Abstract
The precise localization of the epitopes for six monoclonal antibodies specific for the N-terminal region of human platelet glycoprotein IIIa (GPIIIa) was determined. The epitope for P37, a monoclonal antibody that inhibits platelet aggregation, was found at GPIIIa 101-109, flanked by the epitopes for P23-3 (GPIIIa 16-28), P23-4 (GPIIIa 83-91), P23-5 (GPIIIa 67-73), P23-7 (GPIIIa 114-122) and P40 (GPIIIa 262-302), and very close to the early chymotryptic cleavage site of GPIIIa in whole platelets (Phe-100). When the amino acid sequence of GPIIIa was searched for peptide sequences hydropathically complementary to the fibrinogen gamma-chain C-terminal (gamma 400-411) and A alpha-chain RGD-containing peptides, none was found for the gamma 400-411, two (GPIIIa 128-132 and 380-384) were found complementary to fibrinogen A alpha 571-575 and two (GPIIIa 109-113 and 129-133) were found for A alpha 94-99. Two of these putative fibrinogen-binding sites overlap with each other, and a third one overlaps with the epitope for P37. These findings reinforce the earlier suggestion that the N-terminal region of GPIIIa is involved in fibrinogen binding, and suggest the existence in GPIIIa of either multiple or alternative RGD-binding sites or one RGD-binding domain with several moieties. Finally, early chymotryptic cleavage of GPIIIa in whole platelets liberates to the soluble fraction the peptide stretch Ser-101-Tyr-348, which carries the epitope for P37 and the putative binding sites for fibrinogen. The rest of the molecule, together with the GPIIb-resistant moiety, remains membrane-bound. This leads us to propose that the fibrinogen-binding domain of GPIIIa is not involved in the binding to GPIIb to form the Ca2(+)-dependent GPIIb-GPIIIa complex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beer J., Coller B. S. Evidence that platelet glycoprotein IIIa has a large disulfide-bonded loop that is susceptible to proteolytic cleavage. J Biol Chem. 1989 Oct 15;264(29):17564–17573. [PubMed] [Google Scholar]
- Blalock J. E., Smith E. M. Hydropathic anti-complementarity of amino acids based on the genetic code. Biochem Biophys Res Commun. 1984 May 31;121(1):203–207. doi: 10.1016/0006-291x(84)90707-1. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Blalock J. E. Similarity between the corticotropin (ACTH) receptor and a peptide encoded by an RNA that is complementary to ACTH mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1372–1375. doi: 10.1073/pnas.82.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentani R. R., Ribeiro S. F., Potocnjak P., Pasqualini R., Lopes J. D., Nakaie C. R. Characterization of the cellular receptor for fibronectin through a hydropathic complementarity approach. Proc Natl Acad Sci U S A. 1988 Jan;85(2):364–367. doi: 10.1073/pnas.85.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Arias J., Alvarez M. V., Lopez M. M., Henschen A., Gonzalez-Rodriguez J. Further studies on the topography of human platelet glycoprotein IIb. Localization of monoclonal antibody epitopes and the putative glycoprotein IIa- and fibrinogen-binding regions. Biochem J. 1991 Feb 1;273(Pt 3):767–775. doi: 10.1042/bj2730767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Henschen A., González-Rodríguez J. Assignment of disulphide bonds in human platelet GPIIIa. A disulphide pattern for the beta-subunits of the integrin family. Biochem J. 1991 Feb 15;274(Pt 1):63–71. doi: 10.1042/bj2740063. [DOI] [PMC free article] [PubMed] [Google Scholar]
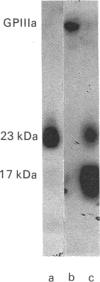
- Calvete J. J., Rivas G., Maruri M., Alvarez M. V., McGregor J. L., Hew C. L., Gonzalez-Rodriguez J. Tryptic digestion of human GPIIIa. Isolation and biochemical characterization of the 23 kDa N-terminal glycopeptide carrying the antigenic determinant for a monoclonal antibody (P37) which inhibits platelet aggregation. Biochem J. 1988 Mar 15;250(3):697–704. doi: 10.1042/bj2500697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Lam S. C., Plow E. F. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988 Oct 7;242(4875):91–93. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- Eirín M. T., Calvete J. J., González-Rodríguez J. New isolation procedure and further biochemical characterization of glycoproteins IIb and IIIa from human platelet plasma membrane. Biochem J. 1986 Nov 15;240(1):147–153. doi: 10.1042/bj2400147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kant J. A., Lord S. T., Crabtree G. R. Partial mRNA sequences for human A alpha, B beta, and gamma fibrinogen chains: evolutionary and functional implications. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3953–3957. doi: 10.1073/pnas.80.13.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornecki E., Chung S. Y., Holt J. C., Cierniewski C. S., Tuszynski G. P., Niewiarowski S. Identification of PlAl alloantigen domain on a 66 kDa protein derived from glycoprotein IIIa of human platelets. Biochim Biophys Acta. 1985 Sep 10;818(3):285–290. doi: 10.1016/0005-2736(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Kornecki E., Lee H., Merlin F., Hershock D., Tuszynski G. P., Niewiarowski S. Comparison of platelet fibrinogen receptors on intact and proteolytically-treated platelets by use of an anti-glycoprotein IIIa monoclonal antibody (MA 123). Thromb Res. 1984 Apr 1;34(1):35–49. doi: 10.1016/0049-3848(84)90104-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Melero J. A., Gonzalez-Rodriguez J. Preparation of monoclonal antibodies against glycoprotein IIIa of human platelets. Their effect on platelet aggregation. Eur J Biochem. 1984 Jun 1;141(2):421–427. doi: 10.1111/j.1432-1033.1984.tb08208.x. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Allen R. W., Kahn R. A., Kunicki T. J. Quantitation of membrane glycoprotein IIIa on intact human platelets using the monoclonal antibody, AP-3. Blood. 1985 Jan;65(1):227–232. [PubMed] [Google Scholar]
- Newman P. J., McEver R. P., Doers M. P., Kunicki T. J. Synergistic action of two murine monoclonal antibodies that inhibit ADP-induced platelet aggregation without blocking fibrinogen binding. Blood. 1987 Feb;69(2):668–676. [PubMed] [Google Scholar]
- Niewiarowski S., Norton K. J., Eckardt A., Lukasiewicz H., Holt J. C., Kornecki E. Structural and functional characterization of major platelet membrane components derived by limited proteolysis of glycoprotein IIIa. Biochim Biophys Acta. 1989 Jul 24;983(1):91–99. doi: 10.1016/0005-2736(89)90384-2. [DOI] [PubMed] [Google Scholar]
- Pasqualini R., Chamone D. F., Brentani R. R. Determination of the putative binding site for fibronectin on platelet glycoprotein IIb-IIIa complex through a hydropathic complementarity approach. J Biol Chem. 1989 Aug 25;264(24):14566–14570. [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Rixon M. W., Chan W. Y., Davie E. W., Chung D. W. Characterization of a complementary deoxyribonucleic acid coding for the alpha chain of human fibrinogen. Biochemistry. 1983 Jun 21;22(13):3237–3244. doi: 10.1021/bi00282a031. [DOI] [PubMed] [Google Scholar]
- Rixon M. W., Chung D. W., Davie E. W. Nucleotide sequence of the gene for the gamma chain of human fibrinogen. Biochemistry. 1985 Apr 9;24(8):2077–2086. doi: 10.1021/bi00329a041. [DOI] [PubMed] [Google Scholar]
- Rosa J. P., Bray P. F., Gayet O., Johnston G. I., Cook R. G., Jackson K. W., Shuman M. A., McEver R. P. Cloning of glycoprotein IIIa cDNA from human erythroleukemia cells and localization of the gene to chromosome 17. Blood. 1988 Aug;72(2):593–600. [PubMed] [Google Scholar]
- Santoro S. A., Lawing W. J., Jr Competition for related but nonidentical binding sites on the glycoprotein IIb-IIIa complex by peptides derived from platelet adhesive proteins. Cell. 1987 Mar 13;48(5):867–873. doi: 10.1016/0092-8674(87)90083-3. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Cheresh D. A. The Arg-Gly-Asp binding domain of the vitronectin receptor. Photoaffinity cross-linking implicates amino acid residues 61-203 of the beta subunit. J Biol Chem. 1988 Dec 15;263(35):18726–18731. [PubMed] [Google Scholar]
- Thurlow P. J., Barlow B., Connellan J. M., McKenzie I. F. Detection of glycoprotein IIb and IIIa by monoclonal antibodies. Br J Haematol. 1983 Sep;55(1):123–134. doi: 10.1111/j.1365-2141.1983.tb01230.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usobiaga P., Calvete J. J., Saíz J. L., Eirín M. T., González-Rodríguez J. Molecular characterization of human platelet glycoproteins IIIa and IIb and the subunits of the latter. Eur Biophys J. 1987;14(4):211–218. doi: 10.1007/BF00256354. [DOI] [PubMed] [Google Scholar]