Abstract
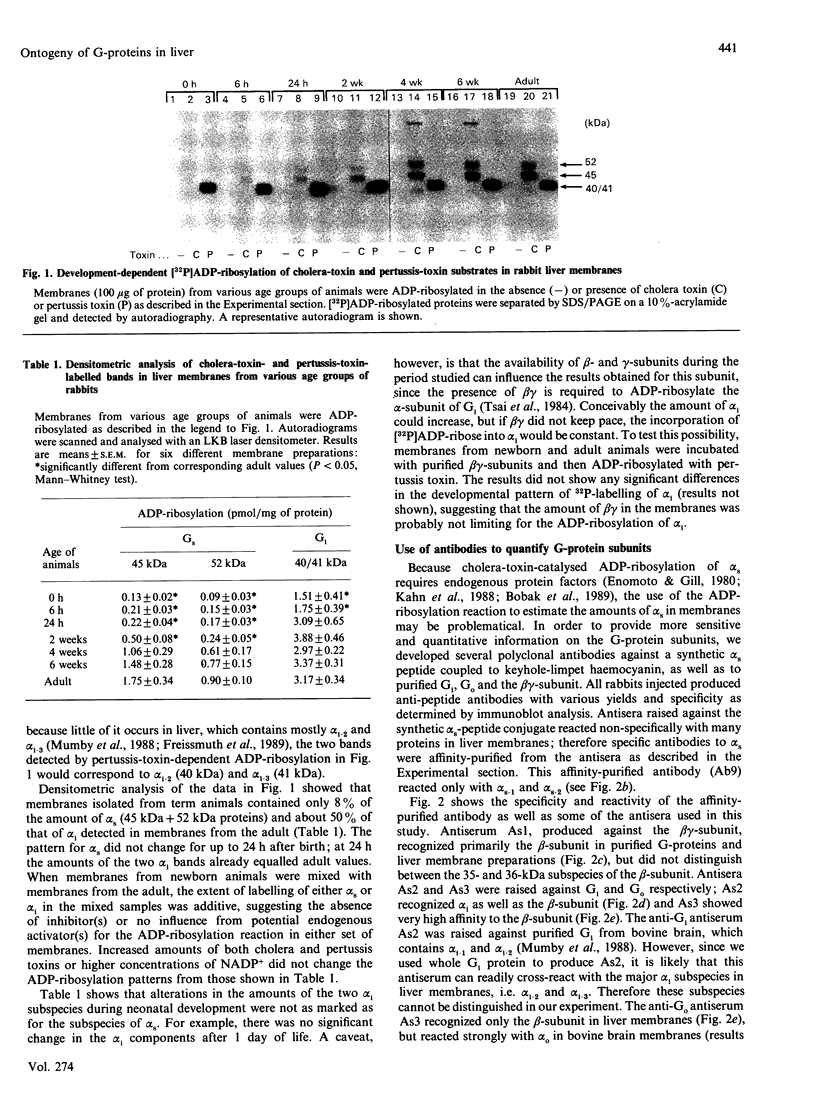
Ontogeny of trimeric GTP-binding regulatory proteins (G-proteins) and their subunits in rabbit liver during neonatal development was studied, by using bacterial-toxin-catalysed ADP-ribosylation of membrane proteins, immunoblot analysis to quantify the alpha-subunit (alpha s and alpha i) of stimulatory (Gs) and inhibitory (Gi) G-protein and the beta-subunit, and reconstitution assay with cyc- membranes (from Gs-deficient variant of S49 lymphoma cell) to measure Gs activity. Under optimal conditions of ADP-ribosylation, little cholera-toxin substrate (alpha s) was detected in membranes from liver of neonatal animals up to 24 h of age. Thereafter ribosylatable alpha s proteins, i.e. 45 kDa (alpha s-1) and 52 kDa (alpha s-2) proteins, were increasingly evident, reaching maximal levels in membranes from animals aged 4-6 weeks. The concentrations of alpha s-1 and alpha s-2, as determined by immunoblotting, were 6.1 +/- 0.8 and 2.7 +/- 0.4 pmol/mg of protein respectively at birth, and did not change during 0-24 h after birth. Thereafter they gradually increased to maximal levels of 22.1 +/- 1.3 and 10.5 +/- 0.7 pmol/mg of protein for alpha s-1 and alpha s-2 respectively, within 6 weeks. The beta-subunit also showed a similar 3-4-fold increase during the same age span. In contrast, the pertussis-toxin substrate (alpha i) was clearly evident even in membranes from term animals and in all age groups studied. Its developmental pattern, as assessed by ADP-ribosylation, was the same as that determined by immunoblot analysis. The functional activity of Gs in cholate extracts of membranes exhibited similar developmental pattern to that of cholera-toxin-mediated labelling. This activity also paralleled the concentrations of alpha s as measured by immunoblotting. These results suggest differential expression of G-protein subunits in liver during neonatal development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda T. T., 3rd, Gautam N., Fong H. K., Northup J. K., Simon M. I. The 35- and 36-kDa beta subunits of GTP-binding regulatory proteins are products of separate genes. J Biol Chem. 1988 Apr 15;263(11):5008–5011. [PubMed] [Google Scholar]
- Ashley P. L., Ellison J., Sullivan K. A., Bourne H. R., Cox D. R. Chromosomal assignment of the murine Gi alpha and Gs alpha genes. Implications for the obese mouse. J Biol Chem. 1987 Nov 5;262(31):15299–15301. [PubMed] [Google Scholar]
- Birnbaumer L., Codina J., Mattera R., Yatani A., Scherer N., Toro M. J., Brown A. M. Signal transduction by G proteins. Kidney Int Suppl. 1987 Dec;23:S14–S42. [PubMed] [Google Scholar]
- Blatt C., Eversole-Cire P., Cohn V. H., Zollman S., Fournier R. E., Mohandas L. T., Nesbitt M., Lugo T., Jones D. T., Reed R. R. Chromosomal localization of genes encoding guanine nucleotide-binding protein subunits in mouse and human. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7642–7646. doi: 10.1073/pnas.85.20.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak D. A., Nightingale M. S., Murtagh J. J., Price S. R., Moss J., Vaughan M. Molecular cloning, characterization, and expression of human ADP-ribosylation factors: two guanine nucleotide-dependent activators of cholera toxin. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6101–6105. doi: 10.1073/pnas.86.16.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Rosenthal W., Hildebrandt J. D., Birnbaumer L., Sekura R. D. Purification of Ns and Ni, the coupling proteins of hormone-sensitive adenylyl cyclases without intervention of activating regulatory ligands. Methods Enzymol. 1985;109:446–465. doi: 10.1016/0076-6879(85)09108-x. [DOI] [PubMed] [Google Scholar]
- Codina J., Stengel D., Woo S. L., Birnbaumer L. Beta-subunits of the human liver Gs/Gi signal-transducing proteins and those of bovine retinal rod cell transducin are identical. FEBS Lett. 1986 Oct 27;207(2):187–192. doi: 10.1016/0014-5793(86)81486-7. [DOI] [PubMed] [Google Scholar]
- Enomoto K., Gill D. M. Cholera toxin activation of adenylate cyclase. Roles of nucleoside triphosphates and a macromolecular factor in the ADP ribosylation of the GTP-dependent regulatory component. J Biol Chem. 1980 Feb 25;255(4):1252–1258. [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Graziano M. P., Freissmuth M., Gilman A. G. Expression of Gs alpha in Escherichia coli. Purification and properties of two forms of the protein. J Biol Chem. 1989 Jan 5;264(1):409–418. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kahn R. A., Goddard C., Newkirk M. Chemical and immunological characterization of the 21-kDa ADP-ribosylation factor of adenylate cyclase. J Biol Chem. 1988 Jun 15;263(17):8282–8287. [PubMed] [Google Scholar]
- Katada T., Oinuma M., Kusakabe K., Ui M. A new GTP-binding protein in brain tissues serving as the specific substrate of islet-activating protein, pertussis toxin. FEBS Lett. 1987 Mar 23;213(2):353–358. doi: 10.1016/0014-5793(87)81521-1. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Graham S. M., Whitsel C., Arinze I. J. Hepatic adenylate cyclase. Development-dependent coupling to the beta-adrenergic receptor in the neonate. J Biol Chem. 1985 Sep 5;260(19):10826–10832. [PubMed] [Google Scholar]
- Kawai Y., Whitsel C., Arinze I. J. NADP+ enhances cholera and pertussis toxin-catalyzed ADP-ribosylation of membrane proteins. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(4):265–274. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang J. Purification and characterization of subforms of the guanine-nucleotide-binding proteins G alpha i and G alpha o. Eur J Biochem. 1989 Aug 15;183(3):687–692. doi: 10.1111/j.1432-1033.1989.tb21099.x. [DOI] [PubMed] [Google Scholar]
- Luetje C. W., Tietje K. M., Christian J. L., Nathanson N. M. Differential tissue expression and developmental regulation of guanine nucleotide binding regulatory proteins and their messenger RNAs in rat heart. J Biol Chem. 1988 Sep 15;263(26):13357–13365. [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S., Pang I. H., Gilman A. G., Sternweis P. C. Chromatographic resolution and immunologic identification of the alpha 40 and alpha 41 subunits of guanine nucleotide-binding regulatory proteins from bovine brain. J Biol Chem. 1988 Feb 5;263(4):2020–2026. [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Ransnäs L. A., Insel P. A. Quantitation of the guanine nucleotide binding regulatory protein Gs in S49 cell membranes using antipeptide antibodies to alpha s. J Biol Chem. 1988 Jul 5;263(19):9482–9485. [PubMed] [Google Scholar]
- Robishaw J. D., Kalman V. K., Moomaw C. R., Slaughter C. A. Existence of two gamma subunits of the G proteins in brain. J Biol Chem. 1989 Sep 25;264(27):15758–15761. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Skorecki K. L., Verkman A. S., Ausiello D. A. Cross talk between stimulatory and inhibitory guanosine 5'-triphosphate binding proteins: role in activation and desensitization of the adenylate cyclase response to vasopressin. Biochemistry. 1987 Jan 27;26(2):639–645. doi: 10.1021/bi00376a040. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Reconstitution of the uncoupled variant of the S40 lymphoma cell. J Biol Chem. 1979 May 10;254(9):3333–3340. [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Strathmann M., Wilkie T. M., Simon M. I. Diversity of the G-protein family: sequences from five additional alpha subunits in the mouse. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7407–7409. doi: 10.1073/pnas.86.19.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Kanaho Y., Hewlett E. L., Moss J. Effects of guanyl nucleotides and rhodopsin on ADP-ribosylation of the inhibitory GTP-binding component of adenylate cyclase by pertussis toxin. J Biol Chem. 1984 Dec 25;259(24):15320–15323. [PubMed] [Google Scholar]
- Watkins D. C., Northup J. K., Malbon C. C. Pertussis toxin treatment in vivo is associated with a decline in G-protein beta-subunits. J Biol Chem. 1989 Mar 5;264(7):4186–4194. [PubMed] [Google Scholar]
- Watkins D. C., Northup J. K., Malbon C. C. Regulation of G-proteins in differentiation. Altered ratio of alpha- to beta-subunits in 3T3-L1 cells. J Biol Chem. 1987 Aug 5;262(22):10651–10657. [PubMed] [Google Scholar]
- Woolkalis M. J., Manning D. R. Structural characteristics of the 35- and 36-kDa forms of the beta subunit common to GTP-binding regulatory proteins. Mol Pharmacol. 1987 Jul;32(1):1–6. [PubMed] [Google Scholar]





