati L., Rappocciolo E., Levi S., Arosio P., Bernelli-Zazzera A. Regulation of ferritin synthesis in malignant and non-malignant lymphoid cells. Biochem Biophys Res Commun. 1986 Sep 14;139(2):652–657. doi: 10.1016/s0006-291x(86)80040-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou C. C., Gatti R. A., Fuller M. L., Concannon P., Wong A., Chada S., Davis R. C., Salser W. A. Structure and expression of ferritin genes in a human promyelocytic cell line that differentiates in vitro. Mol Cell Biol. 1986 Feb;6(2):566–573. doi: 10.1128/mcb.6.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Bevilacqua M. A., Giordano M., Cimino F. Expression of genes of ferritin subunits in human hepatoma cell lines. Biochem Biophys Res Commun. 1989 Jun 15;161(2):902–909. doi: 10.1016/0006-291x(89)92684-3. [DOI] [PubMed] [Google Scholar]
- Cozzi A., Levi S., Bazzigaluppi E., Ruggeri G., Arosio P. Development of an immunoassay for all human isoferritins, and its application to serum ferritin evaluation. Clin Chim Acta. 1989 Oct 16;184(3):197–206. doi: 10.1016/0009-8981(89)90052-1. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dickey L. F., Sreedharan S., Theil E. C., Didsbury J. R., Wang Y. H., Kaufman R. E. Differences in the regulation of messenger RNA for housekeeping and specialized-cell ferritin. A comparison of three distinct ferritin complementary DNAs, the corresponding subunits, and identification of the first processed in amphibia. J Biol Chem. 1987 Jun 5;262(16):7901–7907. [PubMed] [Google Scholar]
- Dickey L. F., Wang Y. H., Shull G. E., Wortman I. A., 3rd, Theil E. C. The importance of the 3'-untranslated region in the translational control of ferritin mRNA. J Biol Chem. 1988 Mar 5;263(7):3071–3074. [PubMed] [Google Scholar]
- Drysdale J. W. Ferritin phenotypes: structure and metabolism. Ciba Found Symp. 1976 Dec 7;(51):41–67. doi: 10.1002/9780470720325.ch3. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W. Human ferritin gene expression. Prog Nucleic Acid Res Mol Biol. 1988;35:127–172. doi: 10.1016/s0079-6603(08)60612-1. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Britten R. J., Davidson E. H. Significance of rare m RNA sequences in liver. Arch Biochem Biophys. 1977 Mar;179(2):584–599. doi: 10.1016/0003-9861(77)90147-3. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. Controlling mRNA lifespan. Nature. 1988 Aug 18;334(6183):567–568. doi: 10.1038/334567a0. [DOI] [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Aziz N., Brown A. J., Munro H. N. Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J Biol Chem. 1984 Apr 10;259(7):4327–4334. [PubMed] [Google Scholar]
- Levi S., Luzzago A., Cesareni G., Cozzi A., Franceschinelli F., Albertini A., Arosio P. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. 1988 Dec 5;263(34):18086–18092. [PubMed] [Google Scholar]
- Linder M. C., Nagel G. M., Roboz M., Hungerford D. M., Jr The size and shape of heart and muscle ferritins analyzed by sedimentation, gel filtration, and electrophoresis. J Biol Chem. 1981 Sep 10;256(17):9104–9110. [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., Ashwell G., van Renswoude J. Multiple post-transcriptional regulatory mechanisms in ferritin gene expression. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1801–1805. doi: 10.1073/pnas.86.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. T., White K., Munro H. N. Conservation of ferritin heavy subunit gene structure: implications for the regulation of ferritin gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7438–7442. doi: 10.1073/pnas.84.21.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A., Rocchi E., Schiaffonati L., Ventura E., Cairo G. Liver gene expression during chronic dietary iron overload in rats. Hepatology. 1990 May;11(5):798–804. doi: 10.1002/hep.1840110513. [DOI] [PubMed] [Google Scholar]
- Schiaffonati L., Rappocciolo E., Tacchini L., Bardella L., Arosio P., Cozzi A., Cantu G. B., Cairo G. Mechanisms of regulation of ferritin synthesis in rat liver during experimental inflammation. Exp Mol Pathol. 1988 Apr;48(2):174–181. doi: 10.1016/0014-4800(88)90054-8. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Torti S. V., Kwak E. L., Miller S. C., Miller L. L., Ringold G. M., Myambo K. B., Young A. P., Torti F. M. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988 Sep 5;263(25):12638–12644. [PubMed] [Google Scholar]
- Ursini M. V., de Franciscis V. TSH regulation of ferritin H chain messenger RNA levels in the rat thyroids. Biochem Biophys Res Commun. 1988 Jan 15;150(1):287–295. doi: 10.1016/0006-291x(88)90518-9. [DOI] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- Worwood M., Hourahane D., Jones B. M. Accumulation and release of isoferritins during incubation in vitro of human peripheral blood mononuclear cells. Br J Haematol. 1984 Jan;56(1):31–43. doi: 10.1111/j.1365-2141.1984.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Worwood M., Summers M., Miller F., Jacobs A., Whittaker J. A. Ferritin in blood cells from normal subjects and patients with leukaemia. Br J Haematol. 1974 Sep;28(1):27–35. [PubMed] [Google Scholar]
- Yap S. H., Strair R. K., Shafritz D. A. Effect of a short term fast on the distribution of cytoplasmic albumin messenger ribonucleic acid in rat liver. Evidence for formation of free albumin messenger ribonucleoprotein particles. J Biol Chem. 1978 Jul 25;253(14):4944–4950. [PubMed] [Google Scholar]