Abstract
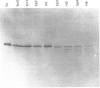
Iron concentration and ferritin distribution have been determined in different organs of pea (Pisum sativum) during development under conditions of continuous iron supply from hydroponic cultures. No ferritin was detected in total protein extracts from roots or leaves. However, a transient iron accumulation in the roots, which corresponds to an increase in iron uptake, was observed when young fruits started to develop. Ferritin was detectable in total protein extracts of flowers and pods, and it accumulated in seeds. In seeds, the same relative amount of ferritin was detected in cotyledons and in the embryo axis. In cotyledons, ferritin and iron concentration decrease progressively during the first week of germination. Ferritin in the embryo axis was processed, and disappeared, during germination, within the first 4 days of radicle and epicotyl growth. This degradation of ferritin in vivo was marked by a shortening of a 28 kDa subunit, giving 26.5 and 25 kDa polypeptides, reminiscent of the radical damage occurring in pea seed ferritin during iron exchange in vitro [Laulhere, Laboure & Briat (1989) J. Biol. Chem. 264, 3629-3635]. Developmental control of iron concentration and ferritin distribution in different organs of pea is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H. Micro methods for the quantitative determination of iron and copper in biological material. Methods Enzymol. 1978;54:435–445. doi: 10.1016/s0076-6879(78)54027-5. [DOI] [PubMed] [Google Scholar]
- Bienfait H. F., van den Briel M. L. Rapid mobilization of ferritin iron by ascorbate in the presence of oxygen. Biochim Biophys Acta. 1980 Sep 1;631(3):507–510. doi: 10.1016/0304-4165(80)90028-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branton D., Jacobson L. Iron Localization in Pea Plants. Plant Physiol. 1962 Jul;37(4):546–551. doi: 10.1104/pp.37.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo D. A., McFadden K. M., Garland T. R., Wildung R. E. Organic Constituents and Complexation of Nickel(II), Iron(III), Cadmium(II), and plutonium(IV) in Soybean Xylem Exudates. Plant Physiol. 1988 Mar;86(3):734–739. doi: 10.1104/pp.86.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Ponce-Ortiz Y., Koch M. H., Parfait R., Stuhrmann H. B. Isolation and characterization of phytoferritin from pea (Pisum sativum) and Lentil (Lens esculenta). Biochem J. 1978 May 1;171(2):349–356. doi: 10.1042/bj1710349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E., Lin S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987 Jul 15;262(20):9902–9907. [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E., Lin S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987 Jul 15;262(20):9902–9907. [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem. 1987 Jul 15;262(20):9908–9913. [PubMed] [Google Scholar]
- HYDE B. B., HODGE A. J., KAHN A., BIRNSTIEL M. L. STUDIES ON PHYTOFERRITIN. I. IDENTIFICATION AND LOCALIZATION. J Ultrastruct Res. 1963 Oct;59:248–258. doi: 10.1016/s0022-5320(63)80005-2. [DOI] [PubMed] [Google Scholar]
- Laulhere J. P., Laboure A. M., Briat J. F. Mechanism of the transition from plant ferritin to phytosiderin. J Biol Chem. 1989 Feb 25;264(6):3629–3635. [PubMed] [Google Scholar]
- Laulhere J. P., Lescure A. M., Briat J. F. Purification and characterization of ferritins from maize, pea, and soya bean seeds. Distribution in various pea organs. J Biol Chem. 1988 Jul 25;263(21):10289–10294. [PubMed] [Google Scholar]
- Laulhère J. P., Labouré A. M., Briat J. F. Photoreduction and incorporation of iron into ferritins. Biochem J. 1990 Jul 1;269(1):79–84. doi: 10.1042/bj2690079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure A. M., Massenet O., Briat J. F. Purification and characterization of an iron-induced ferritin from soybean (Glycine max) cell suspensions. Biochem J. 1990 Nov 15;272(1):147–150. doi: 10.1042/bj2720147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinos N. G. Multifunctional plastids in the meristematic region of potato tuber buds. J Ultrastruct Res. 1967 Jan;17(1):91–113. doi: 10.1016/s0022-5320(67)80023-6. [DOI] [PubMed] [Google Scholar]
- Proudhon D., Briat J. F., Lescure A. M. Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol. 1989 Jun;90(2):586–590. doi: 10.1104/pp.90.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguzzi F., Lesuisse E., Crichton R. R. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 1988 Apr 11;231(1):253–258. doi: 10.1016/0014-5793(88)80742-7. [DOI] [PubMed] [Google Scholar]
- Seckbach J. Studies on the deposition of plant ferritin as influenced by iron supply to iron-deficient beans. J Ultrastruct Res. 1968 Mar;22(5):413–423. doi: 10.1016/s0022-5320(68)90031-2. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- van der Mark F., Bienfait F., van den Ende H. Variable amounts of translatable ferritin mRNA in bean leaves with various iron contents. Biochem Biophys Res Commun. 1983 Sep 15;115(2):463–469. doi: 10.1016/s0006-291x(83)80167-3. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]