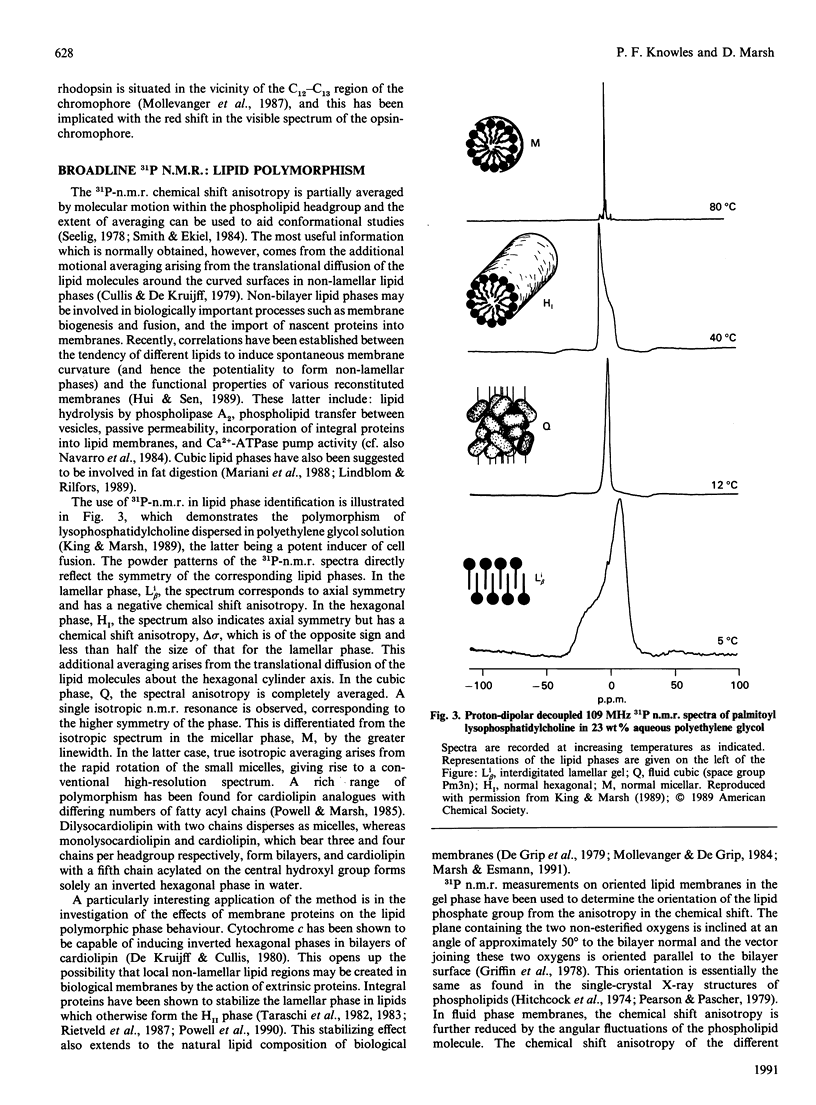
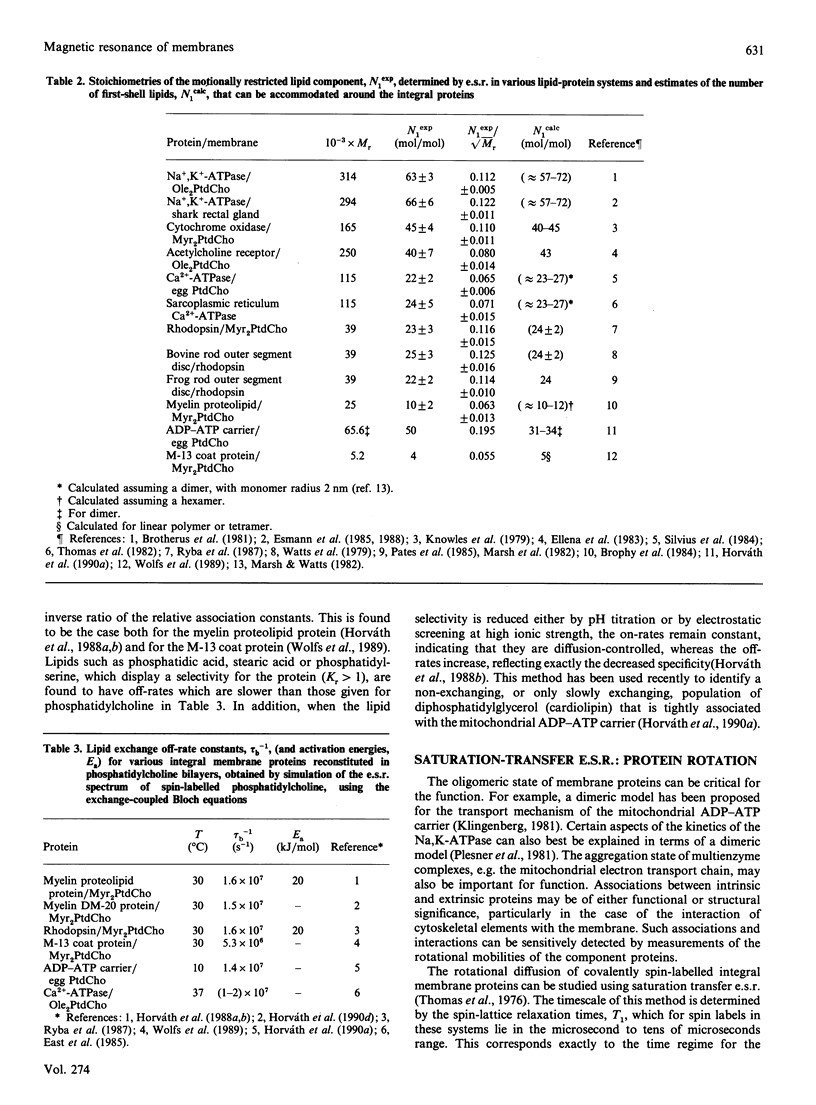
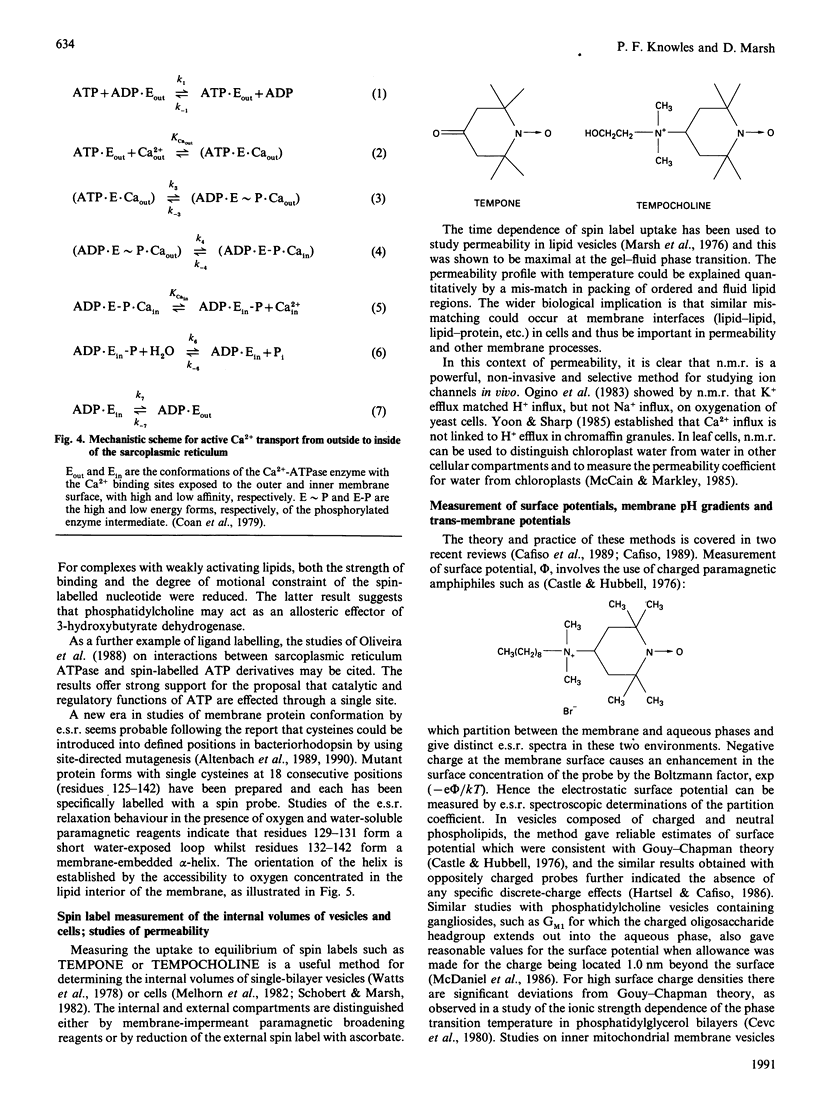
Full text
PDF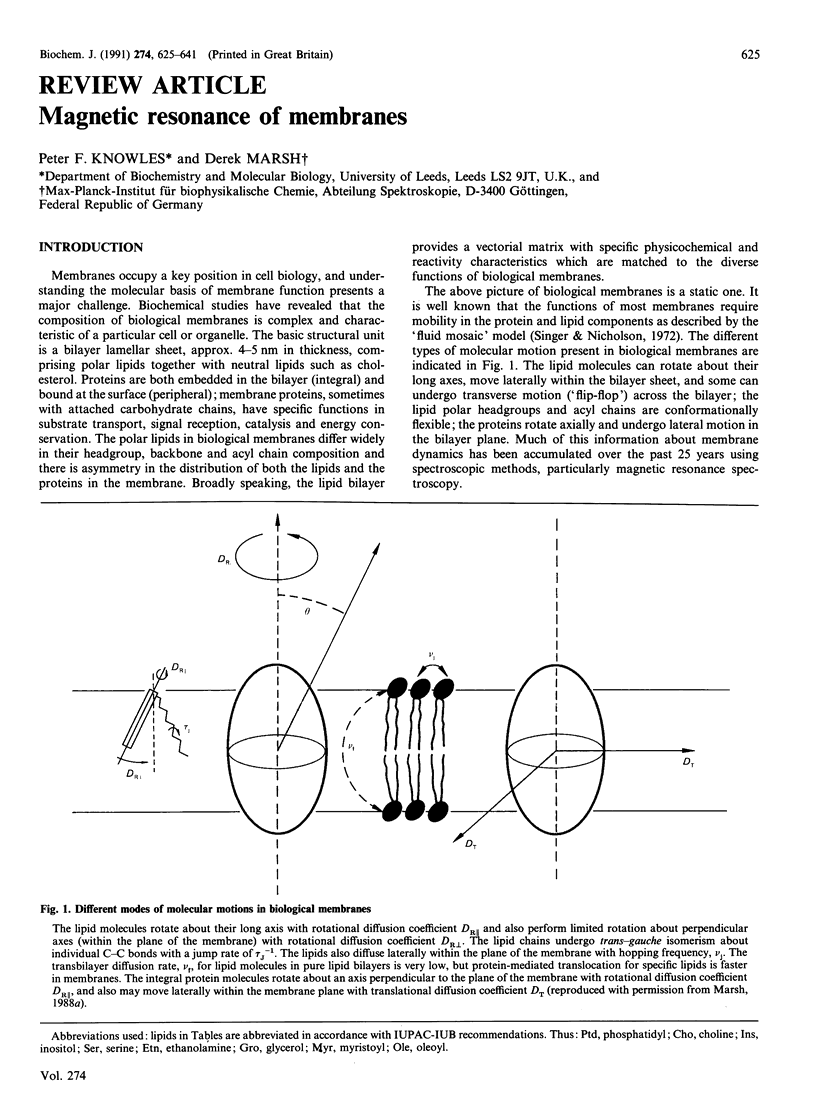










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitch D. A., Marsh D., Powell G. L. Activation of beef-heart cytochrome c oxidase by cardiolipin and analogues of cardiolipin. Biochim Biophys Acta. 1990 Oct 24;1020(1):34–42. doi: 10.1016/0005-2728(90)90090-q. [DOI] [PubMed] [Google Scholar]
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Rees D. C., Deisenhofer J., Michel H., Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by x-ray diffraction. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8589–8593. doi: 10.1073/pnas.83.22.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbach C., Flitsch S. L., Khorana H. G., Hubbell W. L. Structural studies on transmembrane proteins. 2. Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989 Sep 19;28(19):7806–7812. doi: 10.1021/bi00445a042. [DOI] [PubMed] [Google Scholar]
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- Altenbach C., Seelig J. Ca2+ binding to phosphatidylcholine bilayers as studied by deuterium magnetic resonance. Evidence for the formation of a Ca2+ complex with two phospholipid molecules. Biochemistry. 1984 Aug 14;23(17):3913–3920. doi: 10.1021/bi00312a019. [DOI] [PubMed] [Google Scholar]
- Batchelor J. G., Prestegard J. H., Cushley R. J., Lipsky S. R. Conformational analysis of lecithin in vesicles by 13 C NMR. Biochem Biophys Res Commun. 1972 Jul 11;48(1):70–75. doi: 10.1016/0006-291x(72)90345-2. [DOI] [PubMed] [Google Scholar]
- Bazzo R., Tappin M. J., Pastore A., Harvey T. S., Carver J. A., Campbell I. D. The structure of melittin. A 1H-NMR study in methanol. Eur J Biochem. 1988 Apr 5;173(1):139–146. doi: 10.1111/j.1432-1033.1988.tb13977.x. [DOI] [PubMed] [Google Scholar]
- Beinert H. EPR spectroscopy of components of the mitochondrial electron-transfer system. Methods Enzymol. 1978;54:133–150. doi: 10.1016/s0076-6879(78)54014-7. [DOI] [PubMed] [Google Scholar]
- Beinert H. Iron-sulphur clusters: agents of electron transfer and storage, and direct participants in enzymic reactions. Tenth Keilin memorial lecture. Biochem Soc Trans. 1986 Jun;14(3):527–533. doi: 10.1042/bst0140527. [DOI] [PubMed] [Google Scholar]
- Bienvenue A., Bloom M., Davis J. H., Devaux P. F. Evidence for protein-associated lipids from deuterium nuclear magnetic resonance studies of rhodopsin-dimyristoylphosphatidylcholine recombinants. J Biol Chem. 1982 Mar 25;257(6):3032–3038. [PubMed] [Google Scholar]
- Bigelow D. J., Squier T. C., Thomas D. D. Temperature dependence of rotational dynamics of protein and lipid in sarcoplasmic reticulum membranes. Biochemistry. 1986 Jan 14;25(1):194–202. doi: 10.1021/bi00349a028. [DOI] [PubMed] [Google Scholar]
- Bigelow D. J., Thomas D. D. Rotational dynamics of lipid and the Ca-ATPase in sarcoplasmic reticulum. The molecular basis of activation by diethyl ether. J Biol Chem. 1987 Oct 5;262(28):13449–13456. [PubMed] [Google Scholar]
- Blume A., Rice D. M., Wittebort R. J., Griffin R. G. Molecular dynamics and conformation in the gel and liquid-crystalline phases of phosphatidylethanolamine bilayers. Biochemistry. 1982 Nov 23;21(24):6220–6230. doi: 10.1021/bi00267a030. [DOI] [PubMed] [Google Scholar]
- Bogusky M. J., Leo G. C., Opella S. J. Comparison of the dynamics of the membrane-bound form of fd coat protein in micelles and in bilayers by solution and solid-state nitrogen-15 nuclear magnetic resonance spectroscopy. Proteins. 1988;4(2):123–130. doi: 10.1002/prot.340040205. [DOI] [PubMed] [Google Scholar]
- Braun W., Bösch C., Brown L. R., Go N., Wüthrich K. Combined use of proton-proton Overhauser enhancements and a distance geometry algorithm for determination of polypeptide conformations. Application to micelle-bound glucagon. Biochim Biophys Acta. 1981 Feb 27;667(2):377–396. doi: 10.1016/0005-2795(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Brophy P. J., Horváth L. I., Marsh D. Stoichiometry and specificity of lipid-protein interaction with myelin proteolipid protein studied by spin-label electron spin resonance. Biochemistry. 1984 Feb 28;23(5):860–865. doi: 10.1021/bi00300a011. [DOI] [PubMed] [Google Scholar]
- Brotherus J. R., Griffith O. H., Brotherus M. O., Jost P. C., Silvius J. R., Hokin L. E. Lipid--protein multiple binding equilibria in membranes. Biochemistry. 1981 Sep 1;20(18):5261–5267. doi: 10.1021/bi00521a026. [DOI] [PubMed] [Google Scholar]
- Browning J. L., Seelig J. Bilayers of phosphatidylserine: a deuterium and phosphorus nuclear magnetic resonance study. Biochemistry. 1980 Mar 18;19(6):1262–1270. doi: 10.1021/bi00547a034. [DOI] [PubMed] [Google Scholar]
- Brudvig G. W., Beck W. F., de Paula J. C. Mechanism of photosynthetic water oxidation. Annu Rev Biophys Biophys Chem. 1989;18:25–46. doi: 10.1146/annurev.bb.18.060189.000325. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S. Electron paramagnetic resonance methods for measuring pH gradients, transmembrane potentials, and membrane dynamics. Methods Enzymol. 1989;172:331–345. doi: 10.1016/s0076-6879(89)72022-x. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. EPR determination of membrane potentials. Annu Rev Biophys Bioeng. 1981;10:217–244. doi: 10.1146/annurev.bb.10.060181.001245. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Electrogenic H+/OH- movement across phospholipid vesicles measured by spin-labeled hydrophobic ions. Biophys J. 1983 Oct;44(1):49–57. doi: 10.1016/S0006-3495(83)84276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Estimation of transmembrane pH gradients from phase equilibria of spin-labeled amines. Biochemistry. 1978 Sep 5;17(18):3871–3877. doi: 10.1021/bi00611a030. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Estimation of transmembrane potentials from phase equilibria of hydrophobic paramagnetic ions. Biochemistry. 1978 Jan 10;17(1):187–195. doi: 10.1021/bi00594a028. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Light-induced interfacial potentials in photoreceptor membranes. Biophys J. 1980 May;30(2):243–263. doi: 10.1016/S0006-3495(80)85092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Transmembrane electrical currents of spin-labeled hydrophobic ions. Biophys J. 1982 Sep;39(3):263–272. doi: 10.1016/S0006-3495(82)84516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D., McLaughlin A., McLaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Hubbell W. L. Estimation of membrane surface potential and charge density from the phase equilibrium of a paramagnetic amphiphile. Biochemistry. 1976 Nov 2;15(22):4818–4831. doi: 10.1021/bi00667a011. [DOI] [PubMed] [Google Scholar]
- Cevc G., Watts A., Marsh D. Non-electrostatic contribution to the titration of the ordered-fluid phase transition of phosphatidylglycerol bilayers. FEBS Lett. 1980 Nov 3;120(2):267–270. doi: 10.1016/0014-5793(80)80313-9. [DOI] [PubMed] [Google Scholar]
- Champeil P., Büschlen-Boucly S., Bastide F., Gary-Bobo C. Sarcoplasmic reticulum ATPase. Spin labeling detection of ligand-induced changes in the relative reactivities of certain sulfhydryl groups. J Biol Chem. 1978 Feb 25;253(4):1179–1186. [PubMed] [Google Scholar]
- Cherry R. J., Godfrey R. E. Anisotropic rotation of bacteriorhodopsin in lipid membranes. Comparison of theory with experiment. Biophys J. 1981 Oct;36(1):257–276. doi: 10.1016/S0006-3495(81)84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan C. R., Inesi G. Ca2+-dependent effect of ATP on spin-labeled sarcoplasmic reticulum. J Biol Chem. 1977 May 10;252(9):3044–3049. [PubMed] [Google Scholar]
- Coan C., Keating S. Reactivity of sarcoplasmic reticulum adenosinetriphosphatase with iodoacetamide spin-label: evidence for two conformational states of the substrate binding sites. Biochemistry. 1982 Jun 22;21(13):3214–3220. doi: 10.1021/bi00256a028. [DOI] [PubMed] [Google Scholar]
- Coan C. Sensitivity of spin-labeled sarcoplasmic reticulum to the phosphorylation state of the catalytic site in aqueous media and in dimethyl sulfoxide. Biochemistry. 1983 Dec 6;22(25):5826–5836. doi: 10.1021/bi00294a022. [DOI] [PubMed] [Google Scholar]
- Coan C., Verjovski-Almeida S., Inesi G. Ca2+ regulation of conformational states in the transport cycle of spin-labeled sarcoplasmic reticulum ATPase. J Biol Chem. 1979 Apr 25;254(8):2968–2974. [PubMed] [Google Scholar]
- Cornelius F., Skou J. C. Reconstitution of (Na+ + K+)-ATPase into phospholipid vesicles with full recovery of its specific activity. Biochim Biophys Acta. 1984 May 30;772(3):357–373. doi: 10.1016/0005-2736(84)90153-6. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- De Grip W. J., Drenthe E. H., Van Echteld C. J., De Kruijff B., Verkleij A. J. A possible role of rhodopsin in maintaining bilayer structure in the photoreceptor membrane. Biochim Biophys Acta. 1979 Dec 12;558(3):330–337. doi: 10.1016/0005-2736(79)90269-4. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Smith I. C., Jarrell H. C. A 2H-NMR analysis of dihydrosterculoyl-containing lipids in model membranes: structural effects of a cyclopropane ring. Chem Phys Lipids. 1983 Aug;33(2):153–177. doi: 10.1016/0009-3084(83)90019-1. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F. Redox potentiometry in mitochondrial and photosynthetic bioenergetics. Biochim Biophys Acta. 1974 Oct 31;346(2):165–212. doi: 10.1016/0304-4173(74)90008-1. [DOI] [PubMed] [Google Scholar]
- East J. M., Melville D., Lee A. G. Exchange rates and numbers of annular lipids for the calcium and magnesium ion dependent adenosinetriphosphatase. Biochemistry. 1985 May 21;24(11):2615–2623. doi: 10.1021/bi00332a005. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Tollin G. Semiquinone formation in flavo- and metalloflavoproteins. Top Curr Chem. 1983;108:109–138. doi: 10.1007/3-540-11846-2_4. [DOI] [PubMed] [Google Scholar]
- Ellena J. F., Archer S. J., Dominey R. N., Hill B. D., Cafiso D. S. Localizing the nitroxide group of fatty acid and voltage-sensitive spin-labels in phospholipid bilayers. Biochim Biophys Acta. 1988 May 9;940(1):63–70. doi: 10.1016/0005-2736(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Ellena J. F., Blazing M. A., McNamee M. G. Lipid-protein interactions in reconstituted membranes containing acetylcholine receptor. Biochemistry. 1983 Nov 22;22(24):5523–5535. doi: 10.1021/bi00293a012. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Blasie J. K., Wilson D. F. Orientation of the hemes of cytochrome c oxidase and cytochrome c in mitochondria. FEBS Lett. 1977 Apr 15;76(2):235–239. doi: 10.1016/0014-5793(77)80159-2. [DOI] [PubMed] [Google Scholar]
- Esmann M., Hankovszky H. O., Hideg K., Marsh D. A novel spin-label for study of membrane protein rotational diffusion using saturation transfer electron spin resonance. Application to selectively labelled class I and class II-SH groups of the shark rectal gland Na+/K+-ATPase. Biochim Biophys Acta. 1989 Jan 30;978(2):209–215. doi: 10.1016/0005-2736(89)90117-x. [DOI] [PubMed] [Google Scholar]
- Esmann M., Horváth L. I., Marsh D. Saturation-transfer electron spin resonance studies on the mobility of spin-labeled sodium and potassium ion activated adenosinetriphosphatase in membranes from Squalus acanthias. Biochemistry. 1987 Dec 29;26(26):8675–8683. doi: 10.1021/bi00400a028. [DOI] [PubMed] [Google Scholar]
- Esmann M., Watts A., Marsh D. Spin-label studies of lipid-protein interactions in (Na+,K+)-ATPase membranes from rectal glands of Squalus acanthias. Biochemistry. 1985 Mar 12;24(6):1386–1393. doi: 10.1021/bi00327a016. [DOI] [PubMed] [Google Scholar]
- Esposito G., Carver J. A., Boyd J., Campbell I. D. High-resolution 1H NMR study of the solution structure of alamethicin. Biochemistry. 1987 Feb 24;26(4):1043–1050. doi: 10.1021/bi00378a010. [DOI] [PubMed] [Google Scholar]
- Fajer P., Knowles P. F., Marsh D. Rotational motion of yeast cytochrome oxidase in phosphatidylcholine complexes studied by saturation-transfer electron spin resonance. Biochemistry. 1989 Jun 27;28(13):5634–5643. doi: 10.1021/bi00439a045. [DOI] [PubMed] [Google Scholar]
- Feher G., Hoff A. J., Isaacson R. A., Ackerson L. C. ENDOR experiments on chlorophyll and bacteriochlorophyll in vitro and in the photosynthetic unit. Ann N Y Acad Sci. 1975 Apr 15;244:239–259. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. Hydrophobic ion interactions with membranes. Thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys J. 1986 Feb;49(2):531–540. doi: 10.1016/S0006-3495(86)83663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche T. M., McIntyre J. O., Fleischer S., Trommer W. E. Complex formation between nucleotides and D-beta-hydroxybutyrate dehydrogenase studied by fluorescence and EPR spectroscopy. Biochim Biophys Acta. 1984 Dec 7;791(2):173–185. doi: 10.1016/0167-4838(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Froud R. J., Ragan C. I. Cytochrome c mediates electron transfer between ubiquinol-cytochrome c reductase and cytochrome c oxidase by free diffusion along the surface of the membrane. Biochem J. 1984 Jan 15;217(2):561–571. doi: 10.1042/bj2170561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Chemically induced lipid phase separation in model membranes containing charged lipids: a spin label study. Biochim Biophys Acta. 1975 Sep 2;401(3):509–529. doi: 10.1016/0005-2736(75)90249-7. [DOI] [PubMed] [Google Scholar]
- Gao Y., Boyd J., Williams R. J., Pielak G. J. Assignment of proton resonances, identification of secondary structural elements, and analysis of backbone chemical shifts for the C102T variant of yeast iso-1-cytochrome c and horse cytochrome c. Biochemistry. 1990 Jul 31;29(30):6994–7003. doi: 10.1021/bi00482a007. [DOI] [PubMed] [Google Scholar]
- Gawrisch K., Arnold K., Rüger H. J., Kertscher P., Nuhn P. NMR and calorimetric studies of changes in phase transition of head group modified phospholipids. Chem Phys Lipids. 1977 Dec;20(4):285–293. doi: 10.1016/0009-3084(77)90069-x. [DOI] [PubMed] [Google Scholar]
- Gibson J. F., Hall D. O., Thornley J. H., Whatley F. R. The iron complex in spinach ferredoxin. Proc Natl Acad Sci U S A. 1966 Sep;56(3):987–990. doi: 10.1073/pnas.56.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G., Leigh J. S., Jr Distance between the visible copper and cytochrome a in bovine heart cytochrome oxidase. Biochemistry. 1985 Apr 23;24(9):2310–2317. doi: 10.1021/bi00330a028. [DOI] [PubMed] [Google Scholar]
- Griffin R. G., Powers L., Pershan P. S. Head-group conformation in phospholipids: a phosphorus-31 nuclear magnetic resonance study of oriented monodomain dipalmitoylphosphatidylcholine bilayers. Biochemistry. 1978 Jul 11;17(14):2718–2722. doi: 10.1021/bi00607a004. [DOI] [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R., Chazotte B., Gupte S. S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986 Oct;18(5):331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Mulder P. P., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Solid-state 13C NMR studies of retinal in bacteriorhodopsin. Biochemistry. 1984 Jun 5;23(12):2662–2667. doi: 10.1021/bi00307a019. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsel S. C., Cafiso D. S. A test of discreteness-of-charge effects in phospholipid vesicles: measurements using paramagnetic amphiphiles. Biochemistry. 1986 Dec 16;25(25):8214–8219. doi: 10.1021/bi00373a014. [DOI] [PubMed] [Google Scholar]
- Hartsel S. C., Moore C. R., Raines D. E., Cafiso D. S. Time-dependent binding of paramagnetic and fluorescent hydrophobic ions to the acetylcholine receptor from Torpedo. Biochemistry. 1987 Jun 16;26(12):3253–3260. doi: 10.1021/bi00386a003. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Herrmann A., Zachowski A., Devaux P. F. Protein-mediated phospholipid translocation in the endoplasmic reticulum with a low lipid specificity. Biochemistry. 1990 Feb 27;29(8):2023–2027. doi: 10.1021/bi00460a010. [DOI] [PubMed] [Google Scholar]
- Herzfeld J., Mulliken C. M., Siminovitch D. J., Griffin R. G. Contrasting molecular dynamics in red and purple membrane fractions of the Halobacterium halobium. Biophys J. 1987 Nov;52(5):855–858. doi: 10.1016/S0006-3495(87)83278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilden S., Hokin L. Coupled Na+ -K+ transport in vesicles containing a purified (NaK)-ATPase and only phosphatidyl choline. Biochem Biophys Res Commun. 1976 Mar 22;69(2):521–527. doi: 10.1016/0006-291x(76)90552-0. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth L. I., Arias H. R., Hankovszky H. O., Hideg K., Barrantes F. J., Marsh D. Association of spin-labeled local anesthetics at the hydrophobic surface of acetylcholine receptor in native membranes from Torpedo marmorata. Biochemistry. 1990 Sep 18;29(37):8707–8713. doi: 10.1021/bi00489a029. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Brophy P. J., Marsh D. Exchange rates at the lipid-protein interface of myelin proteolipid protein studied by spin-label electron spin resonance. Biochemistry. 1988 Jan 12;27(1):46–52. doi: 10.1021/bi00401a009. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Brophy P. J., Marsh D. Influence of lipid headgroup on the specificity and exchange dynamics in lipid-protein interactions. A spin-label study of myelin proteolipid apoprotein-phospholipid complexes. Biochemistry. 1988 Jul 12;27(14):5296–5304. doi: 10.1021/bi00414a052. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Drees M., Beyer K., Klingenberg M., Marsh D. Lipid-protein interactions in ADP-ATP carrier/egg phosphatidylcholine recombinants studied by spin-label ESR spectroscopy. Biochemistry. 1990 Nov 27;29(47):10664–10669. doi: 10.1021/bi00499a013. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Dux L., Hankovszky H. O., Hideg K., Marsh D. Saturation transfer electron spin resonance of Ca2(+)-ATPase covalently spin-labeled with beta-substituted vinyl ketone- and maleimide-nitroxide derivatives. Effects of segmental motion and labeling levels. Biophys J. 1990 Jul;58(1):231–241. doi: 10.1016/S0006-3495(90)82368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth L. I., Munding A., Beyer K., Klingenberg M., Marsh D. Rotational diffusion of mitochondrial ADP/ATP carrier studied by saturation-transfer electron spin resonance. Biochemistry. 1989 Jan 10;28(1):407–414. doi: 10.1021/bi00427a056. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L. Transbilayer coupling mechanism for the formation of lipid asymmetry in biological membranes. Application to the photoreceptor disc membrane. Biophys J. 1990 Jan;57(1):99–108. doi: 10.1016/S0006-3495(90)82510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Sen A. Effects of lipid packing on polymorphic phase behavior and membrane properties. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5825–5829. doi: 10.1073/pnas.86.15.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell H. C., Jovall P. A., Giziewicz J. B., Turner L. A., Smith I. C. Determination of conformational properties of glycolipid head groups by 2H NMR of oriented multibilayers. Biochemistry. 1987 Apr 7;26(7):1805–1811. doi: 10.1021/bi00381a003. [DOI] [PubMed] [Google Scholar]
- Jarrell H. C., Wand A. J., Giziewicz J. B., Smith I. C. The dependence of glyceroglycolipid orientation and dynamics on head-group structure. Biochim Biophys Acta. 1987 Feb 12;897(1):69–82. doi: 10.1016/0005-2736(87)90316-6. [DOI] [PubMed] [Google Scholar]
- Keniry M. A., Gutowsky H. S., Oldfield E. Surface dynamics of the integral membrane protein bacteriorhodopsin. 1984 Jan 26-Feb 1Nature. 307(5949):383–386. doi: 10.1038/307383a0. [DOI] [PubMed] [Google Scholar]
- Keniry M. A., Kintanar A., Smith R. L., Gutowsky H. S., Oldfield E. Nuclear magnetic resonance studies of amino acids and proteins. Deuterium nuclear magnetic resonance relaxation of deuteriomethyl-labeled amino acids in crystals and in Halobacterium halobium and Escherichia coli cell membranes. Biochemistry. 1984 Jan 17;23(2):288–298. doi: 10.1021/bi00297a018. [DOI] [PubMed] [Google Scholar]
- King M. D., Marsh D. Polymorphic phase behavior of lysopalmitoylphosphatidylcholine in poly(ethylene glycol)-water mixtures. Biochemistry. 1989 Jun 27;28(13):5643–5647. doi: 10.1021/bi00439a046. [DOI] [PubMed] [Google Scholar]
- Kinsey R. A., Kintanar A., Oldfield E. Dynamics of amino acid side chains in membrane proteins by high field solid state deuterium nuclear magnetic resonance spectroscopy. Phenylalanine, tyrosine, and tryptophan. J Biol Chem. 1981 Sep 10;256(17):9028–9036. [PubMed] [Google Scholar]
- Klingenberg M. Membrane protein oligomeric structure and transport function. Nature. 1981 Apr 9;290(5806):449–454. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Watts A., Marsh D. Spin-label studies of lipid immobilization in dimyristoylphosphatidylcholine-substituted cytochrome oxidase. Biochemistry. 1979 Oct 16;18(21):4480–4487. doi: 10.1021/bi00588a005. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Kuchinka E., Seelig J. Interaction of melittin with phosphatidylcholine membranes. Binding isotherm and lipid head-group conformation. Biochemistry. 1989 May 16;28(10):4216–4221. doi: 10.1021/bi00436a014. [DOI] [PubMed] [Google Scholar]
- Kusumi A., Hyde J. S. Spin-label saturation-transfer electron spin resonance detection of transient association of rhodopsin in reconstituted membranes. Biochemistry. 1982 Nov 9;21(23):5978–5983. doi: 10.1021/bi00266a039. [DOI] [PubMed] [Google Scholar]
- Lange A., Marsh D., Wassmer K. H., Meier P., Kothe G. Electron spin resonance study of phospholipid membranes employing a comprehensive line-shape model. Biochemistry. 1985 Jul 30;24(16):4383–4392. doi: 10.1021/bi00337a020. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C., Warren G. B., Roberts G. C. A determination of the mobility gradient in lipid bilayers by 13C nuclear magnetic resonance. Proc R Soc Lond B Biol Sci. 1976 May 18;193(1112):253–274. doi: 10.1098/rspb.1976.0045. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Fitton J. E., Wüthrich K. Nuclear magnetic resonance investigation of the conformation of delta-haemolysin bound to dodecylphosphocholine micelles. Biochim Biophys Acta. 1987 Jan 30;911(2):144–153. doi: 10.1016/0167-4838(87)90003-3. [DOI] [PubMed] [Google Scholar]
- Leo G. C., Colnago L. A., Valentine K. G., Opella S. J. Dynamics of fd coat protein in lipid bilayers. Biochemistry. 1987 Feb 10;26(3):854–862. doi: 10.1021/bi00377a029. [DOI] [PubMed] [Google Scholar]
- Li G., Knowles P. F., Murphy D. J., Nishida I., Marsh D. Spin-label ESR studies of lipid-protein interactions in thylakoid membranes. Biochemistry. 1989 Sep 5;28(18):7446–7452. doi: 10.1021/bi00444a044. [DOI] [PubMed] [Google Scholar]
- Li P. M., Morgan J. E., Nilsson T., Ma M., Chan S. I. Heat treatment of cytochrome c oxidase perturbs the CuA site and affects proton pumping behavior. Biochemistry. 1988 Sep 20;27(19):7538–7546. doi: 10.1021/bi00419a054. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Wennerström H. Amphiphile diffusion in model membrane systems studied by pulsed NMR. Biophys Chem. 1977 Jan;6(2):167–171. doi: 10.1016/0301-4622(77)87006-3. [DOI] [PubMed] [Google Scholar]
- London E., Feigenson G. W. Phosphorus NMR analysis of phospholipids in detergents. J Lipid Res. 1979 Mar;20(3):408–412. [PubMed] [Google Scholar]
- Lugtenburg J., Mathies R. A., Griffin R. G., Herzfeld J. Structure and function of rhodopsins from solid state NMR and resonance Raman spectroscopy of isotopic retinal derivatives. Trends Biochem Sci. 1988 Oct;13(10):388–393. doi: 10.1016/0968-0004(88)90181-8. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Seelig J. Calcium binding to mixed phosphatidylglycerol-phosphatidylcholine bilayers as studied by deuterium nuclear magnetic resonance. Biochemistry. 1987 Mar 10;26(5):1231–1240. doi: 10.1021/bi00379a005. [DOI] [PubMed] [Google Scholar]
- Malthaner M., Hermetter A., Paltauf F., Seelig J. Structure and dynamics of plasmalogen model membranes containing cholesterol: a deuterium NMR study. Biochim Biophys Acta. 1987 Jun 30;900(2):191–197. doi: 10.1016/0005-2736(87)90333-6. [DOI] [PubMed] [Google Scholar]
- Mariani P., Luzzati V., Delacroix H. Cubic phases of lipid-containing systems. Structure analysis and biological implications. J Mol Biol. 1988 Nov 5;204(1):165–189. doi: 10.1016/0022-2836(88)90607-9. [DOI] [PubMed] [Google Scholar]
- Marsh D. Lipid-protein interactions in membranes. FEBS Lett. 1990 Aug 1;268(2):371–375. doi: 10.1016/0014-5793(90)81288-y. [DOI] [PubMed] [Google Scholar]
- Marsh D. Molecular motion in phospholipid bilayers in the gel phase: long axis rotation. Biochemistry. 1980 Apr 15;19(8):1632–1637. doi: 10.1021/bi00549a017. [DOI] [PubMed] [Google Scholar]
- Marsh D. Selectivity of lipid-protein interactions. J Bioenerg Biomembr. 1987 Dec;19(6):677–689. doi: 10.1007/BF00762302. [DOI] [PubMed] [Google Scholar]
- Marsh D., Watts A., Knowles P. F. Evidence for phase boundary lipid. Permeability of Tempo-choline into dimyristoylphosphatidylcholine vesicles at the phase transition. Biochemistry. 1976 Aug 10;15(16):3570–3578. doi: 10.1021/bi00661a027. [DOI] [PubMed] [Google Scholar]
- Marsh D., Watts A. NMR spin-spin splittings in lipid membranes. Headgroup conformation in phosphatidylglycerol bilayers. FEBS Lett. 1978 Jan 1;85(1):124–126. doi: 10.1016/0014-5793(78)81262-9. [DOI] [PubMed] [Google Scholar]
- Marsh D., Watts A., Pates R. D., Uhl R., Knowles P. F., Esmann M. ESR spin-label studies of lipid-protein interactions in membranes. Biophys J. 1982 Jan;37(1):265–274. doi: 10.1016/S0006-3495(82)84675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D., Watts A., Smith I. C. Dynamic structure and phase behavior of dimyristoylphosphatidylethanolamine bilayers studied by deuterium nuclear magnetic resonance. Biochemistry. 1983 Jun 7;22(12):3023–3026. doi: 10.1021/bi00281a036. [DOI] [PubMed] [Google Scholar]
- McCain D. C., Markley J. L. Water permeability of chloroplast envelope membranes. In vivo measurement by saturation-transfer NMR. FEBS Lett. 1985 Apr 22;183(2):353–358. doi: 10.1016/0014-5793(85)80809-7. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- McDaniel R. V., Sharp K., Brooks D., McLaughlin A. C., Winiski A. P., Cafiso D., McLaughlin S. Electrokinetic and electrostatic properties of bilayers containing gangliosides GM1, GD1a, or GT1. Comparison with a nonlinear theory. Biophys J. 1986 Mar;49(3):741–752. doi: 10.1016/S0006-3495(86)83700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee M. G., McConnell H. M. Transmembrane potentials and phospholipid flip-flop in excitable membrane vesicles. Biochemistry. 1973 Jul 31;12(16):2951–2958. doi: 10.1021/bi00740a001. [DOI] [PubMed] [Google Scholar]
- Meier P., Sachse J. H., Brophy P. J., Marsh D., Kothe G. Integral membrane proteins significantly decrease the molecular motion in lipid bilayers: a deuteron NMR relaxation study of membranes containing myelin proteolipid apoprotein. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3704–3708. doi: 10.1073/pnas.84.11.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendz G. L., Moore W. J., Carnegie P. R. Proton N.M.R. evidence for secondary and tertiary structure in myelin basic proteins. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1333–1340. doi: 10.1016/0006-291x(82)90933-0. [DOI] [PubMed] [Google Scholar]
- Mendz G. L., Moore W. J., Martenson R. E. NMR studies of myelin basic protein. IX. Complete assignments of the tyrosine residues by proton NMR of proteins from six species. Biochim Biophys Acta. 1983 Oct 28;748(2):168–175. doi: 10.1016/0167-4838(83)90292-3. [DOI] [PubMed] [Google Scholar]
- Mendz G. L., Moore W. J., Martenson R. E. NMR studies of myelin basic protein. XIII. Assignment of histidine residues in rabbit, bovine and porcine proteins. Biochim Biophys Acta. 1986 Jun 5;871(2):156–166. doi: 10.1016/0167-4838(86)90169-x. [DOI] [PubMed] [Google Scholar]
- Mendz G. L., Moore W. J., Martenson R. E. NMR studies on myelin basic protein. VIII. Complete assignment of the threonine residues by proton NMR of proteins from five species. Biochim Biophys Acta. 1983 Jan 12;742(1):215–223. doi: 10.1016/0167-4838(83)90379-5. [DOI] [PubMed] [Google Scholar]
- Mollevanger L. C., De Grip W. J. Phase behavior of isolated photoreceptor membrane lipids is modulated by bivalent cations. FEBS Lett. 1984 Apr 24;169(2):256–260. doi: 10.1016/0014-5793(84)80329-4. [DOI] [PubMed] [Google Scholar]
- Mollevanger L. C., Kentgens A. P., Pardoen J. A., Courtin J. M., Veeman W. S., Lugtenburg J., de Grip W. J. High-resolution solid-state 13C-NMR study of carbons C-5 and C-12 of the chromophore of bovine rhodopsin. Evidence for a 6-S-cis conformation with negative-charge perturbation near C-12. Eur J Biochem. 1987 Feb 16;163(1):9–14. doi: 10.1111/j.1432-1033.1987.tb10729.x. [DOI] [PubMed] [Google Scholar]
- Morrot G., Hervé P., Zachowski A., Fellmann P., Devaux P. F. Aminophospholipid translocase of human erythrocytes: phospholipid substrate specificity and effect of cholesterol. Biochemistry. 1989 Apr 18;28(8):3456–3462. doi: 10.1021/bi00434a046. [DOI] [PubMed] [Google Scholar]
- Moser M., Marsh D., Meier P., Wassmer K. H., Kothe G. Chain configuration and flexibility gradient in phospholipid membranes. Comparison between spin-label electron spin resonance and deuteron nuclear magnetic resonance, and identification of new conformations. Biophys J. 1989 Jan;55(1):111–123. doi: 10.1016/S0006-3495(89)82784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford C. E., Wright L. C. Organization of lipids in the plasma membranes of malignant and stimulated cells: a new model. Trends Biochem Sci. 1988 May;13(5):172–177. doi: 10.1016/0968-0004(88)90145-4. [DOI] [PubMed] [Google Scholar]
- Mulvey D., King G. F., Cooke R. M., Doak D. G., Harvey T. S., Campbell I. D. High resolution 1H NMR study of the solution structure of the S4 segment of the sodium channel protein. FEBS Lett. 1989 Oct 23;257(1):113–117. doi: 10.1016/0014-5793(89)81799-5. [DOI] [PubMed] [Google Scholar]
- Navarro J., Toivio-Kinnucan M., Racker E. Effect of lipid composition on the calcium/adenosine 5'-triphosphate coupling ratio of the Ca2+-ATPase of sarcoplasmic reticulum. Biochemistry. 1984 Jan 3;23(1):130–135. doi: 10.1021/bi00296a021. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Budil D. E., Gast P., Chang C. H., el-Kabbani O., Schiffer M. Correlation of paramagnetic states and molecular structure in bacterial photosynthetic reaction centers: the symmetry of the primary electron donor in Rhodopseudomonas viridis and Rhodobacter sphaeroides R-26. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4335–4339. doi: 10.1073/pnas.86.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Katz J. J. Models for antenna and reaction center chlorophylls. Ann N Y Acad Sci. 1975 Apr 15;244:260–280. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]
- Ogino T., den Hollander J. A., Shulman R. G. 39K, 23Na, and 31P NMR studies of ion transport in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5185–5189. doi: 10.1073/pnas.80.17.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Harmon H. J., Waring A. J. Electron-paramagnetic-resonance studies on the spatial relationship of redox components in cytochrome oxidase. Biochem Soc Trans. 1985 Jun;13(3):607–611. doi: 10.1042/bst0130607. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., LoBrutto R., Salerno J. C., Bruckner R. C., Frey T. G. Spatial relationship between cytochrome a and a3. J Biol Chem. 1982 Dec 25;257(24):14821–14825. [PubMed] [Google Scholar]
- Ohnishi T., Ragan C. I., Hatefi Y. EPR studies of iron-sulfur clusters in isolated subunits and subfractions of NADH-ubiquinone oxidoreductase. J Biol Chem. 1985 Mar 10;260(5):2782–2788. [PubMed] [Google Scholar]
- Oldfield E., Bowers J. L., Forbes J. High-resolution proton and carbon-13 NMR of membranes: why sonicate? Biochemistry. 1987 Nov 3;26(22):6919–6923. doi: 10.1021/bi00396a009. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Meadows M., Rice D., Jacobs R. Spectroscopic studies of specifically deuterium labeled membrane systems. Nuclear magnetic resonance investigation of the effects of cholesterol in model systems. Biochemistry. 1978 Jul 11;17(14):2727–2740. doi: 10.1021/bi00607a006. [DOI] [PubMed] [Google Scholar]
- Oldfield E. Spectroscopic studies of lipids and biological membranes. Twenty-first Colworth medal lecture. Biochem Soc Trans. 1988 Feb;16(1):1–10. doi: 10.1042/bst0160001. [DOI] [PubMed] [Google Scholar]
- Oliveira C. R., Coan C., Verjovski-Almeida S. Interaction of spin-labeled nucleotides with sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1988 Aug 9;27(16):5923–5927. doi: 10.1021/bi00416a015. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Opella S. J., Stewart P. L., Valentine K. G. Protein structure by solid-state NMR spectroscopy. Q Rev Biophys. 1987 Feb;19(1-2):7–49. doi: 10.1017/s0033583500004017. [DOI] [PubMed] [Google Scholar]
- Pates R. D., Watts A., Uhl R., Marsh D. Lipid-protein interactions in frog rod outer segment disc membranes. Characterization by spin labels. Biochim Biophys Acta. 1985 Apr 11;814(2):389–397. doi: 10.1016/0005-2736(85)90460-2. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Plesner I. W., Plesner L., Nørby J. G., Klodos I. The steady-state kinetic mechanism of ATP hydrolysis catalyzed by membrane-bound (Na+ + K+)-ATPase from ox brain. III. A minimal model. Biochim Biophys Acta. 1981 May 6;643(2):483–494. doi: 10.1016/0005-2736(81)90090-0. [DOI] [PubMed] [Google Scholar]
- Powell G. L., Knowles P. F., Marsh D. Incorporation of cytochrome oxidase into cardiolipin bilayers and induction of nonlamellar phases. Biochemistry. 1990 May 29;29(21):5127–5132. doi: 10.1021/bi00473a018. [DOI] [PubMed] [Google Scholar]
- Powell G. L., Marsh D. Polymorphic phase behavior of cardiolipin derivatives studied by 31P NMR and X-ray diffraction. Biochemistry. 1985 Jun 4;24(12):2902–2908. doi: 10.1021/bi00333a013. [DOI] [PubMed] [Google Scholar]
- Quintanilha A. T., Packer L. Surface potential changes on energization of the mitochondrial inner membrane. FEBS Lett. 1977 Jun 15;78(2):161–165. doi: 10.1016/0014-5793(77)80296-2. [DOI] [PubMed] [Google Scholar]
- Rance M., Jeffrey K. R., Tulloch A. P., Butler K. W., Smith I. C. Orientational order of unsaturated lipids in the membranes of Acholeplasma laidlawii as observed by 2H-NMR. Biochim Biophys Acta. 1980 Aug 4;600(2):245–262. doi: 10.1016/0005-2736(80)90430-7. [DOI] [PubMed] [Google Scholar]
- Renou J. P., Giziewicz J. B., Smith I. C., Jarrell H. C. Glycolipid membrane surface structure: orientation, conformation, and motion of a disaccharide headgroup. Biochemistry. 1989 Feb 21;28(4):1804–1814. doi: 10.1021/bi00430a057. [DOI] [PubMed] [Google Scholar]
- Rietveld A., van Kemenade T. J., Hak T., Verkleij A. J., de Kruijff B. The effect of cytochrome c oxidase on lipid polymorphism of model membranes containing cardiolipin. Eur J Biochem. 1987 Apr 1;164(1):137–140. doi: 10.1111/j.1432-1033.1987.tb11004.x. [DOI] [PubMed] [Google Scholar]
- Rilfors L., Eriksson P. O., Arvidson G., Lindblom G. Relationship between three-dimensional arrays of "lipidic particles" and bicontinuous cubic lipid phases. Biochemistry. 1986 Nov 18;25(23):7702–7711. doi: 10.1021/bi00371a063. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Roux M., Neumann J. M., Hodges R. S., Devaux P. F., Bloom M. Conformational changes of phospholipid headgroups induced by a cationic integral membrane peptide as seen by deuterium magnetic resonance. Biochemistry. 1989 Mar 7;28(5):2313–2321. doi: 10.1021/bi00431a050. [DOI] [PubMed] [Google Scholar]
- Rudy B., Dubois H., Mink R., Trommer W. E., McIntyre J. O., Fleischer S. Coenzyme binding by 3-hydroxybutyrate dehydrogenase, a lipid-requiring enzyme: lecithin acts as an allosteric modulator to enhance the affinity for coenzyme. Biochemistry. 1989 Jun 27;28(13):5354–5366. doi: 10.1021/bi00439a007. [DOI] [PubMed] [Google Scholar]
- Ryba N. J., Horváth L. I., Watts A., Marsh D. Molecular exchange at the lipid-rhodopsin interface: spin-label electron spin resonance studies of rhodopsin-dimyristoylphosphatidylcholine recombinants. Biochemistry. 1987 Jun 2;26(11):3234–3240. doi: 10.1021/bi00385a045. [DOI] [PubMed] [Google Scholar]
- Sakaki T., Tsuji A., Chang C. H., Ohnishi S. Rotational mobility of an erythrocyte membrane integral protein band 3 in dimyristoylphosphatidylcholine reconstituted vesicles and effect of binding of cytoskeletal peripheral proteins. Biochemistry. 1982 May 11;21(10):2366–2372. doi: 10.1021/bi00539a014. [DOI] [PubMed] [Google Scholar]
- Salerno J. C., Osgood M., Liu Y. J., Taylor H., Scholes C. P. Electron nuclear double resonance (ENDOR) of the Qc.- ubisemiquinone radical in the mitochondrial electron transport chain. Biochemistry. 1990 Jul 31;29(30):6987–6993. doi: 10.1021/bi00482a006. [DOI] [PubMed] [Google Scholar]
- Scherer P. G., Seelig J. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry. 1989 Sep 19;28(19):7720–7728. doi: 10.1021/bi00445a030. [DOI] [PubMed] [Google Scholar]
- Schiksnis R. A., Bogusky M. J., Tsang P., Opella S. J. Structure and dynamics of the Pf1 filamentous bacteriophage coat protein in micelles. Biochemistry. 1987 Mar 10;26(5):1373–1381. doi: 10.1021/bi00379a025. [DOI] [PubMed] [Google Scholar]
- Schobert B., Marsh D. Spin label studies on osmotically-induced changes in the aqueous cytoplasm of Phaeodactylum tricornutum. Biochim Biophys Acta. 1982 Feb 10;720(1):87–95. doi: 10.1016/0167-4889(82)90042-8. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. Bilayers of dipalmitoyl-3-sn-phosphatidylcholine. Conformational differences between the fatty acyl chains. Biochim Biophys Acta. 1975 Sep 16;406(1):1–5. doi: 10.1016/0005-2736(75)90037-1. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally G. U., Wohlgemuth R. Orientation and flexibility of the choline head group in phosphatidylcholine bilayers. Biochim Biophys Acta. 1977 Jun 2;467(2):109–119. doi: 10.1016/0005-2736(77)90188-2. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Seelig J., Waespe-Sarcevic N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry. 1978 Aug 8;17(16):3310–3315. doi: 10.1021/bi00609a021. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Zachowski A., Hermann A., Devaux P. F. Asymmetric lipid fluidity in human erythrocyte membrane: new spin-label evidence. Biochemistry. 1984 Sep 11;23(19):4271–4275. doi: 10.1021/bi00314a002. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McMillen D. A., Saley N. D., Jost P. C., Griffith O. H. Competition between cholesterol and phosphatidylcholine for the hydrophobic surface of sarcoplasmic reticulum Ca2+-ATPase. Biochemistry. 1984 Jan 31;23(3):538–547. doi: 10.1021/bi00298a022. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sixl F., Watts A. Deuterium and phosphorus nuclear magnetic resonance studies on the binding of polymyxin B to lipid bilayer-water interfaces. Biochemistry. 1985 Dec 31;24(27):7906–7910. doi: 10.1021/bi00348a009. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Determination of phospholipid head group organization by deuterium and phosphorus nuclear magnetic resonance spectroscopy. Biochemistry. 1979 Dec 25;18(26):5903–5909. doi: 10.1021/bi00593a022. [DOI] [PubMed] [Google Scholar]
- Smith I. C., Tulloch A. P., Stockton G. W., Schreier S., Joyce A., Butler W., Boulanger Y., Blackwell B., Bennett L. G. Determination of membrane properties at the molecular level by carbon-13 and deuterium magnetic resonance. Ann N Y Acad Sci. 1978;308:8–28. doi: 10.1111/j.1749-6632.1978.tb22011.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Courtin J., van den Berg E., Winkel C., Lugtenburg J., Herzfeld J., Griffin R. G. Solid-state 13C NMR of the retinal chromophore in photointermediates of bacteriorhodopsin: characterization of two forms of M. Biochemistry. 1989 Jan 10;28(1):237–243. doi: 10.1021/bi00427a033. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Griffin R. G. High-resolution solid-state NMR of proteins. Annu Rev Phys Chem. 1988;39:511–535. doi: 10.1146/annurev.pc.39.100188.002455. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Palings I., Copié V., Raleigh D. P., Courtin J., Pardoen J. A., Lugtenburg J., Mathies R. A., Griffin R. G. Low-temperature solid-state 13C NMR studies of the retinal chromophore in rhodopsin. Biochemistry. 1987 Mar 24;26(6):1606–1611. doi: 10.1021/bi00380a018. [DOI] [PubMed] [Google Scholar]
- Squier T. C., Bigelow D. J., Thomas D. D. Lipid fluidity directly modulates the overall protein rotational mobility of the Ca-ATPase in sarcoplasmic reticulum. J Biol Chem. 1988 Jul 5;263(19):9178–9186. [PubMed] [Google Scholar]
- Squier T. C., Hughes S. E., Thomas D. D. Rotational dynamics and protein-protein interactions in the Ca-ATPase mechanism. J Biol Chem. 1988 Jul 5;263(19):9162–9170. [PubMed] [Google Scholar]
- Squier T. C., Thomas D. D. Relationship between protein rotational dynamics and phosphoenzyme decomposition in the sarcoplasmic reticulum Ca-ATPase. J Biol Chem. 1988 Jul 5;263(19):9171–9177. [PubMed] [Google Scholar]
- Stubbe J. A. Protein radical involvement in biological catalysis? Annu Rev Biochem. 1989;58:257–285. doi: 10.1146/annurev.bi.58.070189.001353. [DOI] [PubMed] [Google Scholar]
- Sundberg S. A., Hubbell W. L. Investigation of surface potential asymmetry in phospholipid vesicles by a spin label relaxation method. Biophys J. 1986 Feb;49(2):553–562. doi: 10.1016/S0006-3495(86)83665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappin M. J., Pastore A., Norton R. S., Freer J. H., Campbell I. D. High-resolution 1H NMR study of the solution structure of delta-hemolysin. Biochemistry. 1988 Mar 8;27(5):1643–1647. doi: 10.1021/bi00405a038. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., De Kruijff B., Verkleij A., Van Echteld C. J. Effect of glycophorin on lipid polymorphism. A 31P-NMR study. Biochim Biophys Acta. 1982 Feb 23;685(2):153–161. doi: 10.1016/0005-2736(82)90092-x. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., de Kruijff B., Verkleij A. J. The effect of an integral membrane protein on lipid polymorphism in the cardiolipin-Ca2+ system. Eur J Biochem. 1983 Jan 1;129(3):621–625. doi: 10.1111/j.1432-1033.1983.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Bigelow D. J., Squier T. C., Hidalgo C. Rotational dynamics of protein and boundary lipid in sarcoplasmic reticulum membrane. Biophys J. 1982 Jan;37(1):217–225. doi: 10.1016/S0006-3495(82)84671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Tsui F. C., Sundberg S. A., Hubbell W. L. Distribution of charge on photoreceptor disc membranes and implications for charged lipid asymmetry. Biophys J. 1990 Jan;57(1):85–97. doi: 10.1016/S0006-3495(90)82509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Echteld C. J., De Kruijff B., Mandersloot J. G., De Gier J. Effects of lysophosphatidylcholines on phosphatidylcholine and phosphatidylcholine/cholesterol liposome systems as revealed by 31P-NMR, electron microscopy and permeability studies. Biochim Biophys Acta. 1981 Dec 7;649(2):211–220. doi: 10.1016/0005-2736(81)90408-9. [DOI] [PubMed] [Google Scholar]
- Vänngård T. Electron-paramagnetic-resonance studies of structure and function of the two-haem enzymes Pseudomonas cytochrome c peroxidase and beef heart cytochrome c oxidase. Biochem Soc Trans. 1985 Jun;13(3):619–622. doi: 10.1042/bst0130619. [DOI] [PubMed] [Google Scholar]
- Watts A., Marsh D., Knowles P. F. Characterization of dimyristoylphosphatidylcholine vesicles and their dimensional changes through the phase transition: molecular control of membrane morphology. Biochemistry. 1978 May 2;17(9):1792–1801. doi: 10.1021/bi00602a034. [DOI] [PubMed] [Google Scholar]
- Watts A., Volotovski I. D., Marsh D. Rhodopsin-lipid associations in bovine rod outer segment membranes. Identification of immobilized lipid by spin-labels. Biochemistry. 1979 Oct 30;18(22):5006–5013. doi: 10.1021/bi00589a031. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W., Devaux P. F., Bienvenue A. Phosphatidylcholine exchange protein catalyzes the net transfer of phosphatidylcholine to model membranes. Biochemistry. 1980 Jul 8;19(14):3395–3399. doi: 10.1021/bi00555a046. [DOI] [PubMed] [Google Scholar]
- Wolfs J. A., Horváth L. I., Marsh D., Watts A., Hemminga M. A. Spin-label ESR of bacteriophage M13 coat protein in mixed lipid bilayers. Characterization of molecular selectivity of charged phospholipids for the bacteriophage M13 coat protein in lipid bilayers. Biochemistry. 1989 Dec 26;28(26):9995–10001. doi: 10.1021/bi00452a018. [DOI] [PubMed] [Google Scholar]
- Xu Z. C., Cafiso D. S. Phospholipid packing and conformation in small vesicles revealed by two-dimensional 1H nuclear magnetic resonance cross-relaxation spectroscopy. Biophys J. 1986 Mar;49(3):779–783. doi: 10.1016/S0006-3495(86)83705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L., Hutton W. C., Huang C. H., Martin R. B. Structure in the polar head region of phospholipid bilayers: A 31P [1H] nuclear Overhauser effect study. Biochemistry. 1976 May 18;15(10):2121–2124. doi: 10.1021/bi00655a014. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Hutton W. C., Huang C., Martin R. B. Phospholipid head-group conformations; intermolecular interactions and cholesterol effects. Biochemistry. 1977 Oct 4;16(20):4344–4349. doi: 10.1021/bi00639a003. [DOI] [PubMed] [Google Scholar]
- Yoon P. S., Sharp R. R. Ca2+ and proton transport in chromaffin granule membranes: a proton NMR study. Biochemistry. 1985 Dec 3;24(25):7269–7273. doi: 10.1021/bi00346a037. [DOI] [PubMed] [Google Scholar]
- Zachowski A., Fellman P., Devaux P. F. Absence of transbilayer diffusion of spin-labeled sphingomyelin on human erythrocytes. Comparison with the diffusion of several spin-labeled glycerophospholipids. Biochim Biophys Acta. 1985 May 28;815(3):510–514. doi: 10.1016/0005-2736(85)90380-3. [DOI] [PubMed] [Google Scholar]
- de Jongh H. H., Hemminga M. A., Marsh D. ESR of spin-labeled bacteriophage M13 coat protein in mixed phospholipid bilayers. Biochim Biophys Acta. 1990 May 9;1024(1):82–88. doi: 10.1016/0005-2736(90)90210-f. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R. Cytochrome c specifically induces non-bilayer structures in cardiolipin-containing model membranes. Biochim Biophys Acta. 1980 Nov 18;602(3):477–490. doi: 10.1016/0005-2736(80)90327-2. [DOI] [PubMed] [Google Scholar]
- van Meer G., Op den Kamp J. A. Transbilayer movement of various phosphatidylcholine species in intact human erythrocytes. J Cell Biochem. 1982;19(2):193–204. doi: 10.1002/jcb.240190209. [DOI] [PubMed] [Google Scholar]