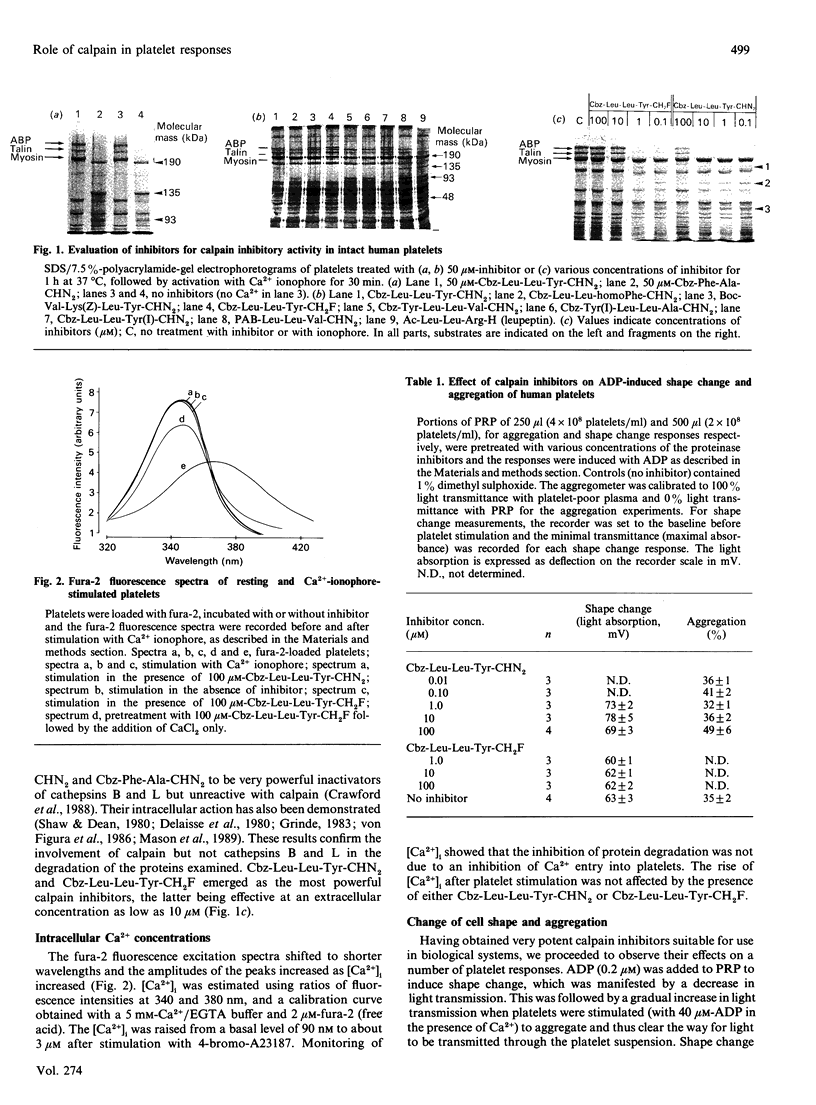
Abstract
A series of peptidyl diazomethanes and monofluoromethane with structures specific for calpain have been synthesized and tested for their ability to inhibit calpain activity in vivo, using human platelets as a model system. Calpain activity in vivo was determined by observing proteolysis of actin-binding protein and talin, two known substrates of calpain. Very potent inhibitors, which emerged from this study, were used to investigate the role of calpain in some platelet response processes. Our results show that calpain-mediated proteolysis in platelets is not an obligatory event leading to change of cell shape, adhesion to glass and spreading, aggregation and 5-hydroxytryptamine release. Two of the inhibitors were iodinated with 125I and used to radiolabel the enzyme in vivo. To our knowledge, this work also represents the first report describing the affinity labelling of calpain in human platelets using irreversible radioactive inhibitors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angliker H., Wikstrom P., Kirschke H., Shaw E. The inactivation of the cysteinyl exopeptidases cathepsin H and C by affinity-labelling reagents. Biochem J. 1989 Aug 15;262(1):63–68. doi: 10.1042/bj2620063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angliker H., Wikstrom P., Rauber P., Shaw E. The synthesis of lysylfluoromethanes and their properties as inhibitors of trypsin, plasmin and cathepsin B. Biochem J. 1987 Feb 1;241(3):871–875. doi: 10.1042/bj2410871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angliker H., Wikström P., Rauber P., Stone S., Shaw E. Synthesis and properties of peptidyl derivatives of arginylfluoromethanes. Biochem J. 1988 Dec 1;256(2):481–486. doi: 10.1042/bj2560481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle M. C., O'Halloran T., Burridge K. Demonstration of a relationship between talin and P235, a major substrate of the calcium-dependent protease in platelets. J Cell Biochem. 1986;30(3):259–270. doi: 10.1002/jcb.240300307. [DOI] [PubMed] [Google Scholar]
- Billger M., Wallin M., Karlsson J. O. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium. 1988 Feb;9(1):33–44. doi: 10.1016/0143-4160(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Cong J., Goll D. E., Peterson A. M., Kapprell H. P. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J Biol Chem. 1989 Jun 15;264(17):10096–10103. [PubMed] [Google Scholar]
- Crawford C., Mason R. W., Wikstrom P., Shaw E. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem J. 1988 Aug 1;253(3):751–758. doi: 10.1042/bj2530751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C., Willis A. C., Gagnon J. The effects of autolysis on the structure of chicken calpain II. Biochem J. 1987 Dec 1;248(2):579–588. doi: 10.1042/bj2480579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. Inhibition of bone resorption in culture by inhibitors of thiol proteinases. Biochem J. 1980 Oct 15;192(1):365–368. doi: 10.1042/bj1920365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elce J. S., Sigmund L., Fox M. J. Calpain I activation is not correlated with aggregation in human platelets. Biochem J. 1989 Aug 1;261(3):1039–1042. doi: 10.1042/bj2611039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Kawasaki H., Sugihara H., Imajoh S., Kawashima S., Suzuki K. Isolation and sequence analyses of cDNA clones for the large subunits of two isozymes of rabbit calcium-dependent protease. J Biol Chem. 1986 Jul 15;261(20):9465–9471. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Morrow J. S., Phillips D. R. Spectrin is associated with membrane-bound actin filaments in platelets and is hydrolyzed by the Ca2+-dependent protease during platelet activation. Blood. 1987 Feb;69(2):537–545. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- Green G. D., Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem. 1981 Feb 25;256(4):1923–1928. [PubMed] [Google Scholar]
- Grinde B. The thiol proteinase inhibitors, Z-Phe-PheCHN2 and Z-Phe-AlaCHN2, inhibit lysosomal protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1983 May 4;757(1):15–20. doi: 10.1016/0304-4165(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hatanaka M., Yoshimura N., Murakami T., Kannagi R., Murachi T. Evidence for membrane-associated calpain I in human erythrocytes. Detection by an immunoelectrophoretic blotting method using monospecific antibody. Biochemistry. 1984 Jul 3;23(14):3272–3276. doi: 10.1021/bi00309a023. [DOI] [PubMed] [Google Scholar]
- Herman B., Roe M. W., Harris C., Wray B., Clemmons D. Platelet-derived growth factor-induced alterations in vinculin distribution in porcine vascular smooth muscle cells. Cell Motil Cytoskeleton. 1987;8(2):91–105. doi: 10.1002/cm.970080202. [DOI] [PubMed] [Google Scholar]
- Imajoh S., Aoki K., Ohno S., Emori Y., Kawasaki H., Sugihara H., Suzuki K. Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease. Biochemistry. 1988 Oct 18;27(21):8122–8128. doi: 10.1021/bi00421a022. [DOI] [PubMed] [Google Scholar]
- Imperiali B. Synthetic fluoropeptides as pharmacologically useful compounds. Adv Biotechnol Processes. 1988;10:97–129. [PubMed] [Google Scholar]
- Ishii H., Kuboki M., Fujii J., Hiraishi S., Kazama M. Thiolprotease inhibitor, EST, can inhibit thrombin-induced platelet activation. Thromb Res. 1990 Mar 15;57(6):847–861. doi: 10.1016/0049-3848(90)90152-3. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y., Tsujinaka T., Sakon M., Kambayashi J., Ohshiro T., Murachi T., Mori T. Elucidation of calpain dependent phosphorylation of myosin light chain in human platelets. Biochem Int. 1987 Nov;15(5):935–944. [PubMed] [Google Scholar]
- Kirschke H., Wikstrom P., Shaw E. Active center differences between cathepsins L and B: the S1 binding region. FEBS Lett. 1988 Feb 8;228(1):128–130. doi: 10.1016/0014-5793(88)80600-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laubscher A., Pletscher A. Shape change and uptake of 5-hydroxytryptamine in human blood platelets: action of neuropsychotropic drugs. Life Sci. 1979 May 14;24(20):1833–1840. doi: 10.1016/0024-3205(79)90233-9. [DOI] [PubMed] [Google Scholar]
- Lynch G., Baudry M. Brain spectrin, calpain and long-term changes in synaptic efficacy. Brain Res Bull. 1987 Jun;18(6):809–815. doi: 10.1016/0361-9230(87)90220-6. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E. B., Becker E., Detwiler T. C. Inhibition of calpain in intact platelets by the thiol protease inhibitor E-64d. Biochem Biophys Res Commun. 1989 Jan 31;158(2):432–435. doi: 10.1016/s0006-291x(89)80065-8. [DOI] [PubMed] [Google Scholar]
- Miyake S., Emori Y., Suzuki K. Gene organization of the small subunit of human calcium-activated neutral protease. Nucleic Acids Res. 1986 Nov 25;14(22):8805–8817. doi: 10.1093/nar/14.22.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Okita J. R., Frojmovic M. M., Kristopeit S., Wong T., Kunicki T. J. Montreal platelet syndrome: a defect in calcium-activated neutral proteinase (calpain). Blood. 1989 Aug 1;74(2):715–721. [PubMed] [Google Scholar]
- Parkes C., Kembhavi A. A., Barrett A. J. Calpain inhibition by peptide epoxides. Biochem J. 1985 Sep 1;230(2):509–516. doi: 10.1042/bj2300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perides G., Kühn S., Scherbarth A., Traub P. Probing of the structural stability of vimentin and desmin-type intermediate filaments with Ca2+-activated proteinase, thrombin and lysine-specific endoproteinase Lys-C. Eur J Cell Biol. 1987 Jun;43(3):450–458. [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Puri R. N., Zhou F. X., Bradford H., Hu C. J., Colman R. F., Colman R. W. Thrombin-induced platelet aggregation involves an indirect proteolytic cleavage of aggregin by calpain. Arch Biochem Biophys. 1989 Jun;271(2):346–358. doi: 10.1016/0003-9861(89)90284-1. [DOI] [PubMed] [Google Scholar]
- Rao G. H., White J. G., Cox C. A. Influence of a calcium dependent protease inhibitor on platelet activation and secretion. Thromb Res. 1987 Sep 15;47(6):625–637. doi: 10.1016/0049-3848(87)90101-0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Horne W. C. Ionomycin can elevate intraplatelet Ca2+ and activate phospholipase A without activating phospholipase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):393–397. doi: 10.1016/0006-291x(84)90426-1. [DOI] [PubMed] [Google Scholar]
- Schollmeyer J. E. Calpain II involvement in mitosis. Science. 1988 May 13;240(4854):911–913. doi: 10.1126/science.2834825. [DOI] [PubMed] [Google Scholar]
- Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-oka T., Shin W. S., Okai Y., Dan Y., Morita M., Iizuka M., Sugimoto T. Collagen-stimulated human platelet aggregation is mediated by endogenous calcium-activated neutral protease. Circ Res. 1989 Feb;64(2):407–410. doi: 10.1161/01.res.64.2.407. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsuchida K., Aihara H., Isogai K., Hanada K., Shibata N. Degradation of myocardial structural proteins in myocardial infarcted dogs is reduced by Ep459, a cysteine proteinase inhibitor. Biol Chem Hoppe Seyler. 1986 Jan;367(1):39–45. doi: 10.1515/bchm3.1986.367.1.39. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T., Kajiwara Y., Kambayashi J., Sakon M., Higuchi N., Tanaka T., Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin). Biochem Biophys Res Commun. 1988 Jun 30;153(3):1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- Verhallen P. F., Bevers E. M., Comfurius P., Zwaal R. F. Correlation between calpain-mediated cytoskeletal degradation and expression of platelet procoagulant activity. A role for the platelet membrane-skeleton in the regulation of membrane lipid asymmetry? Biochim Biophys Acta. 1987 Sep 18;903(1):206–217. doi: 10.1016/0005-2736(87)90170-2. [DOI] [PubMed] [Google Scholar]
- Verhallen P. F., Bevers E. M., Comfurius P., Zwaal R. F. Fluoride-dependent calcium-induced platelet procoagulant activity shows that calpain is involved in increased phospholipid transbilayer movement. Biochim Biophys Acta. 1988 Jul 7;942(1):150–158. doi: 10.1016/0005-2736(88)90284-2. [DOI] [PubMed] [Google Scholar]
- Ware J. A., Smith M., Salzman E. W. Synergism of platelet-aggregating agents. Role of elevation of cytoplasmic calcium. J Clin Invest. 1987 Jul;80(1):267–271. doi: 10.1172/JCI113058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. C., 2nd Calcium-dependent proteins in platelets: response of calcium-activated protease in normal and thrombasthenic platelets to aggregating agents. Biochim Biophys Acta. 1980 Aug 1;631(1):130–138. doi: 10.1016/0304-4165(80)90061-6. [DOI] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Conary J., Hasilik A., Shaw E. Heterogeneity in late-onset metachromatic leukodystrophy. Effect of inhibitors of cysteine proteinases. Am J Hum Genet. 1986 Sep;39(3):371–382. [PMC free article] [PubMed] [Google Scholar]