Abstract
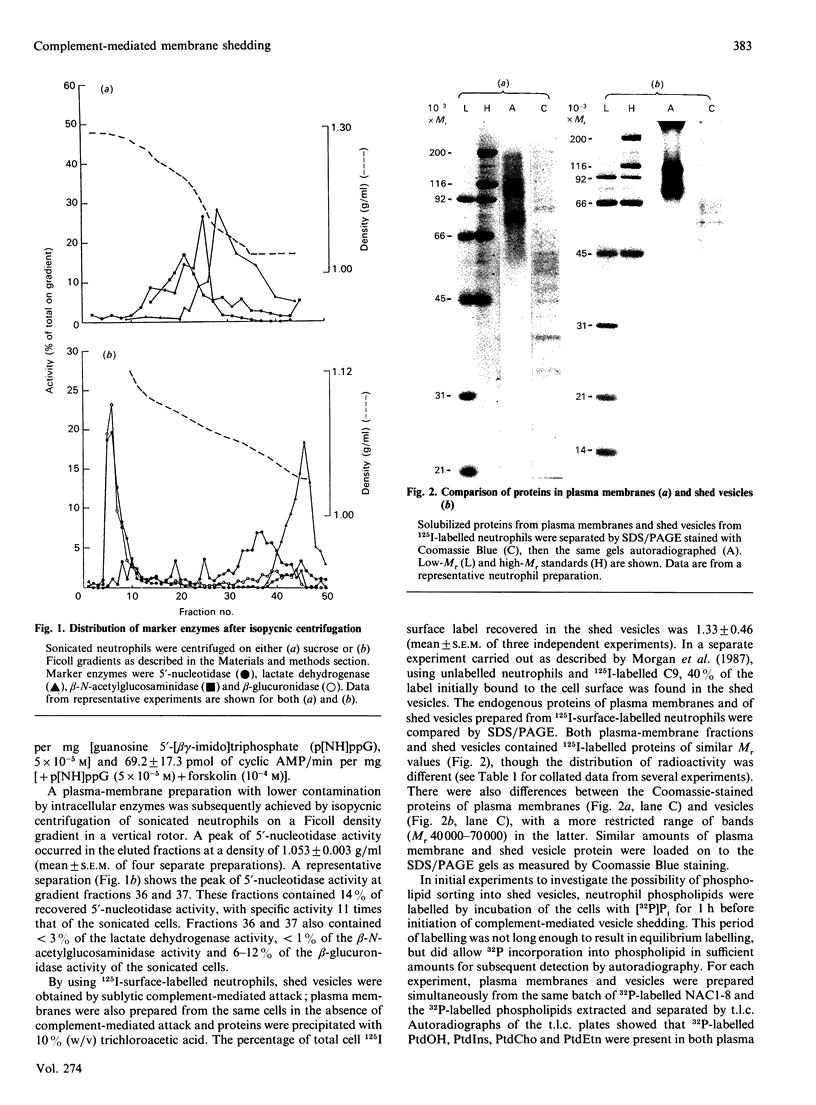
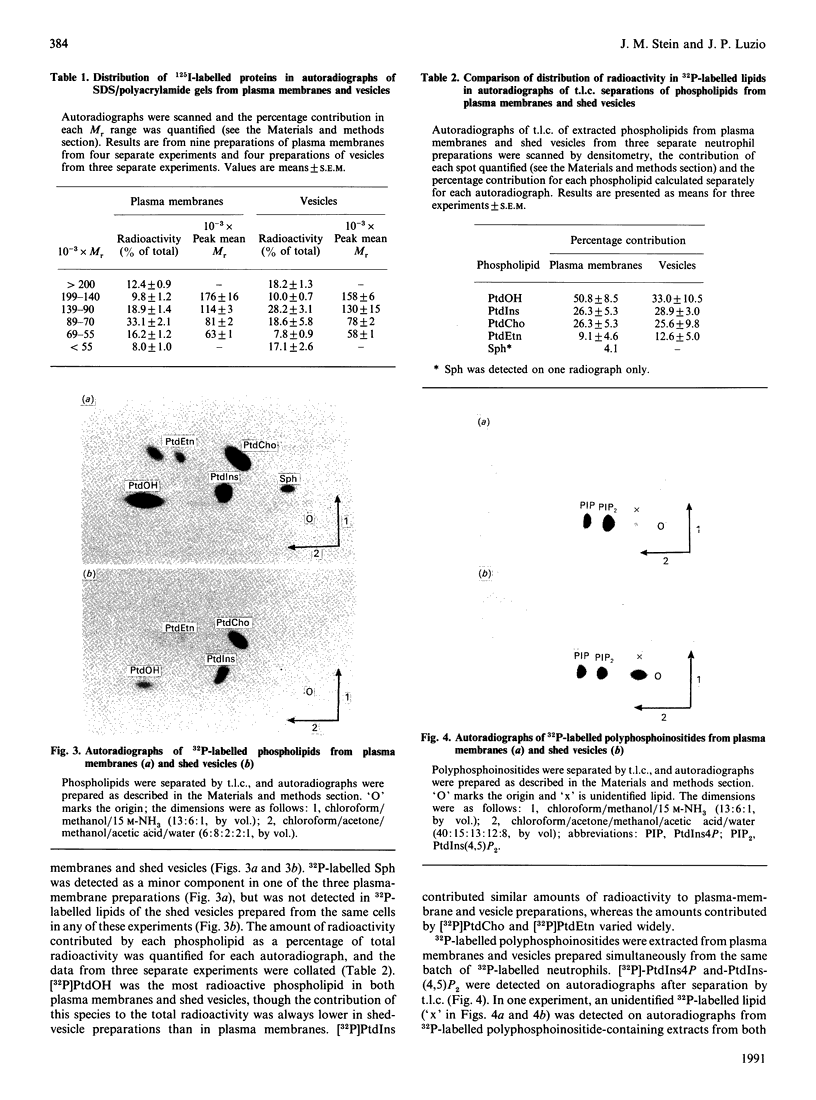
During sublytic complement attack on human neutrophils, plasma-membrane vesicles are shed from the cell surface as a cell-protection mechanism. By using surface-iodinated neutrophils it was found that less than 2% of surface label was recovered in shed vesicles under conditions where 40% of complement component C9 was shed. SDS/PAGE of 125I-labelled shed vesicles and plasma membranes showed differences in iodination pattern, demonstrating the sorting of membrane proteins into the shed vesicles. Analysis of 32P-labelled phospholipids after labeling of neutrophils with [32P]Pi before sublytic complement attack showed the presence of phosphatidic acid, phosphatidylcholine, phosphatidyl-ethanolamine, phosphatidylinositol and polyphosphoinositides in shed vesicles. Quantitative analysis using [3H]acetic anhydride-labelling method showed that the molar proportions of phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine and sphingomyelin were the same in shed vesicles as in plasma membranes. In contrast, the molar proportions of cholesterol and diacylglycerol relative to sphingomyelin were almost twice those found in plasma membranes. The data demonstrate the existence of protein and lipid sorting mechanisms during the formation of shed vesicles when neutrophils are subject to sublytic complement attack. The term 'ectocytosis' is proposed to describe triggered shedding of right-side-out membrane vesicles from the surface of eukaryotic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Cockcroft S. The fatty acid composition of 1,2-diacylglycerol and polyphosphoinositides from human erythrocyte membranes. Biochem J. 1983 Aug 1;213(2):555–557. doi: 10.1042/bj2130555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Accumulation of 1,2-diacylglycerol in the plasma membrane may lead to echinocyte transformation of erythrocytes. Nature. 1975 Nov 27;258(5533):348–349. doi: 10.1038/258348a0. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. The possible role of lipids in control of membrane fusion during secretion. Symp Soc Exp Biol. 1979;33:323–336. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baron M. D., Pope B., Luzio J. P. The membrane topography of ecto-5'-nucleotidase in rat hepatocytes. Biochem J. 1986 Jun 1;236(2):495–502. doi: 10.1042/bj2360495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Caswell A. H., Gomperts B. D. Plasma-membrane location of phosphatidylinositol hydrolysis in rabbit neutrophils stimulated with formylmethionyl-leucylphenylalanine. Biochem J. 1982 Dec 15;208(3):801–808. doi: 10.1042/bj2080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Black P. H. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Branch W. J., Mullock B. M., Luzio J. P. Rapid subcellular fractionation of the rat liver endocytic compartments involved in transcytosis of polymeric immunoglobulin A and endocytosis of asialofetuin. Biochem J. 1987 Jun 1;244(2):311–315. doi: 10.1042/bj2440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. K., Luzio J. P. Intracellular free calcium as a pathogen in cell damage initiated by the immune system. Experientia. 1981 Oct 15;37(10):1110–1112. doi: 10.1007/BF02085041. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Morgan B. P. Monoclonal antibodies demonstrate protection of polymorphonuclear leukocytes against complement attack. Nature. 1985 Sep 12;317(6033):164–166. doi: 10.1038/317164a0. [DOI] [PubMed] [Google Scholar]
- Carney D. F., Hammer C. H., Shin M. L. Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+. J Immunol. 1986 Jul 1;137(1):263–270. [PubMed] [Google Scholar]
- Carney D. F., Lang T. J., Shin M. L. Multiple signal messengers generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol. 1990 Jul 15;145(2):623–629. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Hauser G., Eichberg J., Gonzalez-Sastre F. Regional distribution of polyphosphoinositides in rat brain. Biochim Biophys Acta. 1971 Oct 5;248(1):87–95. doi: 10.1016/0005-2760(71)90078-6. [DOI] [PubMed] [Google Scholar]
- Hoerl B. J., Scott R. E. Plasma membrane vesiculation: a cellular response to injury. Virchows Arch B Cell Pathol. 1978 Jun 19;27(4):335–345. doi: 10.1007/BF02889005. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Plasma-membrane components can be removed from isolated lymphocytes by the bile salts glycocholate and taurocholate without cell lysis. Biochem J. 1976 Aug 15;158(2):493–495. doi: 10.1042/bj1580493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Stanley K. K. The isolation of endosome-derived vesicles from rat hepatocytes. Biochem J. 1983 Oct 15;216(1):27–36. doi: 10.1042/bj2160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G. A., Docherty K., Hales C. N. Sugar transport in rat liver lysosomes. Direct demonstration by using labelled sugars. Biochem J. 1983 Apr 15;212(1):211–218. doi: 10.1042/bj2120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985 Oct 1;231(1):205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Dankert J. R., Esser A. F. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987 Jan 1;138(1):246–253. [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Luzio J. P., Campbell A. K. Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium. 1986 Dec;7(5-6):399–411. doi: 10.1016/0143-4160(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Morgan B. P. Non-lethal complement-membrane attack on human neutrophils: transient cell swelling and metabolic depletion. Immunology. 1988 Jan;63(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Newby A. C. Role of adenosine deaminase, ecto-(5'-nucleotidase) and ecto-(non-specific phosphatase) in cyanide-induced adenosine monophosphate catabolism in rat polymorphonuclear leucocytes. Biochem J. 1980 Mar 15;186(3):907–918. doi: 10.1042/bj1860907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. J., Luzio J. P. Complement-mediated production of plasma-membrane vesicles from rat fat-cells. Biochem J. 1980 Mar 15;186(3):897–906. doi: 10.1042/bj1860897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Maercklein P. B. Plasma membrane vesiculation in 3T3 and SV3T3 cells. II. Factors affecting the process of vesiculation. J Cell Sci. 1979 Feb;35:245–252. doi: 10.1242/jcs.35.1.245. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Perkins R. G., Zschunke M. A., Hoerl B. J., Maercklein P. B. Plasma membrane vesiculation in 3T3 and SV3T3 cells. I. Morphological and biochemical characterization. J Cell Sci. 1979 Feb;35:229–243. doi: 10.1242/jcs.35.1.229. [DOI] [PubMed] [Google Scholar]
- Shirley P. S., Wang P., DeChatelet L. R., Waite M. Absence of the membrane marker enzyme 5'-nucleotidase in human polymorphonuclear leukocytes. Biochem Med. 1976 Jun;15(3):289–295. doi: 10.1016/0006-2944(76)90060-0. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Berriman J., Coleman R., Finean J. B., Michell R. H. Membrane protein segregation during release of microvesicles from human erythrocytes. FEBS Lett. 1978 Jun 15;90(2):289–292. doi: 10.1016/0014-5793(78)80388-3. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Faioni E. M., Wiedmer T., Shattil S. J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988 Dec 5;263(34):18205–18212. [PubMed] [Google Scholar]
- Stein J. M., Smith G. A., Luzio J. P. An acetylation method for the quantification of membrane lipids, including phospholipids, polyphosphoinositides and cholesterol. Biochem J. 1991 Mar 1;274(Pt 2):375–379. doi: 10.1042/bj2740375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer T., Shattil S. J., Cunningham M., Sims P. J. Role of calcium and calpain in complement-induced vesiculation of the platelet plasma membrane and in the exposure of the platelet factor Va receptor. Biochemistry. 1990 Jan 23;29(3):623–632. doi: 10.1021/bi00455a005. [DOI] [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]