Abstract
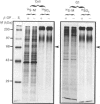
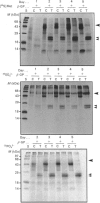
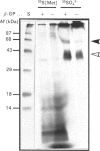
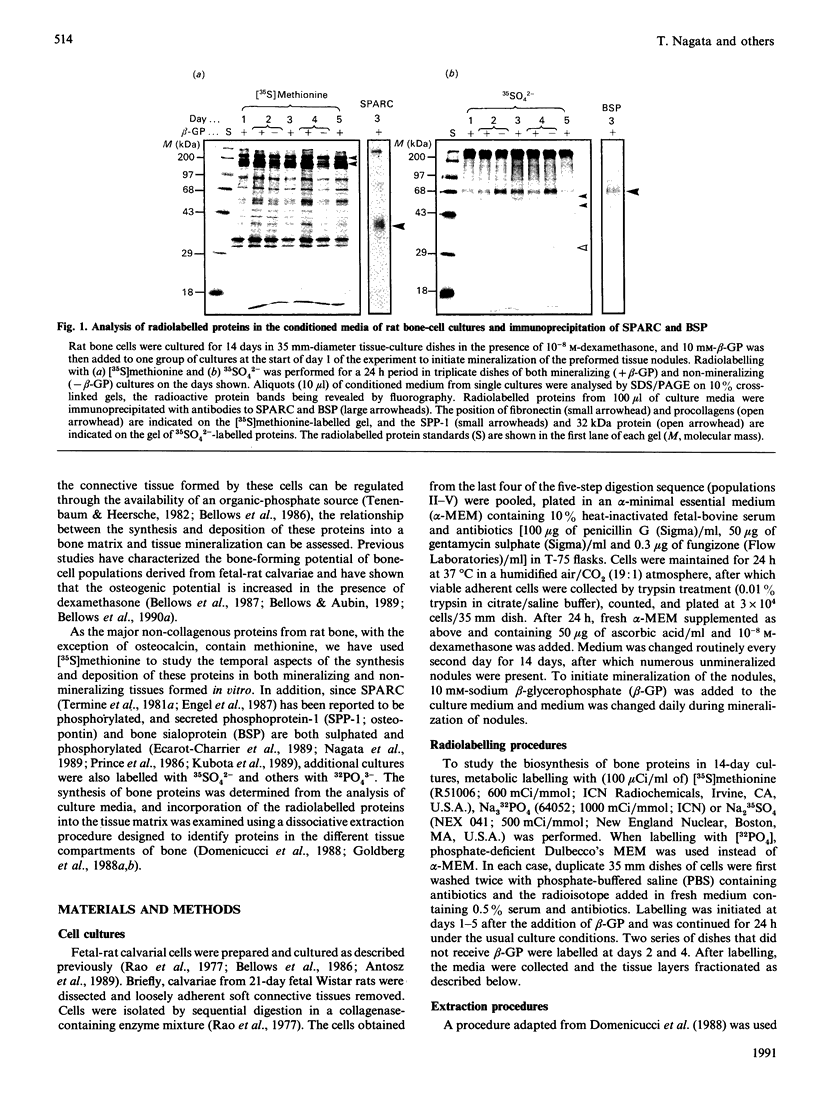
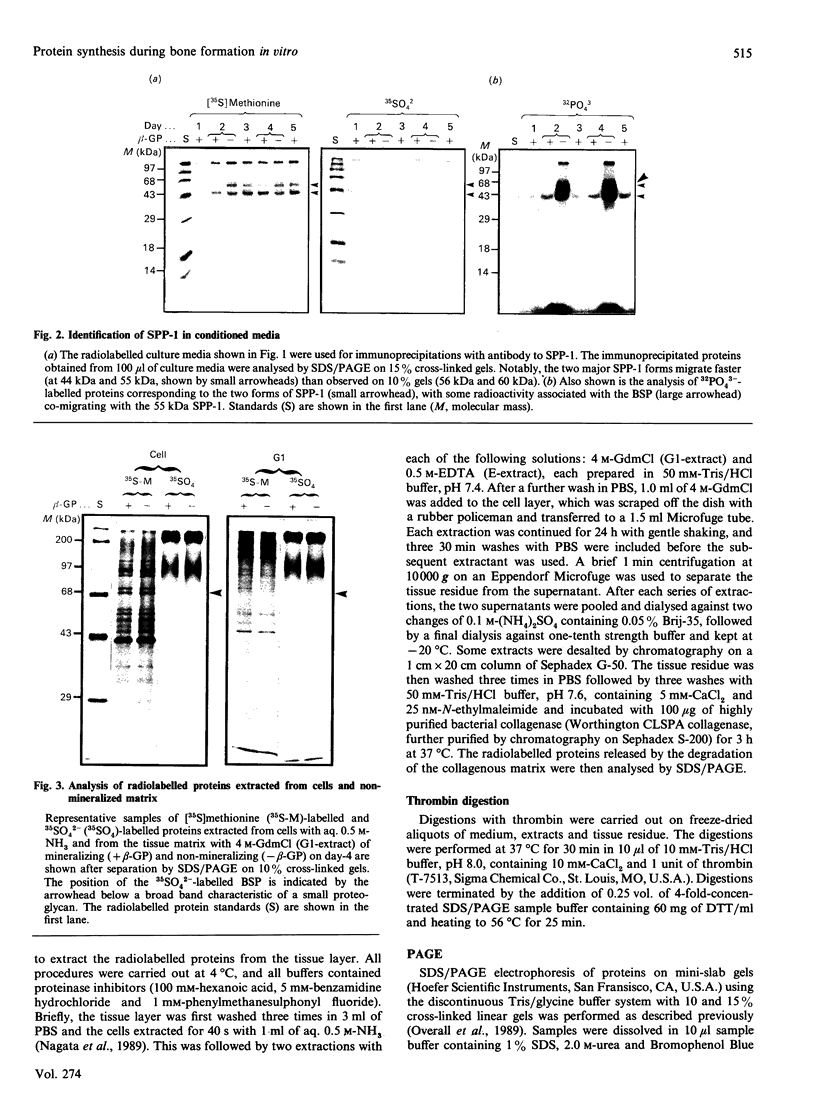
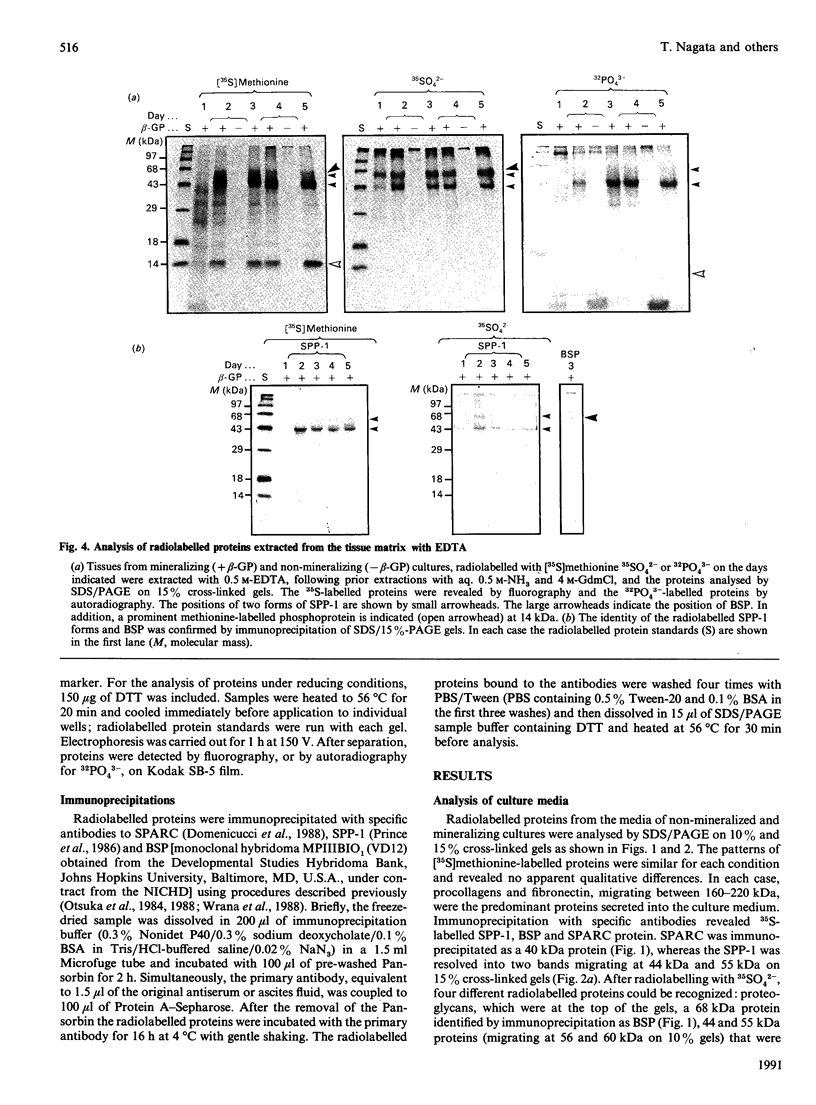
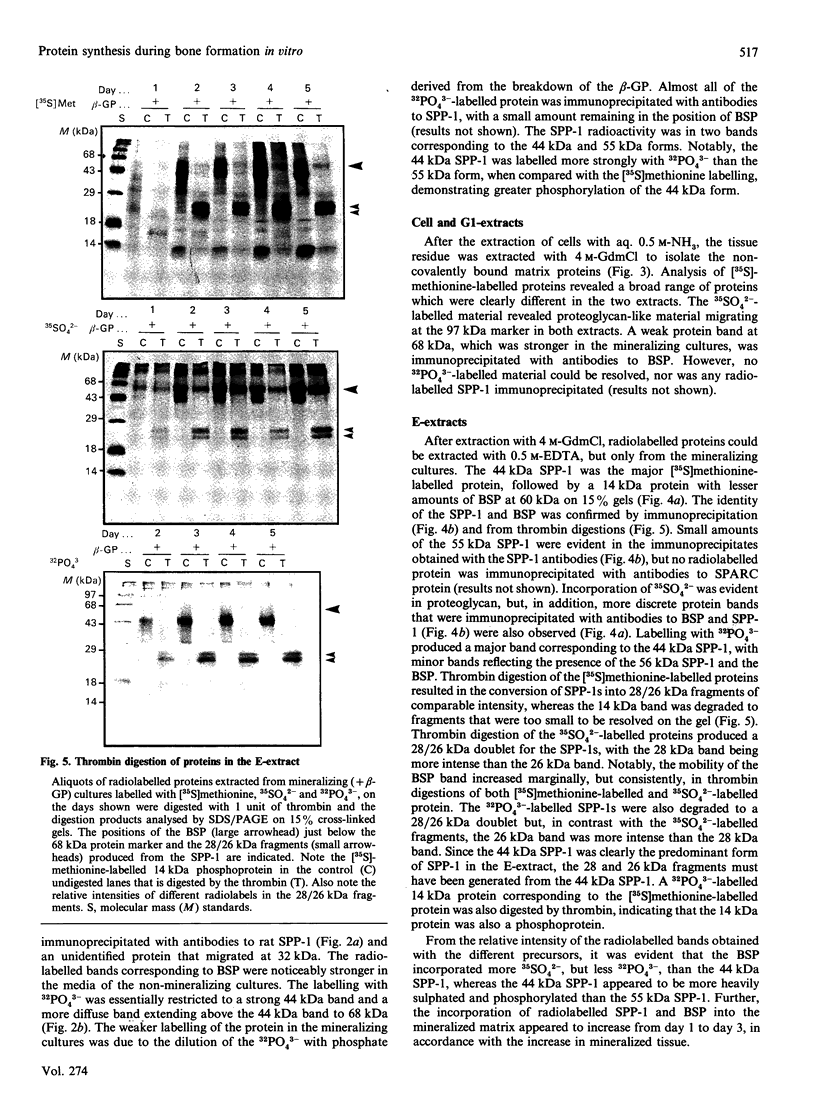
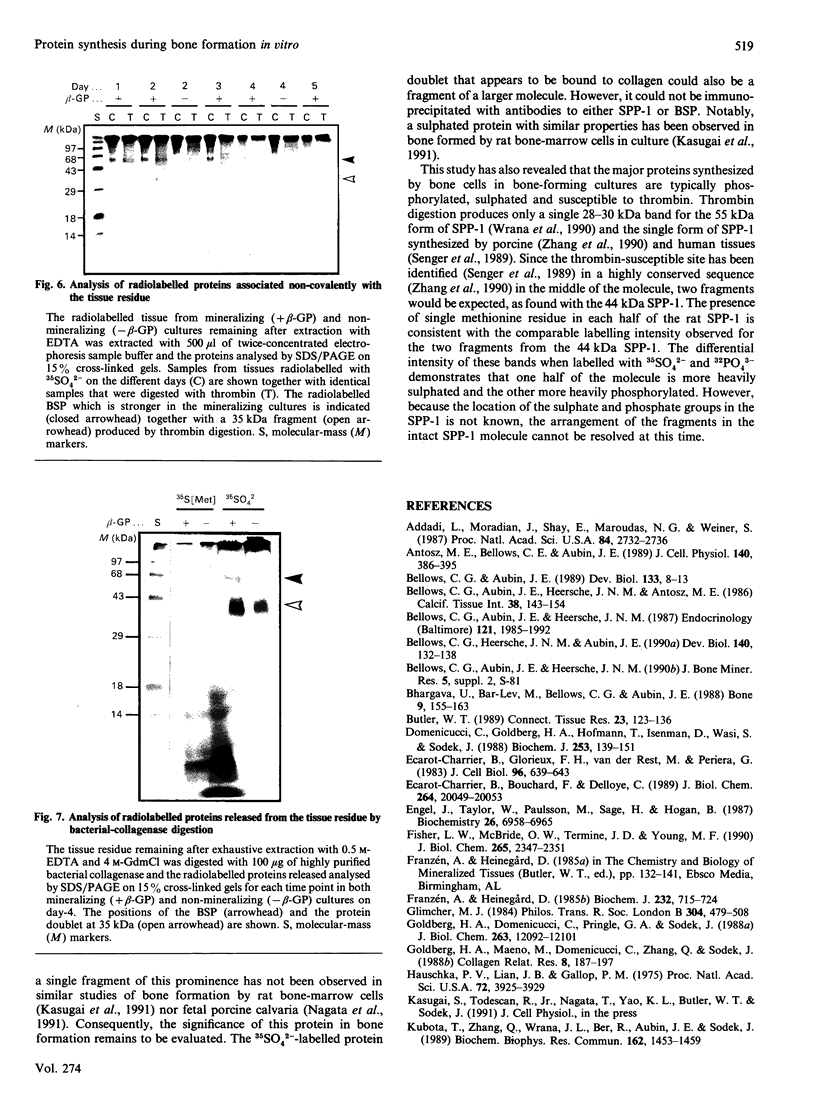
To determine the relationship between the expression of bone proteins and the formation of mineralized-tissue matrix, the biosynthesis of non-collagenous bone proteins was studied in cultures of fetal-rat calvarial cells, which form mineralized nodules of bone-like tissue in the presence of beta-glycerophosphate. The temporal pattern of protein synthesis in both mineralizing and non-mineralizing cultures was studied by metabolic labelling with [35S]methionine, 35SO4(2-) or 32PO4(3-) over a 5-day period. After a 24 h labelling period, the culture media were harvested and the cell layers extracted sequentially with aq. 0.5 M-NH3, followed by 4 M-guanidinium chloride (GdmCl), 0.5 M-EDTA and a second extraction with 4 M-GdmCl. Protein associated with collagenous bone matrix was analysed after digestion with bacterial collagenase. On the basis of [35S]methionine labelling, the major proteins extracted from the mineralizing matrix were secreted phosphoprotein-1 (SPP-1; osteopontin), bone sialoprotein (BSP) and a 14 kDa phosphoprotein. The presence of SPP-1 and BSP in the conditioned media of both mineralizing and non-mineralizing cultures and their incorporation into the mineralizing nodules indicated that these proteins associate with preformed mineral crystals. However, some BSP was also present in GdmCl extracts and, together with a 35 kDa sulphated protein, was released from a bacterial-collagenase digestion of the tissue residue in both non-mineralizing and mineralizing cultures. Two forms of sulphated SPP-1 were identified, a highly phosphorylated 44 kDa species being the predominant form in the mineralized matrix. The BSP was more highly sulphated but less phosphorylated than SPP-1. Bone SPARC (secreted protein, acid and rich in cysteine) protein (osteonectin) was present almost entirely in the conditioned media and did not incorporate 32PO4(3-) or 35SO4(2-). The SPP-1 and the 14 kDa protein were susceptible to thrombin digestion, the 44 kDa SPP-1 being specifically cleaved into 28 and 26 kDa fragments. The fragments were labelled uniformly with [35S]methionine, but the 28 kDa fragment incorporated more 35SO4(2-), but less 32PO4(3-), than the 26 kDa fragment. These studies demonstrate that SPP-1 and BSP are the major osteoblast-derived bone proteins to bind to the bone mineral. That BSP also binds to the collagenous bone matrix indicates a potential role for this protein in linking the hydroxyapatite with collagen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addadi L., Moradian J., Shay E., Maroudas N. G., Weiner S. A chemical model for the cooperation of sulfates and carboxylates in calcite crystal nucleation: Relevance to biomineralization. Proc Natl Acad Sci U S A. 1987 May;84(9):2732–2736. doi: 10.1073/pnas.84.9.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosz M. E., Bellows C. G., Aubin J. E. Effects of transforming growth factor beta and epidermal growth factor on cell proliferation and the formation of bone nodules in isolated fetal rat calvaria cells. J Cell Physiol. 1989 Aug;140(2):386–395. doi: 10.1002/jcp.1041400225. [DOI] [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E. Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol. 1989 May;133(1):8–13. doi: 10.1016/0012-1606(89)90291-1. [DOI] [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E., Heersche J. N., Antosz M. E. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986 Mar;38(3):143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E., Heersche J. N. Physiological concentrations of glucocorticoids stimulate formation of bone nodules from isolated rat calvaria cells in vitro. Endocrinology. 1987 Dec;121(6):1985–1992. doi: 10.1210/endo-121-6-1985. [DOI] [PubMed] [Google Scholar]
- Bellows C. G., Heersche J. N., Aubin J. E. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol. 1990 Jul;140(1):132–138. doi: 10.1016/0012-1606(90)90060-v. [DOI] [PubMed] [Google Scholar]
- Bhargava U., Bar-Lev M., Bellows C. G., Aubin J. E. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 1988;9(3):155–163. doi: 10.1016/8756-3282(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Domenicucci C., Goldberg H. A., Hofmann T., Isenman D., Wasi S., Sodek J. Characterization of porcine osteonectin extracted from foetal calvariae. Biochem J. 1988 Jul 1;253(1):139–151. doi: 10.1042/bj2530139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Bouchard F., Delloye C. Bone sialoprotein II synthesized by cultured osteoblasts contains tyrosine sulfate. J Biol Chem. 1989 Nov 25;264(33):20049–20053. [PubMed] [Google Scholar]
- Ecarot-Charrier B., Glorieux F. H., van der Rest M., Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol. 1983 Mar;96(3):639–643. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Taylor W., Paulsson M., Sage H., Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987 Nov 3;26(22):6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., McBride O. W., Termine J. D., Young M. F. Human bone sialoprotein. Deduced protein sequence and chromosomal localization. J Biol Chem. 1990 Feb 5;265(4):2347–2351. [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher M. J. Recent studies of the mineral phase in bone and its possible linkage to the organic matrix by protein-bound phosphate bonds. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):479–508. doi: 10.1098/rstb.1984.0041. [DOI] [PubMed] [Google Scholar]
- Goldberg H. A., Domenicucci C., Pringle G. A., Sodek J. Mineral-binding proteoglycans of fetal porcine calvarial bone. J Biol Chem. 1988 Aug 25;263(24):12092–12101. [PubMed] [Google Scholar]
- Goldberg H. A., Maeno M., Domenicucci C., Qi Z. N., Sodek J. Identification of small collagenous proteins with properties of procollagen alpha 1 (I) pN-propeptide in fetal porcine calvarial bone. Coll Relat Res. 1988 May;8(3):187–197. doi: 10.1016/s0174-173x(88)80039-6. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Zhang Q., Wrana J. L., Ber R., Aubin J. E., Butler W. T., Sodek J. Multiple forms of SppI (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1453–1459. doi: 10.1016/0006-291x(89)90837-1. [DOI] [PubMed] [Google Scholar]
- Linde A., Bhown M., Butler W. T. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980 Jun 25;255(12):5931–5942. [PubMed] [Google Scholar]
- Linde A., Lussi A. Mineral induction by polyanionic dentin and bone proteins at physiological ionic conditions. Connect Tissue Res. 1989;21(1-4):197–203. doi: 10.3109/03008208909050009. [DOI] [PubMed] [Google Scholar]
- Maniatopoulos C., Sodek J., Melcher A. H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988 Nov;254(2):317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Oosawa T., Gay S., Bronckers A. L., Butler W. T. Immunohistochemical demonstration of a 44-KD phosphoprotein in developing rat bones. J Histochem Cytochem. 1987 Jul;35(7):707–715. doi: 10.1177/35.7.3295029. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menanteau J., Neuman W. F., Neuman M. W. A study of bone proteins which can prevent hydroxyapatite formation. Metab Bone Dis Relat Res. 1982;4(2):157–162. doi: 10.1016/0221-8747(82)90030-3. [DOI] [PubMed] [Google Scholar]
- Nagata T., Todescan R., Goldberg H. A., Zhang Q., Sodek J. Sulphation of secreted phosphoprotein I (SPPI, osteopontin) is associated with mineralized tissue formation. Biochem Biophys Res Commun. 1989 Nov 30;165(1):234–240. doi: 10.1016/0006-291x(89)91059-0. [DOI] [PubMed] [Google Scholar]
- Nefussi J. R., Boy-Lefevre M. L., Boulekbache H., Forest N. Mineralization in vitro of matrix formed by osteoblasts isolated by collagenase digestion. Differentiation. 1985;29(2):160–168. doi: 10.1111/j.1432-0436.1985.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988 Dec 25;263(36):19430–19432. [PubMed] [Google Scholar]
- Otsuka K., Pitaru S., Overall C. M., Aubin J. E., Sodek J. Biochemical comparison of fibroblast populations from different periodontal tissues: characterization of matrix protein and collagenolytic enzyme synthesis. Biochem Cell Biol. 1988 Mar;66(3):167–176. doi: 10.1139/o88-023. [DOI] [PubMed] [Google Scholar]
- Otsuka K., Yao K. L., Wasi S., Tung P. S., Aubin J. E., Sodek J., Termine J. D. Biosynthesis of osteonectin by fetal porcine calvarial cells in vitro. J Biol Chem. 1984 Aug 10;259(15):9805–9812. [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Price P. A., Poser J. W., Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Oosawa T., Butler W. T., Tomana M., Bhown A. S., Bhown M., Schrohenloher R. E. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987 Feb 25;262(6):2900–2907. [PubMed] [Google Scholar]
- Rao L. G., Ng B., Brunette D. M., Heersche J. N. Parathyroid hormone- and prostaglandin E1-response in a selected population of bone cells after repeated subculture and storage at -80C. Endocrinology. 1977 May;100(5):1233–1241. doi: 10.1210/endo-100-5-1233. [DOI] [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Lollar P., Riggs B. L., Mann K. G. Isolation and characterization of native adult osteonectin. J Biol Chem. 1985 Mar 10;260(5):2728–2736. [PubMed] [Google Scholar]
- Sato S., Rahemtulla F., Prince C. W., Tomana M., Butler W. T. Proteoglycans of adult bovine compact bone. Connect Tissue Res. 1985;14(1):65–75. doi: 10.3109/03008208509089844. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., Heersche J. N. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int. 1982 Jan;34(1):76–79. doi: 10.1007/BF02411212. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981 Oct 25;256(20):10403–10408. [PubMed] [Google Scholar]
- Wrana J. L., Kubota T., Zhang Q., Overall C. M., Aubin J. E., Butler W. T., Sodek J. Regulation of transformation-sensitive secreted phosphoprotein (SPPI/osteopontin) expression by transforming growth factor-beta. Comparisons with expression of SPARC (secreted acidic cysteine-rich protein). Biochem J. 1991 Feb 1;273(Pt 3):523–531. doi: 10.1042/bj2730523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Maeno M., Hawrylyshyn B., Yao K. L., Domenicucci C., Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988 Mar;106(3):915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Domenicucci C., Goldberg H. A., Wrana J. L., Sodek J. Characterization of fetal porcine bone sialoproteins, secreted phosphoprotein I (SPPI, osteopontin), bone sialoprotein, and a 23-kDa glycoprotein. Demonstration that the 23-kDa glycoprotein is derived from the carboxyl terminus of SPPI. J Biol Chem. 1990 May 5;265(13):7583–7589. [PubMed] [Google Scholar]
- Zung P., Domenicucci C., Wasi S., Kuwata F., Sodek J. Osteonectin is a minor component of mineralized connective tissues in rat. Biochem Cell Biol. 1986 Apr;64(4):356–362. doi: 10.1139/o86-049. [DOI] [PubMed] [Google Scholar]